Abstract
The Middle East respiratory syndrome coronavirus (MERS-CoV) was first discovered in late 2012 and has gone on to cause over 1800 infections and 650 deaths. There are currently no approved therapeutics or vaccinations for MERS-CoV. The MERS-CoV spike (S) protein is responsible for receptor binding and virion entry to cells, is immunodominant and induces neutralizing antibodies in vivo, all of which, make the S protein an ideal target for anti-MERS-CoV vaccines. In this study, we demonstrate protection induced by vaccination with a recombinant MERS-CoV S nanoparticle vaccine and Matrix-M1 adjuvant combination in mice. The MERS-CoV S nanoparticle vaccine produced high titer anti-S neutralizing antibody and protected mice from MERS-CoV infection in vivo.
Keywords: MERS-CoV, Nanoparticle vaccine, Mouse model, Coronavirus
1. Introduction
The Middle East respiratory syndrome coronavirus (MERS-CoV) is a highly pathogenic respiratory virus that was first identified in the Kingdom of Saudi Arabia in 2012 [1]. As of February 10th 2017, there have been 1905 confirmed MERS-CoV cases, with 677 deaths across 27 countries (http://www.who.int/). Currently, there are no approved vaccines or treatments for MERS-CoV [2], [3].
MERS-CoV spike (S) protein on the virion surface is the main attachment factor for virion entry through binding to dipeptidyl peptidase IV (DPP4) on the host cell [4]. As such, MERS-CoV S has been a prime target for vaccination strategies [reviewed in 5] and we have previously reported an S protein vaccine candidate that was able to induce high levels of anti-MERS-CoV neutralizing antibodies in mice [6].
A major problem for MERS-CoV treatment or vaccine testing in vivo is that mice are not susceptible to MERS-CoV infection [7] because mouse (m)DPP4 is not a functional MERS-CoV receptor [8]. MERS-CoV S binding to mDPP4 is inhibited by glycosylation on two mDPP4 residues which are hypothesized to physically inhibit binding of MERS-CoV S to mDPP4 [9]. Therefore, mouse model development has focused on humanizing mDPP4. Mice can be made susceptible to MERS-CoV infection by adenovirus transduction of human (h)DPP4 into the lungs of mice [10]. Though hDPP4 expressing mice display only mild clinical symptoms of MERS-CoV infection, there is robust MERS-CoV replication in the lungs of the mice [10], making them useful for testing treatments or vaccines that block MERS-CoV replication in the lungs [11], [12], [13], [14], [15], [16].
In this study we vaccinated mice with MERS-CoV S nanoparticles and showed protection from MERS-CoV infection in vivo.
2. Materials and methods
2.1. Viruses, cells and mice
The Jordan strain of MERS-CoV (MERS-CoV- Hu/Jordan-N3/2012) was kindly provided by Dr. Kanta Subbarao (NIH, Bethesda, MD), Dr. Gabriel Defang (NAMRU-3, Cairo, EG), Dr. Michael Cooper (AFHSC) and Dr. Emad Mohereb (NAMRU-3).
Stocks of MERS-CoV(Jordan) were grown and quantified using Vero E6 cells (ATCC #CRL-1586) as described previously [17]. All experiments with live MERS-CoV(Jordan) were performed under biosafety level 3 conditions at the University of Maryland School of Medicine. Adenovirus expressing hDPP4 (AdCMVhDPP4) was obtained from the Gene Transfer Vector Core at the University of Iowa.
BALB/c mice were obtained from Charles River Laboratories and all mouse experiments were performed at the University of Maryland School of Medicine in accordance with protocols approved by the local institutional animal care and use committee (IACUC).
2.2. Vaccination and serum collection
BALB/c mice, 8–10 weeks of age, were intramuscularly vaccinated with either PBS, 1 μg, 3 μg or 10 μg MERS-CoV S nanoparticles with or without 5 μg of Matrix-M1™ adjuvant (Novavax AB, Uppsala, Sweden), which has been previously described [18]. Mice were vaccinated at days 0 and 21. At 0, 21 and 29 days post-initial vaccination, blood was collected by retro-orbital bleeding.
2.3. MERS-CoV S ELISA
MERS-CoV S-specific antibodies in mouse sera were evaluated in an ELISA as described previously [19], except that 96-well MaxiSorp microtiter plates (Thermo Scientific) were coated with 2 µg/ml of purified MERS-CoV S protein and a horseradish peroxidase conjugated goat anti-mouse IgG (Southern Biotech) was used as the secondary antibody.
2.4. MERS-CoV neutralization assays
MERS-CoV neutralization assays using days 0, 21 and 29 sera from vaccinated mice were performed as described previously [6].
2.5. MERS-CoV mouse infections and quantification of MERS-CoV replication in the lung
On day 30 post-initial vaccination, vaccinated mice were transduced with AdCMVhDPP4 as described previously [10]. Mice were then intranasally inoculated with 2.5 × 103 pfu of MERS-CoV(Jordan), as described previously [16]. During the experiments, mice were weighed on the day of MERS-CoV infection and every subsequent day. At 3 days post-infection, mice were sacrificed and lungs were harvested as previously described [7].
Lung MERS-CoV titers and levels of MERS-CoV genomic RNA and Leader containing mRNA were determined as previously described [16], [17]. Relative RNA levels were quantified using the ΔΔCt method compared to PBS vaccinated controls.
2.6. Statistics
Data were analyzed using the 1-way ANOVA with Bonnferoni post-test in Graphpad Prism 5. Statistical significance was achieved where P < 0.05.
3. Results
3.1. MERS-CoV S nanoparticle vaccinated mice produce anti-MERS-CoV S antibodies
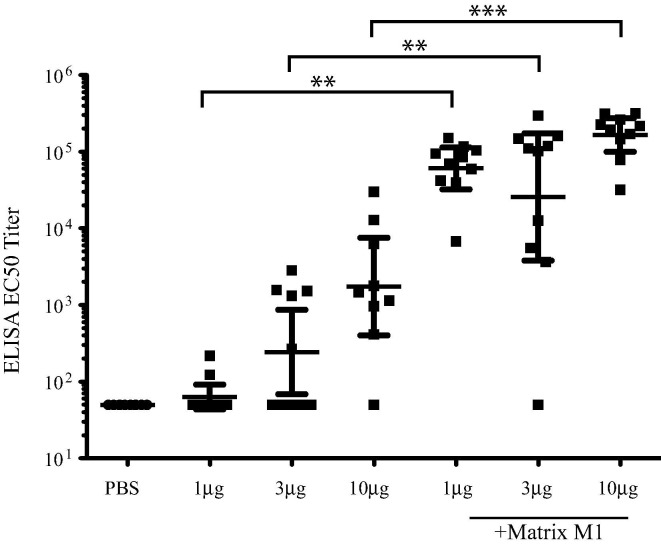
Sera collected at day 29 post-initial vaccination were analyzed for anti-MERS-CoV S antibody titers by ELISA (Fig. 1 ). As expected, PBS vaccinated mice had no detectable anti-S antibody. While some mice in the non-adjuvanted 1 μg, 3 μg and 10 μg MERS S nanoparticles groups had measurable anti-MERS-CoV S antibody, this response was not statistically significant (P > 0.05 in all cases) when compared to PBS vaccinated controls. Sera from mice vaccinated with the Matrix-M1 adjuvant in combination with 1 μg, 3 μg or 10 μg of MERS S nanoparticles demonstrate significantly higher anti-S titers (for example, P < 0.0001 for 10 μg MERS-CoV S nanoparticles) compared to PBS vaccinated controls with an apparent dose dependent increase. These data demonstrate that the MERS-CoV S nanoparticles along with Matrix-M1 adjuvant produce high titer anti-MERS-CoV S antibody in vaccinated mice.
Fig. 1.
Vaccination of MERS S nanoparticles plus Matrix M1 produces high titer anti-MERS-CoV S antibody. Sera from mice at day 29 post-initial vaccination from PBS vaccinated, MERS S nanoparticle vaccinated or MERS S nanoparticle plus Matrix M1 vaccinated mice was analyzed by ELISA for MERS-CoV S specific antibodies. ELISA EC50 titer is graphed for each group of 10 mice. Mean ± standard deviation are graphed for each cohort. Dots represent individual mice. ** = P < 0.01, *** = P < 0.001.
3.2. MERS-CoV S nanoparticle vaccinated mice produce MERS-CoV neutralizing antibodies
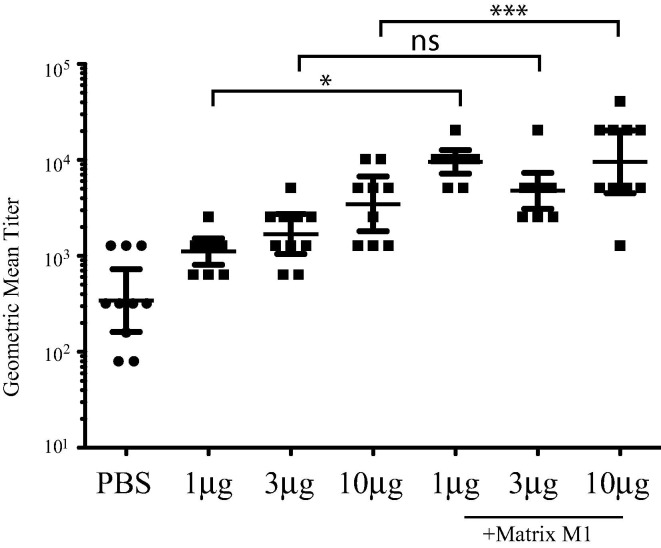
Neutralizing antibody levels in sera from day 29 post-initial vaccination were assessed using a live MERS-CoV neutralization assay (Fig. 2 ). Sera from mice vaccinated with 1 μg, 3 μg or 10 μg MERS S nanoparticles alone showed no significant neutralization of MERS-CoV (P > 0.05 in all cases) compared to PBS vaccinated controls. Sera from PBS vaccinated mice showed some anti-MERS-CoV neutralizing activity probably as a result of non-specific antiviral activity. Sera from mice vaccinated with Matrix-M1 adjuvant in combination with the 1 μg, 3 μg or 10 μg MERS S nanoparticles showed significantly higher neutralizing antibody titers compared to PBS vaccinated controls (P < 0.05) and also to the corresponding MERS-CoV S nanoparticles alone (for example, for P < 0.01 for 10 μg MERS-CoV S nanoparticles), with the exception of the 3 μg dose (P > 0.05) because 2 mice showed low levels of antibody in the 3 μg MERS-CoV S nanoparticles plus Matrix M1 group. Within the adjuvant groups, there was no significant difference (P > 0.05) between MERS-CoV neutralizing antibody levels in mice vaccinated with 1 μg, 3 μg or 10 μg MERS S nanoparticles. These data suggest that mice vaccinated with MERS S nanoparticles in combination with Matrix-M1 produce MERS-CoV neutralizing antibodies.
Fig. 2.
Vaccination of MERS S nanoparticles plus Matrix M1 produces high titer MERS-CoV neutralizing antibody. Sera from mice at day 29 post-initial vaccination from PBS vaccinated, MERS S nanoparticle vaccinated or MERS S nanoparticle plus Matrix M1 vaccinated mice was analyzed by neutralization assay against live MERS-CoV. GMT ± standard deviation is graphed for each group of 10 mice. Dots represent individual mice. * = P < 0.05, *** = P < 0.001, ns = not significant.
3.3. MERS-CoV S nanoparticle vaccine protects mice from MERS-CoV challenge
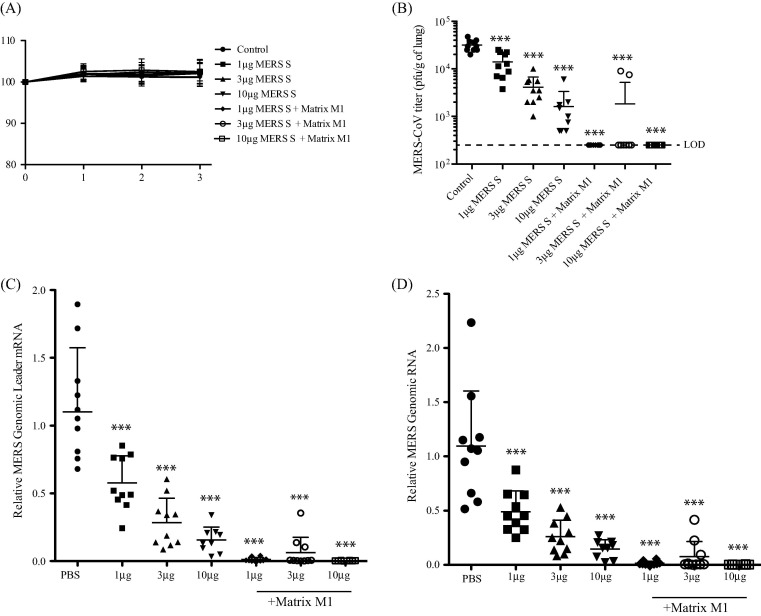
We utilized a MERS-CoV model in which an adenovirus expressing hDPP4 is used to transduce mice [10] to assess MERS-CoV replication in vaccinated mice (Fig. 3 ). As expected, there was no weight loss observed from the infection (Fig.3A), but we achieved efficient replication of MERS-CoV in PBS vaccinated mice, as demonstrated by MERS-CoV titer (Fig.3B), MERS-CoV Leader mRNA expression (Fig.3C) and genomic RNA expression (Fig.3D). Mice vaccinated with 1 μg, 3 μg or 10 μg MERS-CoV S nanoparticles showed statistically significant (P < 0.001) reductions in MERS-CoV titer (Fig.3B), MERS-CoV genomic RNA (Fig.3C) and MERS-CoV M mRNA (Fig.3D), however, in all cases, there was detectable MERS-CoV in the lungs of the vaccinated mice. When mice were vaccinated with Matrix-M1 in combination with 1 μg, 3 μg or 10 μg MERS-CoV S nanoparticles, with the exception of two mice in the 3 μg group, MERS-CoV titer (Fig.3B) and MERS-CoV genomic RNA (Fig.3C) and MERS-CoV M mRNA (Fig.3D) were reduced to baseline levels ( P< 0.001), suggesting that MERS-CoV replication was completely blocked in the lungs of the vaccinated mice. These data suggest that the MERS-CoV S nanoparticle vaccination, in combination with the Matrix M1 adjuvant, is able to block MERS-CoV replication in the lungs of vaccinated mice.
Fig. 3.
Vaccination of MERS S nanoparticle plus Matrix M1 protects mice from MERS-CoV challenge. Vaccinated mice were intranasally infected with Ad/hDPP4, challenged with MERS-CoV and then sacrificed at day 3 post-MERS-CoV infection. (A) Mice were weighed daily to determine if there were effects of weight loss on MERS-CoV infection. Lung MERS-CoV replication was determined by plaque assay (B), MERS-CoV specific Leader mRNA expression (C) and MERS-CoV genomic RNA expression (D). Mean ± standard deviation are graphed for each group of 10 mice. Dots represent individual mice. LOD means limit of detection. *** = P < 0.001.
4. Discussion
Highly pathogenic coronaviruses have caused significant problems to public health with the emergence of SARS-CoV and MERS-CoV. There are currently no approved vaccines or treatments for MERS-CoV, however both vaccine and drug candidates have been shown to be effective in vitro and/or in in vivo animal models [2], [3].
The S protein of MERS-CoV is a prime target for vaccination strategies because it is the main attachment factor and is expressed on the virion surface. Therefore, various vaccination strategies have been tested for eliciting an anti-S response [5]. We have previously described that MERS-CoV S nanoparticles are able to induce a strong neutralizing antibody response to MERS-CoV [6], however these were not tested in an in vivo model of MERS-CoV infection.
Mice are not susceptible to MERS-CoV infection because mDPP4 is not a functional receptor for MERS-CoV [8]. DPP4 susceptibility to MERS-CoV S binding is correlated with the glycosylation of critical DPP4 residues [9], therefore there are few options to make mice susceptible to MERS-CoV. hDPP4 can be expressed in the lungs of mice using an adenovirus vector [10]. In this model, there is robust replication of MERS-CoV in the lungs of the transduced mice that can be detected by both plaque assay and MERS-CoV RNA expression [16]. Therefore, we used this model to test the MERS-CoV S nanoparticle vaccine in vivo.
In agreement with our previous study [6], we found that mice vaccinated with MERS-CoV S nanoparticles developed a MERS-CoV neutralizing antibody response targeted to the MERS-CoV S. In our neutralization assays we see low level non-specific neutralization activity of PBS vaccinated mice however all adjuvanted MERS-CoV S nanoparticle vaccinated mice had significantly higher neutralization activity. Furthermore, Matrix-M1 adjuvant enhances the anti-MERS-CoV S neutralizing antibody response in vaccinated mice. Finally, we demonstrated that mice vaccinated with MERS-CoV S nanoparticles with Matrix-M1 are able to efficiently and completely block MERS-CoV replication in the lungs. The MERS-CoV S + Matrix-M1 nanoparticle formulation is a prime candidate for further development for use in camels or humans.
Conflict of interest
Novavax Inc. is a commercial enterprise with an interest in commercial vaccine development and funded this study.
Author contributions
CMC, TV and MBF were responsible for the vaccination of the mice and collection of serum. CMC was responsible for the MERS-CoV neutralization assay, MERS-CoV mouse infection and quantification of MERS-CoV infection. YVL and GMG were responsible for creating and producing the nanoparticle vaccine used in this study and for performing the MERS-CoV S ELISA. MBF, GES and DCF were responsible for funding and coordinating this study. CMC, MBF, GES and DCF contributed to the writing and editing of the manuscript.
Acknowledgments
This work was supported by funding from Novavax Inc. and in part by NIH grant R01 AI095569(MBF).
References
- 1.Zaki A.M., van Boheemen S., Bestebroer T.M., Osterhaus A.D., Fouchier R.A. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Eng J Med. 2012;367:1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- 2.Uyeki T.M., Erlandson K.J., Korch G., O'Hara M., Wathen M., Hu-Primmer J. Development of medical countermeasures to Middle East respiratory syndrome coronavirus. Emerg Infect Dis. 2016;22 doi: 10.3201/eid2207.160022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Excler J.L., Delvecchio C.J., Wiley R.E., Williams M., Yoon I.K., Modjarrad K. Toward Developing a Preventive MERS-CoV Vaccine-Report from a Workshop Organized by the Saudi Arabia Ministry of Health and the International Vaccine Institute, Riyadh, Saudi Arabia, November 14–15, 2015. Emerg Infect Dis. 2016;22 doi: 10.3201/eid2208.160229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raj V.S., Mou H., Smits S.L., Dekkers D.H., Muller M.A., Dijkman R. Dipeptidyl peptidase 4 is a functional receptor for the emerging human coronavirus-EMC. Nature. 2013;495:251–254. doi: 10.1038/nature12005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Q., Wong G., Lu G., Yan J., Gao G.F. MERS-CoV spike protein: targets for vaccines and therapeutics. Antiviral Res. 2016;133:165–177. doi: 10.1016/j.antiviral.2016.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coleman C.M., Liu Y.V., Mu H., Taylor J.K., Massare M., Flyer D.C. Purified coronavirus spike protein nanoparticles induce coronavirus neutralizing antibodies in mice. Vaccine. 2014;32:3169–3174. doi: 10.1016/j.vaccine.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coleman C.M., Matthews K.L., Goicochea L., Frieman M.B. Wild-type and innate immune-deficient mice are not susceptible to the Middle East respiratory syndrome coronavirus. J Gen Virol. 2014;95:408–412. doi: 10.1099/vir.0.060640-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cockrell A.S., Peck K.M., Yount B.L., Agnihothram S.S., Scobey T., Curnes N.R. Mouse dipeptidyl peptidase 4 is not a functional receptor for Middle East respiratory syndrome coronavirus infection. J Virol. 2014;88:5195–5199. doi: 10.1128/JVI.03764-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peck K.M., Cockrell A.S., Yount B.L., Scobey T., Baric R.S., Heise M.T. Glycosylation of mouse DPP4 plays a role in inhibiting Middle East respiratory syndrome coronavirus infection. J Virol. 2015;89:4696–4699. doi: 10.1128/JVI.03445-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao J., Li K., Wohlford-Lenane C., Agnihothram S.S., Fett C., Gale M.J., Jr. Rapid generation of a mouse model for Middle East respiratory syndrome. Proc Natl Acad Sci USA. 2014;111:4970–4975. doi: 10.1073/pnas.1323279111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Channappanavar R., Lu L., Xia S., Du L., Meyerholz D.K., Perlman S. Protective effect of intranasal regimens containing peptidic Middle East respiratory syndrome coronavirus fusion inhibitor against MERS-CoV infection. J Infect Dis. 2015;212:1894–1903. doi: 10.1093/infdis/jiv325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corti D., Zhao J., Pedotti M., Simonelli L., Agnihothram S., Fett C. Prophylactic and postexposure efficacy of a potent human monoclonal antibody against MERS coronavirus. Proc Natl Acad Sci USA. 2015;112:10473–10478. doi: 10.1073/pnas.1510199112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luke T., Wu H., Zhao J., Channappanavar R., Coleman C.M., Jiao J.A. Human polyclonal immunoglobulin G from transchromosomic bovines inhibits MERS-CoV in vivo. Sci Transl Med. 2016;8:326ra21. doi: 10.1126/scitranslmed.aaf1061. [DOI] [PubMed] [Google Scholar]
- 14.Zhang N., Channappanavar R., Ma C., Wang L., Tang J., Garron T. Identification of an ideal adjuvant for receptor-binding domain-based subunit vaccines against Middle East respiratory syndrome coronavirus. Cell Mol Immunol. 2016;13:180–190. doi: 10.1038/cmi.2015.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Y., Wan Y., Liu P., Zhao J., Lu G., Qi J. A humanized neutralizing antibody against MERS-CoV targeting the receptor-binding domain of the spike protein. Cell Res. 2015;25:1237–1249. doi: 10.1038/cr.2015.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wirblich C., Coleman C.M., Kurup D., Abraham T.S., Bernbaum J.G., Jahrling P.B. One-health: a safe, efficient dual-use vaccine for humans and animals against MERS-CoV and Rabies virus. J Virol. 2016 doi: 10.1128/JVI.02040-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coleman C.M., Frieman M.B. Growth and quantification of MERS-CoV infection. Curr Protoc Microbiol. 2015;37 doi: 10.1002/9780471729259.mc15e02s37. 15E 2 1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lovgren Bengtsson K., Morein B., Osterhaus A.D. ISCOM technology-based Matrix M adjuvant: success in future vaccines relies on formulation. Expert Rev Vaccin. 2011;10:401–403. doi: 10.1586/erv.11.25. [DOI] [PubMed] [Google Scholar]
- 19.Smith G., Raghunandan R., Wu Y., Liu Y., Massare M., Nathan M. Respiratory syncytial virus fusion glycoprotein expressed in insect cells form protein nanoparticles that induce protective immunity in cotton rats. PLoS ONE. 2012;7:e50852. doi: 10.1371/journal.pone.0050852. [DOI] [PMC free article] [PubMed] [Google Scholar]