Vascular complications of diabetes are the most serious manifestations of the disease, and account for most of its morbidity and mortality. Diabetic retinopathy (DR) is one of the most common microvascular complications of diabetes. Symptoms of DR are minimal until structural abnormalities are established. Microaneurysms and pericyte loss are found in earlier stages of DR, while capillary non-perfusion, angiogenesis and vascular leakage mark the more progressed stages of the disease. On a global scale, the escalating health care costs related to diabetic complications threaten to become unmanageable in the foreseeable future. Ideally, early detection of DR before structural injury to the retina would allow treatment before irreversible damage occurs, potentially leading to better prognosis for patients and containment of healthcare costs related to diabetes management.
Attempts are being made to improve the application of conventional diagnostic techniques – funduscopy, optical coherence tomography (OCT) and its more advanced marker-free OCT angiography[1] for early diagnosis of DR. Adaptive optics scanning light ophthalmoscopy[2] (AOSLO), an experimental technique that removes optical aberrations, delineates photoreceptors and provides highly detailed images of microvascular structures. Although varied in nature, these techniques have in common the feature that they depict morphological and functional changes that corroborate with clinical manifestations of DR. Further improvements of these imaging techniques will increase their spatial and temporal resolutions, yet detection of the earliest pathological molecular events in DR remain out of reach with these methods alone. Furthermore, the development of therapeutic approaches for DR and other diabetic complications is hampered by the lack of biomarkers for tracking the onset, progression and extent of tissue damage. None of the anatomical (caliber, branching, tortuosity) and functional (blood oxygenation, flow rate) parameters have yielded a predictive or therapeutic biomarkers for DR or other manifestations of diabetes in other organs.[3]
The article by Frimmel et al[4] in the current issue of Journal of Ophthalmic and Vision Research is one in a series of articles from Ali Hafezi-Moghadam's lab at Harvard Medical School and the Brigham and Women's Hospital that offer a diametrical departure from existing approaches to early detection of DR. Their work has systematically paved the way toward addressing the important unmet medical need of subclinical diagnosis of DR.[5,6,7,8,9,10] To visualize the earliest vascular changes in diabetes, Dr. Hafezi-Moghadam developed a novel and nature-inspired molecular imaging approach, as the authors explain in their discussion. Like other chronic conditions, DR starts off with subtle molecular changes that often remain morphologically undetectable. When exposed to higher glucose, lipid, and cytokine values, which are characteristic conditions in the plasma of diabetic individuals, the retinal microvascular endothelium responds with expression of molecules that could be revealing for diagnosis. The immune cells are the first to notice molecular changes in the organism and respond to them. In DR, this amounts to more frequent tethering, rolling and adhesion of innate immune cells in the retinal vessels.
Dr. Hafezi-Moghadam's laboratory developed the first generation of fluorescent nano-probes with a variety of surface moieties that mimic leukocyte rolling and adhesion to the vascular endothelium. The nano-probes injected into the blood stream of live animals circulate throughout the animal's vasculature, including the retinal vessels. The probes' interactions with the inner vessel wall are tracked by epifluorescence microscopy, and provide an unprecedented temporal and spatial resolution that gives precise knowledge about the presence of target molecules in the retinal microvessels. Using their custom-designed probes in combination with scanning laser ophthalmoscopy, Frimmel et al[4] in their current work elegantly visualize early molecular signs of DR in vivo. These results could ominously foretell the course of the disease before it becomes too late to intervene.
The efficacy of light-based molecular imaging in experimental DR shown in the current work reveals the great potential for this technology in future management of diabetic complications. All eyes will be on translation. To make the leap for this approach to humans, three areas need further development [Figure 1].
Figure 1.
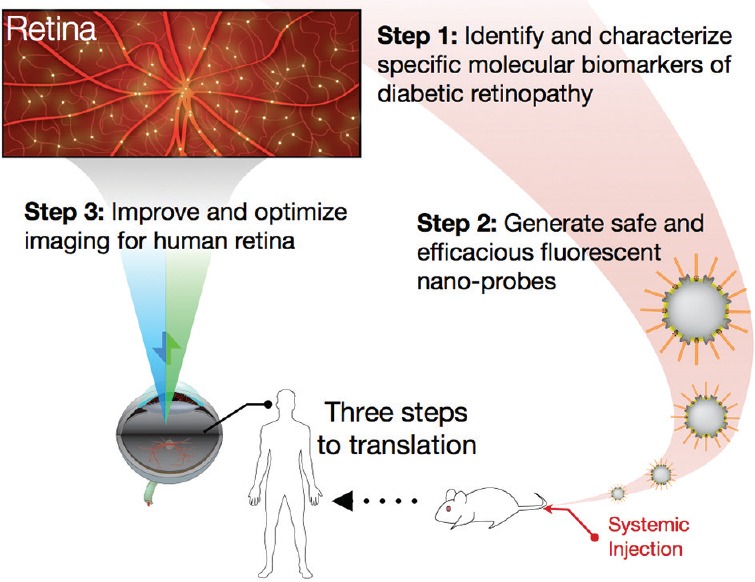
Schematic diagram of the steps to improve and optimize retinal molecular imaging.
Existing candidate molecular markers need to be validated and new ones identified. The Hafezi-Moghadam lab introduced the growth factor receptor, VEGFR-2 in a recent work,[10] and the adhesion molecule, ICAM-1 in the current work for early detection of diabetic retinopathy. Higher VEGFR-2 was found in human diabetic retinas, which makes it particularly well-suited for translation.
The next generation injectable nano-probes need to be re-engineered and optimized for human use. Generation of biocompatible nano-probes is feasible using FDA-approved components with well-known safety profile in humans. Additionally, rationale-design of biomaterials, such as combinatorial approaches in synthesizing and selecting various polymers will lead to more sophisticated nano-probe designs. For example, polymeric materials that respond to specific endogenous or exogenous stimuli (e.g., light responsive) can further modulate the functionality of nano-probes in vivo. Also, one can envision multi-functional systems that include imaging probes combined with therapeutic intervention or reporting strategies in one system.
The imaging requirements for detection of individual nano-probes in the human retina are considerably higher as compared to existing clinical modalities. Detectors with higher fluorescence sensitivity, and better temporal and spatial resolutions need to be developed and optimized for the human eye. A variety of technologies were developed to enhance fluorescence detection, including multiple-pulse pumping[11] or lock-in-detection.[12] Futures studies will show if these technologies are suitable for real-world clinical applications. Besides fluorescence sensitivity, quantification of fluorescence sensitivity by reference standards[13] is useful for longitudinal comparisons or between different patients. Although this technology is used for fundus autofluorescence, it could be very useful for marker-based fluorescence as well. The recently introduced slit scan ophthalmoscope (SSO) technology sets a promising trend.[14] In addition to its significantly larger field of view combined with improved imaging quality, the SSO could accomplish a higher success rate despite presence of cataract, or involuntary eye movements.
With the ability to diagnose subclinical DR, we anticipate rapid progress in all these three identified pillars for translation. Subsequent clinical studies will reveal, if beyond DR, this technology could become useful for early diagnosis of other eye diseases, such as age-related macular degeneration, glaucoma, and retinitis pigmentosa. Molecular changes in the retina could further become a useful surrogate for pathologies in other organs of the body. The hope exists that one day evasive conditions such as Alzheimer's[15] and atherosclerosis could be diagnosed and staged through molecular imaging in the retina [Figure 1].
Acknowledgements
*Authors have contributed equally to this editorial.
REFERENCES
- 1.Lee J, Rosen R. Optical Coherence Tomography Angiography in Diabetes. Curr Diab Rep. 2016;16:123. doi: 10.1007/s11892-016-0811-x. [DOI] [PubMed] [Google Scholar]
- 2.Chui TY, Mo S, Krawitz B, Menon NR, Choudhury N, Gan A, et al. Human retinal microvascular imaging using adaptive optics scanning light ophthalmoscopy. Int J Retina Vitreous. 2016;2:11. doi: 10.1186/s40942-016-0037-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ikram MK, Cheung CY, Lorenzi M, Klein R, Jones TL, Wong TY. Retinal vascular caliber as a biomarker for diabetes microvascular complications. Diabetes Care. 2013;36:750–759. doi: 10.2337/dc12-1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frimmel S, Zandi S, Sun D, Zhang Z, Schering A, Melhorn MI, et al. Molecular imaging of retinal endothelial injury in diabetic animals. J Ophthalmic Vis Res. 2017:12. doi: 10.4103/jovr.jovr_243_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miyahara S, Almulki L, Noda K, Nakazawa T, Hisatomi T, Nakao S, et al. In vivo imaging of endothelial injury in choriocapillaris during endotoxin-induced uveitis. FASEB J. 2008;22:1973–1980. doi: 10.1096/fj.07-096891. [DOI] [PubMed] [Google Scholar]
- 6.Sun D, Nakao S, Xie F, Zandi S, Schering A, Hafezi-Moghadam A. Superior sensitivity of novel molecular imaging probe: Simultaneously targeting two types of endothelial injury markers. FASEB J. 2010;24:1532–1540. doi: 10.1096/fj.09-148981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xie F, Su D, Schering A, Nakao S, Zandi S, Liu P, et al. Novel molecular imaging approach for subclinical detection of iritis and evaluation of therapeutic success. Am J Pathol. 2010;177:39–48. doi: 10.2353/ajpath.2010.100007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garland RC, Sun D, Zandi S, Xie F, Faez S, Tayyari F, et al. Noninvasive molecular imaging reveals role of PAF in leukocyte-endothelial interaction in LPS-induced ocular vascular injury. FASEB J. 2011;25:1284–1294. doi: 10.1096/fj.10-160051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakao S, Zandi S, Hata Y, Kawahara S, Arita R, Schering A, et al. Blood vessel endothelial VEGFR-2 delays lymphangiogenesis: An endogenous trapping mechanism links lymph- and angiogenesis. Blood. 2011;117:1081–1090. doi: 10.1182/blood-2010-02-267427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun D, Nakao S, Xie F, Zandi S, Bagheri A, Kanavi MR, et al. Molecular imaging reveals elevated VEGFR-2 expression in retinal capillaries in diabetes: A novel biomarker for early diagnosis. FASEB J. 2014;28:3942–3951. doi: 10.1096/fj.14-251934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rich RM, Gryczynski I, Fudala R, Borejdo J, Stankowska DL, Krishnamoorthy RR, et al. Multiple-pulse pumping for enhanced fluorescence detection and molecular imaging in tissue. Methods. 2014;66:292–298. doi: 10.1016/j.ymeth.2013.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Komar K, Stremplewski P, Motoczynska M, Szkulmowski M, Wojtkowski M. Multimodal instrument for high-sensitivity autofluorescence and spectral optical coherence tomography of the human eye fundus. Biomed Opt Express. 2013;4:2683–2695. doi: 10.1364/BOE.4.002683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Delori F, Greenberg JP, Woods RL, Fischer J, Duncker T, Sparrow J, et al. Quantitative measurements of autofluorescence with the scanning laser ophthalmoscope. Invest Ophthalmol Vis Sci. 2011;52:9379–9390. doi: 10.1167/iovs.11-8319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kunert KS, Taeubig K, Blum M, Saur S, O'Hara K, Durbin MK, et al. Wide field imaging of the retina using a new slit scan ophthalmoscope (SSO) imager. Invest Ophthalmol Vis Sci. 2016;57:1677. [Google Scholar]
- 15.van Wijngaarden P, Hadoux X, Alwan M, Keel S, Dirani M. Emerging ocular biomarkers of Alzheimer disease. Clin Exp Ophthalmol. 2017;45:54–61. doi: 10.1111/ceo.12872. [DOI] [PubMed] [Google Scholar]


