Abstract
Purpose:
Diabetic retinopathy is a leading cause of vision loss. There is a great need for early diagnosis prior to the occurrence of irreversible structural damages. Expression of endothelial adhesion molecules is observed before the onset of diabetic vascular damage; however, to date, these molecules cannot be visualized in vivo.
Methods:
To quantify the expression of endothelial surface molecules, we generated imaging probes that bind to ICAM-1. The α-ICAM-1 probes were characterized via flow cytometry under microfluidic conditions. Probes were systemically injected into normal and diabetic rats, and their adhesion in the retinal microvessels was visualized via confocal scanning laser ophthalmoscopy. Histology was performed to validate in vivo imaging results. Vascular pathologies were visualized using trypsin-digested retinal preparations.
Results:
The α-ICAM-1 probes showed significantly higher adhesion to retinal microvessels in diabetic rats than in normal controls (P < 0.01), whereas binding of control probes did not differ between the two groups. Western blotting results showed higher ICAM-1 expression in retinas of T1D animals than in normal controls. Retinal endothelial ICAM-1 expression was observed via molecular imaging before markers of structural damage, such as pericyte ghosts and acellular capillaries.
Conclusion:
Results indicate that molecular imaging can be used to detect subtle changes in the diabetic retina prior to the occurrence of irreversible pathology. Thus, ICAM-1 could serve as a diagnostic target in patients with diabetes. This study provides a proof of principle for non-invasive subclinical diagnosis in experimental diabetic retinopathy. Further development of this technology could improve management of diabetic complications.
Keywords: Biomarkers, Diabetic Retinopathy, Early Diagnosis, ICAM-1
INTRODUCTION
The prevalence of diabetes is rapidly increasing worldwide.[1] The vascular complications of diabetes, which develop over many years, are the major causes of morbidity and mortality. A microvascular manifestation of diabetes is diabetic retinopathy (DR), a leading cause of vision loss.[2] Detection during the subclinical period leads to a higher chance of the prevention of disease progression to the clinical stage.[3] An urgent need thus exists to develop strategies to diagnose DR prior to the occurrence of structural damage.
Retinal vessels can be visualized through non-invasive means, such as funduscopy, angiography, and optical coherence tomography (OCT); thus, in the past decades, several studies have attempted to identify early changes serving as predictors of retinopathy,[3] focusing on morphological characteristics, such as retinal vascular dimensions or functional characteristics, including blood flow rate, oxygenation, or leakage of systemically applied dyes, such as those used in fluorescein angiography.[3] However, an effective biomarker for DR was not successfully identified.[3] Indeed, results of the Wisconsin Epidemiologic Study of Diabetic Retinopathy (WESDR)[4] and the New Jersey 725 study revealed no correlation between retinal vascular caliber and the risk of DR.[5] It is uncertain whether continued use of the existing architectural and functional vascular measurements with more rigor would provide a reliable biomarker for early diagnosis of DR.
Therefore, we have chosen a categorically different strategy to visualize molecular biomarkers for DR. The current requirement for a biomarker is objective measurement as an indicator of pathogenic processes.[3] For effective and timely prevention of retinopathy, a biomarker should be non-invasively detectable in the early subclinical disease stage, such as in the period before capillaries obliterate. Furthermore, the ideal biomarker should be causally linked to retinal pathology development.[6]
In diabetes, the microvascular endothelium is known to express ICAM-1.[7,8] Endothelial ICAM-1 binds to leukocyte β2 integrins and results in leukocyte accumulation in retinal microvessels. Binding of the β2 integrins to the endothelial ICAM-1 in experimental DR subsequently leads to opening of the blood retinal barrier and leakage.[9] Our current approach is to target retinal endothelial ICAM-1 as an indicator of early pathology.
We introduce a new imaging technique capable of detecting single molecules in the retina.[10] This was accomplished by generating imaging probes targeting specific endothelial surface molecules;[10,11,12,13] once in circulation, the probes interact with their targets, located on the intra-luminal vessel surfaces.[13] The binding interactions are then visualized using light-based imaging,[10,11] which provides quantitative, highly sensitive and specific expression and localization information for the targets.[10] Using this approach, we have previously shown that VEGFR-2 is upregulated in the retinal vessels of diabetic animals.[14] The non-invasive nature of the described imaging approach can be further applied in longitudinal investigations, which is not highly feasible using currently existing endpoint techniques.[15]
Considering that leukocyte adhesion in retinal vessels precedes structural damage in DR,[16] we hypothesized that mimicking immune cell recruitment using our custom-designed imaging probes can reveal the earliest signs of DR. In this study, we evaluated endothelial ICAM-1 in normal and diabetic animals as a potential indicator for endothelial injury.
METHODS
Streptozotocin-induced Diabetes
All experiments were performed in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. Male Long-Evans rats (180–200 g, six to seven weeks old) were obtained from Charles River Laboratory (Wilmington, MA). After 16 h of overnight fasting, diabetes was induced in each animal via intraperitoneal injection of 12 mg of streptozotocin (STZ, minimum 98%, HPLC, Sigma-Aldrich, St. Louis, MO) diluted in 0.2 ml of citrate buffer (0.1 M, pH = 4.5). Control animals received an intraperitoneal injection of vehicle (citrate buffer). Animals with blood glucose levels greater than 250 mg/dl at 24 h after STZ injection were considered diabetic. Body weights and blood glucose levels were regularly measured. Animals were maintained in an air-conditioned room under a 12-h light/dark cycle and were given free access to water and food.
Preparation of the Molecular Imaging Probes
Carboxylated microspheres (MS, 1 and 2 μm, Polysciences, Inc.; Warrington, PA) were covalently conjugated to Protein G as previously described.[14] Conjugated probes were then incubated with anti-Intercellular-Adhesion Molecule-1 (α-ICAM-1 mAb, Santa Cruz Biotechnology, Inc., Santa Cruz, CA) or human IgG (hIgG, ZYMED Laboratories, Carlsbad, CA) for 2 hours at room temperature (approximately 22 degrees Celsius) on a rotary shaker. Probes were then washed with PBS containing 0.1% BSA.
Interaction and Accumulation of the Molecular Imaging Probes In vivo
To evaluate the adhesion of the imaging probes under physiological flow conditions in the rat retina during diabetes, high-resolution images of the fundus were captured continuously using a scanning laser ophthalmoscope (SLO; HRA2; Heidelberg Engineering, Dossenheim, Germany) coupled with a computer-assisted image analysis system.
Rats were anesthetized with xylazine hydrochloride (10 mg/kg) and ketamine hydrochloride (50 mg/kg). Pupils were dilated with 0.5% tropicamide and 2.5% phenylephrine hydrochloride. A contact lens was used to retain corneal clarity throughout the experiment. Probes were injected into the femoral vein. Thereafter, animals were placed on a platform in front of the SLO. Movement of the probes through the vessels and their interactions with the endothelium were captured in fluorescent mode. An argon blue laser with a regular emission filter for fluorescein angiography was used as the illumination source, because the spectral properties of the imaging probes are similar to those of sodium fluorescein. Images were obtained at a 30° angle at a rate of 15 frames per second and recorded on a computer for further analysis. Videos were obtained for 10 minutes after probe injections. The number of accumulated probes in the retina was quantified as distinct stationary fluorescent marks with very high contrast against the non-fluorescent background.[10,11,12] The number of the interacting probes were counted using Image J software (1.41o, Java 1.60_15; NIH).[17]
Ex vivo Evaluation of the Accumulation of the Imaging Probes
To directly visualize the interactions between the imaging probes and the retinal endothelium, retinal flatmounts were prepared. Rats were perfused 30 minutes after probe injection with PBS and rhodamine-labeled concanavalin A lectin (ConA, Vector Laboratories, Burlingame, CA). For upper body perfusion, the chest cavity was opened; the vena cava inferior and the abdominal aorta were clamped, after which a 24-gauge needle was inserted from the apex of the left ventricle into the aorta. Drainage was achieved by opening the right atrium. Animals were perfused with 30 ml PBS to wash out intravascular content and unbound probes, followed by washing with 0.15 ml of ConA in 15 ml of PBS to stain the vascular endothelium and firmly adhering leukocytes and again with 15 ml of PBS to remove the excess ConA. Immediately after perfusion, eyes were enucleated, and the retinas were microdissected and flatmounted using a fluorescence anti-fading medium (Vectashield, Vector Laboratories, Burlingame, CA). Tissues were then observed under an epifluorescence microscope (DM RXA; Leica, Deerfield, IL) with both an FITC- (excitation, 488 nm; detection, 505–530 nm) and a rhodamine filter (excitation, 543 nm; detection, >560 nm). Images were acquired using a high-sensitivity digital camera connected to a computer-assisted image analysis system. Openlab image analysis software (Improvision, Boston, MA) was used to merge images of the probes (green) with those of the retinal vessels (red). The number of probes was counted using Image J software. In each preparation, micrographs (10×) from five different fields of view (optic disc, nasally, temporally, superiorly, and inferiorly) were obtained, and the number of probes were counted and averaged.
Flow Cytometry
The average number of α-ICAM-1 mAbs on the surfaces of the imaging probes was determined using flow cytometry. Non-fluorescent microspheres (Polysciences, Inc., Warrington, PA) conjugated to α-ICAM or IgG were incubated with either FITC conjugated goat-α-mouse Ab or its isotype control. Microspheres were centrifuged, washed twice, and resuspended in PBS. The fluorescence intensities of the microspheres were measured using a FACScan (Coulter EPICS XL) equipped with the ‘System Work II’ software.
To quantify the number of copies of the molecules conjugated on the surface of the microspheres, mean fluorescence Intensity (MFI) was plotted against a calibration curve constructed as previously described.[11]
Adhesion of the Imaging Probes to Immobilized ICAM-1 Under Flow Conditions
Adhesion properties of the conjugated probes were examined using the micro flow chamber assay.[18] Glass microchambers were coated with recombinant rat ICAM-1 (5 μg/ml; R and D, 583-IC-050) at 4°C overnight. The next day, the ICAM-1-coated microfluidic chambers were connected to biocompatible polyester tubings (Small Parts Inc. #TGY-010-C) and a 1-ml syringe filled with α-ICAM-1 probes or control probes (1:200 dilution compared to animal experiments). A syringe pump injected α-ICAM-1 or human IgG-conjugated probes into the flow chambers at 2 dynes/cm2 shear stress, which were prepared as previously described.[19] Video microscopic images of the flow through the chambers were obtained for further analysis.
Western Blotting
Animals were perfused with PBS and eyes subsequently enucleated. The retina was carefully isolated and placed in 100 μl of lysis buffer (mammalian cell lysis kit MCL 1, Sigma Chemical Co, St. Louis, MO) supplemented with protease inhibitors and phosphatase inhibitors (Sigma) and then sonicated. The lysate was centrifuged, and the supernatant was collected. Samples containing equal amounts of total protein were separated via SDS-PAGE (sodium dodecyl sulfate–polyacrylamide gel electrophoresis) and electro-blotted onto PVDF (polyvinylidene fluoride) membranes (Life Technologies, Carlsbad, CA). Non-specific binding was blocked with 5% skim milk. Afterwards, membranes were incubated with a rabbit anti ICAM-1 polyclonal antibody (1:200 Santa Cruz Biotechnology, Santa Cruz, CA) or an anti β-tubulin mAb (1:2000; Abcam, Cambridge, MA) at 4°C overnight, followed by incubation with horseradish peroxidase-conjugated donkey or sheep anti rabbit or mouse IgG (1:2000; GE Healthcare UK limited Buckinghamshire, UK). Signals were visualized based on chemiluminescence (ECL kit; GE Healthcare UK limited, Buckinghamshire, UK) according to the manufacturer's protocol.
Trypsin Digestion and Analysis of Retinal Vasculature
Trypsin digestion of retinal vessels is the standard technique currently used to detect structural pathologies in diabetes.[19] The eyes of normal and diabetic rats were enucleated and fixed in 10% neutral buffered formalin. Eyes were placed in six-well plates in PBS. Retinas were then dissected under a surgical microscope and washed 4-5 times using filtered water to facilitate separation of the neural retinal layers from the blood vessels. Subsequently, retinas were incubated in 0.1 M Tris buffer (pH 7.8, Sigma, T8193) containing 3% trypsin (Difco 1:250, Amresco LLC, 0458) at 37°C for 90 min. Water washes were used to disintegrate the neuronal tissues and debris from the vascular network. The retinal vascular network was then flatmounted on a slide and stained with periodic acid solution (PAS, Sigma, 3951), Schiff's reagent (Sigma, 3952016), and hematoxylin (Sigma, GHS316).
Statistical Analysis
Values were expressed as mean ± SEM. Data were analyzed using Student's t-test. Statistical significance was considered at P < 0.05. Unless specified otherwise, n indicates the number of independent animal experiments.
RESULTS
Diabetic Retinal Leukocyte Accumulation and ICAM-1 Expression
Leukocyte accumulation is a key component of experimental diabetic retinopathy (DR).[20] To enumerate the firmly adhering leukocytes, histological flatmounts from the retinas of normal and diabetic animals were prepared [Figure 1a]. Retinal arteries and veins of diabetic animals showed more pronounced leukocyte accumulation compared to those of normal controls [Figure 1b].
Figure 1.
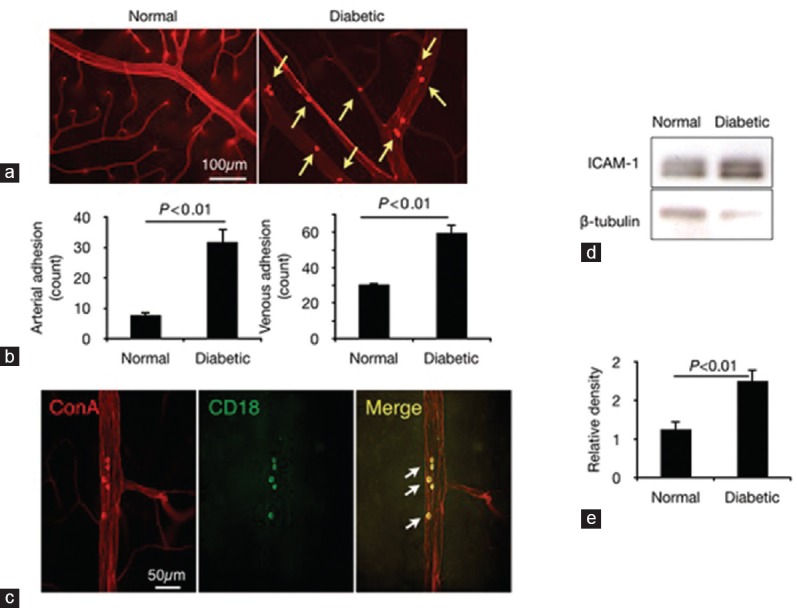
Leukocyte accumulation and ICAM-1 expression in the diabetic retina. (a) Leukocyte adhesion in retinal vessels of normal and diabetic rats. (b) Quantification of leukocytes in retinal arteries and veins (n = 6). (c) Immunohistochemistry of firmly adhering leukocytes (red) for CD18, a ligand for the endothelial ICAM-1. (d) Western blot analysis for ICAM-1 using retinal extracts of normal and diabetic rats (three weeks after STZ injection). (e) Western blotting results showing higher ICAM-1 expression in retinal tissues of diabetic rats (n = 8 animals) than in those of normal controls (n = 7 animals).
Next, we stained for the leukocyte activation marker CD18, a ligand for endothelial ICAM-1. Firmly adhering leukocytes in the retinal vessels expressed CD18 [Figure 1c]. Western blotting was performed to quantify endothelial ICAM-1 expression in the diabetic retinas [Figure 1d]. ICAM-1 protein levels in the retinal tissues of three-week diabetic rats were significantly higher than those in normal rats [Figure 1e]
Characterization of Molecular Imaging Probes
The number of ligands on the surface of the imaging probes was quantified following our previously established protocol.[10,12] PE-conjugated anti-murine IgG and isotype-matched control IgG were used to stain the antibodies on the imaging probes [Figure 2a]. Fluorescence intensities of the probes and calibration microspheres with known site densities of PE-conjugated IgG were measured via flow cytometry.[13] A calibration curve was generated (R2 =0.99) and used to determine the average number of α-ICAM-1 antibodies (n = 2.6 × 103) on the probe surface.
Figure 2.
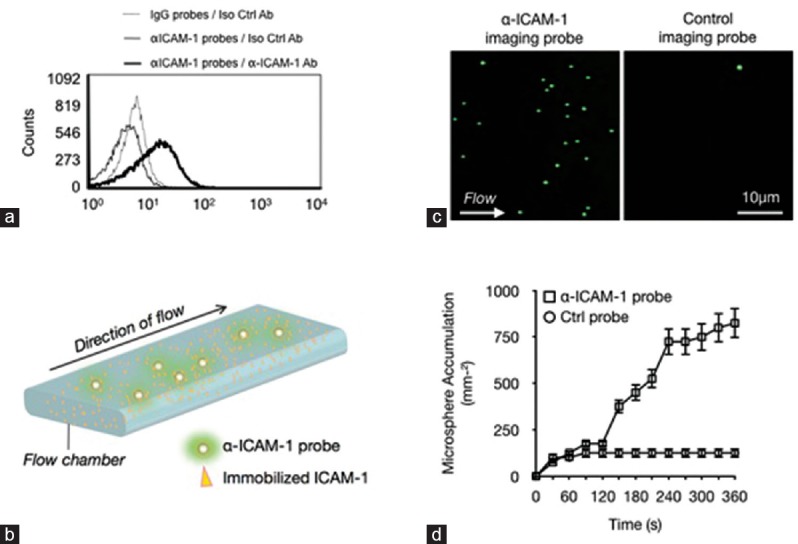
Characterization of the molecular imaging probes. (a) Flow cytometry analysis of non-fluorescent α-ICAM-1 probes labeled with FITC-conjugated mAb or isotype control and IgG-conjugated probes with isotype control. (b) Schematic of the microfluidic experiments. Recombinant ICAM-1 was immobilized, and probe interactions were visualized via live microscopy. (c) Representative video micrographs showing the interactions of the imaging probes with the immobilized ICAM-1 at 2 dynes/cm2. (d) Quantification of probe adhesion illustrates specific adhesion of probes to immobilized ICAM-1.
To examine the specificity and adhesion properties of our imaging probes under controlled flow conditions, we performed in vitro flow studies following our previously described protocols [Figure 2b].[18] Recombinant ICAM-1 protein was immobilized on the inner chamber surfaces. Subsequently, live video microscopy of the flow of the imaging probes through the chambers was recorded for 6 min, and numbers of adhering probes were quantified every 30 s [Figure 2c]. At 6 min after the start of imaging, the ICAM-1-coated flow chambers showed significantly more pronounced accumulation of α-ICAM-1 probes than those of IgG-conjugated control probes under the same conditions (P < 0.01), thereby demonstrating the specificity of the accumulated probes for ICAM-1 [Figure 2d].
Molecular Imaging of Endothelial Injury in Diabetic Animals
To monitor endothelial injury in vivo, we used our new molecular imaging approach[10,12] designed for early diagnosis[12] and use in mechanistic studies to monitor the retinal microvessels.[15] Imaging probes were generated by conjugating the binding ligands of endothelial ICAM-1 onto the surfaces of injectable fluorescent microspheres. These α-ICAM-1 or control probes were then injected into the bloodstreams of normal and diabetic animals. Accumulation of the firmly adhering probes in vivo was visualized using SLO [Figure 3a]. Firmly adhering probes were visible as bright dots in the fundus images of the examined eyes [Figure 3b]. The number of α-ICAM-1 and IgG-conjugated probes, which were used as negative controls, did not differ significantly between diabetic and normal rats. However, a significantly higher number of α-ICAM-1 probes adhered to the retinal vessels of the diabetic rats than those in normal controls [Figure 3c]. A significant difference in adhesion of α-ICAM-1 probes was also observed between normal and six-week diabetic animals injected with α-ICAM-1 probes [Figure 3d]. To examine the effect of probe size on binding efficacy, we used probes with 1- and 2-μm diameters. In diabetic animals, the smaller probes had a higher binding ratio than the control probes [Figure 3e].
Figure 3.
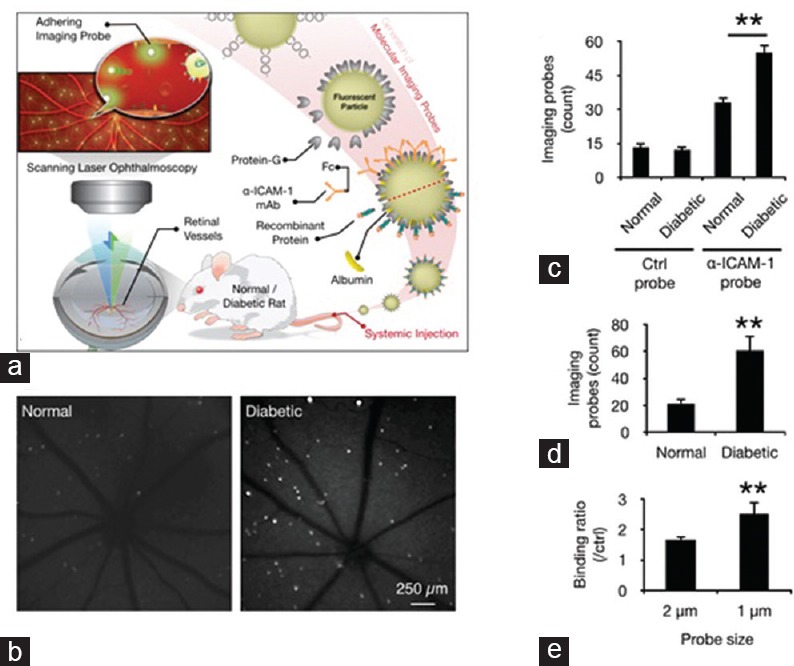
In-vivo detection of endothelial injury using molecular imaging. (a) Schematic of our in vivo molecular imaging approach. (b) Representative SLO-micrographs from the retinas of normal and diabetic animals. White dots represent firmly adhering probes. (c) In-vivo probe adhesion in normal and three-week diabetic animals (n = 5, **P < 0.01). (d) Molecular imaging of retinal endothelial ICAM-1 in 6-diabetic animals (n = 6, **P < 0.01). (e) Comparison between the binding of two differently sized a-ICAM-1 imaging probes (1 and 2 μm) in diabetic retinas (n = 5).
Ex vivo Visualization of Probe Accumulation in Retinal Micro Vessels
To verify the interaction of the imaging probes with the retinal endothelium, histological flatmounts were prepared and subsequently examined via epifluorescence microscopy [Figure 4a]. Consistent with our in vivo findings, retinal vessels of normal and diabetic animals showed no difference in the adhesion of IgG probes, which were used as negative controls. However, retinal microvessels of diabetic rats showed significantly more pronounced adhesion of the α-ICAM-1 probes compared to those of normal rats [Figure 4b].
Figure 4.
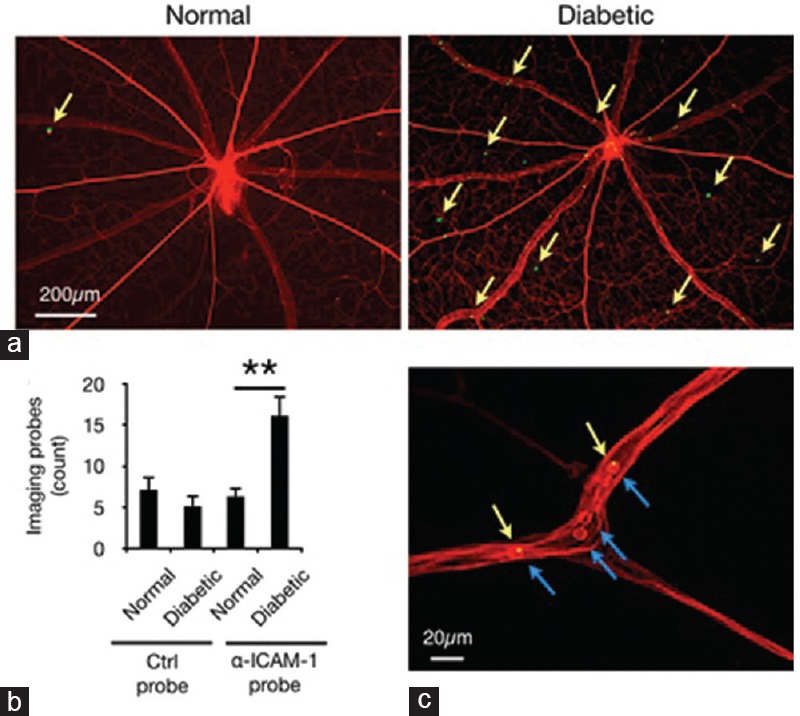
Ex-vivo evaluation of imaging probe accumulation. Retinal flatmounts were prepared from animals perfused with rhodamine-ConA (red). Imaging probes that resisted perfusion are visible as green spots in fluorescence microscopy. (a) Representative retinal micrographs show probe adhesion in retinal vessels of normal and diabetic rats. (b) Quantification of α-ICAM-1 probe adhesion in retinal vessels (n = 6–8, **P < 0.01). (c) Imaging probes bound to firmly adhering leukocytes in retinal vessels. Micrograph shows rhodamine-stained leukocytes (blue arrows) and a-ICAM-1 probes (green/yellow).
Leukocytes constitutively express ICAM-1, which was also targeted by the imaging probes in the diabetic retina. A fraction of the α-ICAM-1 imaging probes was firmly bound to adhering leukocytes [Figure 4c], consistent with our previous reports using a lipopolysaccharide-induced model of acute inflammation.[10,12] The capability of molecular imaging to detect accumulated leukocytes could potentially be used for quantification of specific immune responses in the retina.
Imaging probe accumulation precedes structural injury in the retina
A key morphological feature of the diabetic retina is the formation of acellular, nonperfused capillaries,[21] which is caused by microenvironmental damage to the endothelium, matrix, and pericyte.[21] Early diagnosis is required for therapeutically effective treatment before irreversible structural damage occurs. To investigate these structural changes, we performed trypsin digestion of the retinas of normal and diabetic animals. Retinas of normal rats showed patent capillaries containing endothelial cells and surrounding pericytes [Figure 5a]. Similarly, no evident structural pathologies, such as pericyte loss or acellular capillaries, were observed in the three-week diabetic animals [Figure 5b]. In contrast, obliterated acellular capillaries, a classic sign of DR pathology, was frequently observed in long-term diabetic animals [Figure 5c]. Thus, molecular imaging can detect endothelial injury in the diabetic retina at an early time point when no visible structural pathology is evident.
Figure 5.
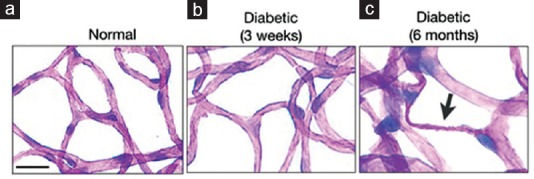
Early detection by molecular imaging precedes structural damage. Retinas of normal and diabetic animals were trypsin-digested to visualize vascular changes in the diabetic retina. PAS and hematoxylin-stained flatmounts of trypsin-digested normal retinas show patent retinal capillaries, which are comprised of endothelial cells and are surrounded by pericytes (a). At three weeks of diabetes, retinas of diabetic animals show no signs of structural damage (b). In contrast, long-term diabetic animals (six months) display obliterated acellular capillaries (arrow) (c). Bar, 50 μm.
DISCUSSION
The progression from physiological to pathological conditions in DR involves a sequence of events initiated by molecular changes, followed by cellular responses eventually leading to structural damage. Clinically detectable structural changes, such as acellular capillaries, microaneurysms, or neovascularization, severely compromise disease prognosis. Therefore, detection of the early molecular changes in the retinal endothelium during diabetes onset is critical as it can ideally enable adequate treatment before irreversible structural damage occurs. An urgent need thus exists to identify biomarkers for early DR and to develop practical strategies for subclinical diagnosis.[3] However, retinal endothelial injury in diabetic patients[3] cannot be visualized using existing techniques, which only detect structural damage.
To address the unmet need for an early diagnostic tool for DR, we developed an imaging technique measuring in vivo mono molecular interactions using custom-designed molecular imaging probes.[10,11,12,15] Our approach is based on the principle that one of the earliest responses of the retina to hyperglycemia is immune cell accumulation in the retinal vasculature. Leukocyte accumulation is caused by the expression of adhesion molecules, such as ICAM-1, in the retinal endothelium of diabetic animals. Higher ICAM-1 expression has been observed in the eyes of diabetic individuals, making ICAM-1 a clinically relevant target.[7] To visualize ICAM-1, we developed imaging probes that mimic early leukocyte endothelial interactions and which can be detected using fluorescent signals [Figure 6].
Figure 6.
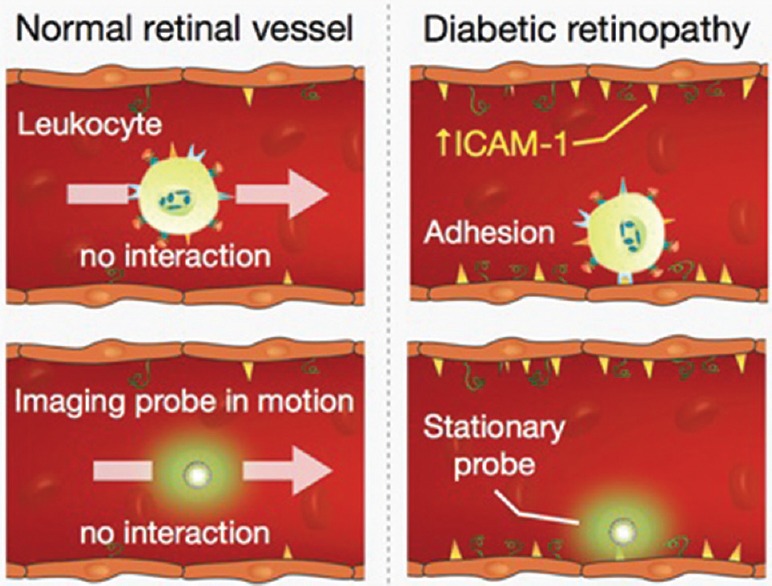
Sensitive detection of endothelial injury by mimicking leukocyte function. Under normal conditions, leukocytes freely flow in the bloodstream and do not interact with healthy endothelia (upper left). Diabetic endothelia express ICAM-1, which mediates firm leukocyte adhesion (upper right). Imaging probes that target endothelial ICAM-1 apply this principle to detect early vascular changes in vivo. α-ICAM-1 probes that target endothelial ICAM-1 show significantly higher interaction with diabetic endothelia (lower right) than in those of normal animals (lower left).
Our current approach is ideal for visualization of retinal endothelial molecules, since the structure of the eye provides a unique platform for light-based molecular imaging. The high sensitivity and specificity of the molecular imaging probes make them highly suitable for the detection of low-grade inflammation, as observed in DR.[10,11] Using this non-invasive approach, we demonstrated elevated ICAM-1 expression levels in the retinal vessels of T1D animals. This novel approach represents a great advancement that can be applied to humans.
Clinical use of this technique is feasible as the imaging probes can be constructed entirely from FDA-approved components safe for human use, such as biodegradable polymers including poly(epsilon-caprolactone) (PCL) and poly (D, L-lactide-co-glycolide) (PLGA),[22,23] both of which are approved for clinical use in targeted drug delivery systems. The FDA-approved dyes indocyanine green (ICG) and fluorescein[24] have established safety profiles and can thus be utilized as fluorophores for encapsulation in the imaging probes.
We demonstrate that non-invasive molecular imaging can distinguish between normal and diabetic retinas at an early time point when structural damage is not yet evident. In this study, ICAM-1 was selected as the imaging target because it mediates leukocyte accumulation in the retina.[7,8] However, other endothelial surface molecules, such as growth factor receptors and glycocalyx components, can be targeted using the same principles. We recently demonstrated the in vivo upregulation of VEGFR-2 in both the retinal microvessels of diabetic animals and human eyes.[14]
The high sensitivity and specificity of the molecular probes,[10] the quantitative nature of the detected signals,[11] and the automated processing[12] makes this technique a potentially powerful tool for monitoring early events in diabetic retinal microangiopathy that can meet the need for an early diagnostic tool for DR detection. Molecular imaging of retinal endothelial ICAM-1 could provide an early warning signal before clinical symptoms develop, and effective treatments at this early stage could prevent disease progression.
Financial Support and Sponsorship
Nil.
Conflicts of Interest
There are no conflicts of interest.
Acknowledgments
We thank Rebecca C. Garland for editorial help in the preparation of this manuscript. This work was supported by the NIDDK Diabetes Complication award #25732-30, the NIH Impact Award (DK108238-01), and JDRF Innovation award to A.H.M., Dr. Ernst und Anita Bauer – Stiftung (S.F.) and Deutscher Akademischer Austausch Dienst (S.F. and M.I.M).
REFERENCES
- 1.Misra R, Patel T, Kotha P, Raji A, Ganda O, Banerji M, et al. Prevalence of diabetes, metabolic syndrome, and cardiovascular risk factors in US Asian Indians: Results from a national study. J Diabetes Complications. 2010;24:145–153. doi: 10.1016/j.jdiacomp.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 2.Mohamed Q, Gillies MC, Wong TY. Management of diabetic retinopathy: A systematic review. JAMA. 2007;298:902–916. doi: 10.1001/jama.298.8.902. [DOI] [PubMed] [Google Scholar]
- 3.Ikram MK, Cheung CY, Lorenzi M, Klein R, Jones TL, Wong TY, et al. Retinal vascular caliber as a biomarker for diabetes microvascular complications. Diabetes Care. 2013;36:750–759. doi: 10.2337/dc12-1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klein R, Klein BE, Moss SE, Wong TY, Hubbard L, Cruickshanks KJ, et al. The relation of retinal vessel caliber to the incidence and progression of diabetic retinopathy: XIX: The Wisconsin Epidemiologic Study of Diabetic Retinopathy. Arch Ophthalmol. 2004;122:76–83. doi: 10.1001/archopht.122.1.76. [DOI] [PubMed] [Google Scholar]
- 5.Roy MS, Klein R, Janal MN. Retinal venular diameter as an early indicator of progression to proliferative diabetic retinopathy with and without high-risk characteristics in African Americans with type 1 diabetes mellitus. Arch Ophthalmol. 2011;129:8–15. doi: 10.1001/archophthalmol.2010.340. [DOI] [PubMed] [Google Scholar]
- 6.Steyerberg EW, Pencina MJ, Lingsma HF, Kattan MW, Vickers AJ, Van Calster B. Assessing the incremental value of diagnostic and prognostic markers: A review and illustration. Eur J Clin Invest. 2012;42:216–228. doi: 10.1111/j.1365-2362.2011.02562.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McLeod DS, Lefer DJ, Merges C, Lutty GA. Enhanced expression of intracellular adhesion molecule-1 and P-selectin in the diabetic human retina and choroid. Am J Pathol. 1995;147:642–653. [PMC free article] [PubMed] [Google Scholar]
- 8.Miyamoto K, Khosrof S, Bursell SE, Rohan R, Murata T, Clermont AC, et al. Prevention of leukostasis and vascular leakage in streptozotocin-induced diabetic retinopathy via intercellular adhesion molecule-1 inhibition. Proc Natl Acad Sci U S A. 1999;96:10836–10841. doi: 10.1073/pnas.96.19.10836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Skondra D, Noda K, Almulki L, Tayyari F, Frimmel S, Nakazawa T, et al. Characterization of azurocidin as a permeability factor in the retina: Involvement in VEGF-induced and early diabetic blood-retinal barrier breakdown. Invest Ophthalmol Vis Sci. 2008;49:726–731. doi: 10.1167/iovs.07-0405. [DOI] [PubMed] [Google Scholar]
- 10.Sun D, Nakao S, Xie F, Zandi S, Schering A, Hafezi-Moghadam A. Superior sensitivity of novel molecular imaging probe: Simultaneously targeting two types of endothelial injury markers. Faseb J. 2010;24:1532–1540. doi: 10.1096/fj.09-148981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miyahara S, Almulki L, Noda K, Nakazawa T, Hisatomi T, Nakao S, et al. In vivo imaging of endothelial injury in choriocapillaris during endotoxin-induced uveitis. FASEB J. 2008;22:1973–1980. doi: 10.1096/fj.07-096891. [DOI] [PubMed] [Google Scholar]
- 12.Xie F, Sun D, Schering A, Nakao S, Zandi S, Liu P, Hafezi-Moghadam A. Novel molecular imaging approach for subclinical detection of iritis and evaluation of therapeutic success. Am J Pathol. 2010;177:39–48. doi: 10.2353/ajpath.2010.100007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hafezi-Moghadam A, Thomas KL, Prorock AJ, Huo Y, Ley K. L-selectin shedding regulates leukocyte recruitment. J Exp Med. 2001;193:863–872. doi: 10.1084/jem.193.7.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun D, Nakao S, Xie F, Zandi S, Bagheri A, Kanavi MR, et al. Molecular imaging reveals elevated VEGFR-2 expression in retinal capillaries in diabetes: A novel biomarker for early diagnosis. FASEB J. 2014;28:3942:3951. doi: 10.1096/fj.14-251934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garland RC, Sun D, Zandi S, Xie F, Faez S, Tayyari F, et al. Noninvasive molecular imaging reveals role of PAF in leukocyte-endothelial interaction in LPS-induced ocular vascular injury. FASEB J. 2011;25:1284–1294. doi: 10.1096/fj.10-160051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arita R, Hata Y, Nakao S, Kita T, Miura M, Kawahara S, et al. Rho kinase inhibition by fasudil ameliorates diabetes-induced microvascular damage. Diabetes. 2009;58:215–226. doi: 10.2337/db08-0762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abramoff MD, Magelhaes PJ, Ram SJ. Image processing with ImageJ. Biophotonics Int. 2004;11:36–42. [Google Scholar]
- 18.Hafezi-Moghadam A, Thomas KL, Cornelssen C. A novel mouse-driven ex vivo flow chamber for the study of leukocyte and platelet function. Am J Physiol Cell Physiol. 2004;286:C876–892. doi: 10.1152/ajpcell.00500.2003. [DOI] [PubMed] [Google Scholar]
- 19.Chou JC, Rollins SD, Fawzi AA. Trypsin digest protocol to analyze the retinal vasculature of a mouse model. J Vis Exp. 2013:e50489. doi: 10.3791/50489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Noda K, Nakao S, Zandi S, Sun D, Hayes KC, Hafezi-Moghadam A. Retinopathy in a novel model of metabolic syndrome and type 2 diabetes: New insight on the inflammatory paradigm. FASEB J. 2014;28:2038–2046. doi: 10.1096/fj.12-215715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hammes HP, Feng Y, Pfister F, Brownlee M. Diabetic retinopathy: Targeting vasoregression. Diabetes. 2011;60:9–16. doi: 10.2337/db10-0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iyer AK, Ganta S, Amiji MM. Handbook of Materials for Nanomedicine. Vol. 1. Pan Stanford Publishing; 2010. Polymeric nanoparticles as target-specific delivery systems; pp. 81–130. [Google Scholar]
- 23.Iftimia N, Amiji M, Milane L, Oldenburg A. Nanotechnology approaches for contrast enhancement in optical imaging and disease targeted therapy. In: Iftimia N, Brugge W, Hammer DX, editors. Advances in Optical Imaging for Clinical Medicine. Wiley Publishing; 2011. pp. 455–504. [Google Scholar]
- 24.Merian J, Gravier J, Navarro F, Texier I. Fluorescent nanoprobes dedicated to in vivo imaging: From preclinical validations to clinical translation. Molecules. 2012;17:5564–5591. doi: 10.3390/molecules17055564. [DOI] [PMC free article] [PubMed] [Google Scholar]


