Abstract
New technological progress in robotics has brought many beneficial clinical applications. Currently, computer integrated robotic surgery has gained clinical acceptance for several surgical procedures. Robotically assisted eye surgery is envisaged as a promising solution to overcome the shortcomings inherent to conventional surgical procedures as in vitreoretinal surgeries. Robotics by its high precision and fine mechanical control can improve dexterity, cancel tremor, and allow highly precise remote surgical capability, delicate vitreoretinal manipulation capabilities. Combined with magnified three-dimensional imaging of the surgical site, it can enhance surgical precision. Tele-manipulation can provide the ability for tele-surgery or haptic feedback of forces generated by the manipulation of intraocular tissues. It presents new solutions for some sight-threatening conditions such as retinal vein cannulation where, due to physiological limitations of the surgeon's hand, the procedure cannot be adequately performed. In this paper, we provide an overview of the research and advances in robotically assisted vitreoretinal eye surgery. Additionally the barriers to the integration of this method in the field of ocular surgery are summarized. Finally, we discuss the possible applications of the method in the area of vitreoretinal surgery.
Keywords: Minimally Invasive Surgical Procedures, Robotic Surgical Procedures, Vitreoretinal Surgery
INTRODUCTION
The introduction of minimally invasive surgery (MIS) has revolutionized conventional surgical procedures.[1] Through the use of special surgical instruments and techniques, MIS allows surgeons to operate through small incisions minimizing tissue trauma. Use of this technique as a substitution for conventional methods has resulted in lower rates of injury, scarring, and pain in addition to faster recovery times and reduced health care costs.[2] The advantages of MIS make it an excellent method for vitreoretinal surgeries. Vitreoretinal surgeries are performed through small incisions through the sclera, allowing the insertion of fine surgical instruments which are delicately manipulated by the surgeon inside the patient's eye while observing the operation through a microscope equipped with a magnifying lens. The surgical site can also be viewed indirectly by watching a monitor coupled to a camera mounted on the surgical microscope. Since the 1970s when the first pars plana vitrectomy was performed, the method has been constantly refined, developing new techniques and surgical tools capable of increasing precision and accuracy.[3] Figure 1 shows a surgeon performing vitreoretinal surgery and a schematic view of the surgical site inside the eye. Despite the appreciable benefits of MIS for vitreoretinal surgeries, the method has several shortcomings, mostly related to the surgeon. In vitreoretinal surgeries, it is sometimes necessary to position the instrument very precisely relative to the target site of surgery; within about 10 microns.[4] This degree of precision is far below the 100-micron amplitude of the natural tremor of a human hand.[5] Distance between the entry site into the eye, and the surgical field means that any precise movement from the surgeon's hand close to the entry site will be enhanced at the point of contact with ocular tissue. Surgery as it is carried out presently is not ergonomic. The head position, unsupported hands at the edge of the head lead to frequent complaints of neck, shoulder, and back pain a decade or so into a surgeon's practice.[6,7] Lack of force perception is a third problem in vitreoretinal surgeries; the surgeon does not have a direct sense of tissue stiffness as it is beyond the limits of human perception.[8] The surgeon must instead rely on optical feedback to gauge stiffness. Moreover, to become a skillful vitreoretinal surgeon demands several years of training. In particular, it takes time to learn how to adjust one's movements to the biomechanical properties of intraocular tissues particularly when these are affected by disease. Furthermore fine motor skills unfortunately diminish as one matures, and any surgeons skilled in this procedure find that their physical abilities decreased much faster than hoped due to age, while their understanding and skill in performing these delicate operations increase.
Figure 1.
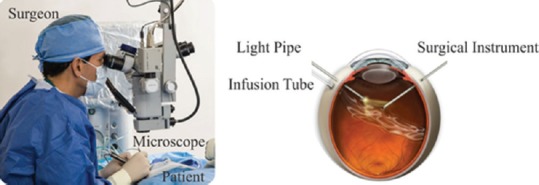
Surgeon performing vitreoretinal surgery.
Recent advances in robotics technology and computer graphics have introduced means to overcome some of the limitations of vitreoretinal surgery mentioned above. Using a robotic surgical system, surgeons can perform operations from a distance while sitting in an ergonomic position. It is also possible for several surgeons simultaneously to help perform a single surgery. The tremor of the surgeon's hand can be filtered out using a robotic system and the surgery can be performed with a high degree of precision. It is believed that the next major advancement in ophthalmology will be the integration of robotic surgery.[9]
Robotically assisted eye surgery was first introduced in 1989 by Guerrouad and Vidan.[10] The feasibility and applicability of the robotic vitreoretinal surgeries has been analyzed in several studies.[11,12,13] The first experimental robotic vitreoretinal eye surgery on a human eye was done at Oxford University in 2016 by Professor Robert MacLaren. Surgeons successfully removed a membrane from the retina with a thickness of only 10 microns.[14] This achievement of robotic-assisted eye surgery in its first clinical application was a breakthrough in vitreoretinal surgery, but there are still many barriers to overcome before the method can be clinically accepted.
Robotic-assisted Eye Surgery
Robotically assisted eye surgery was sparked by the introduction of the Stereo-Taxical Micro-Manipulator (SMOS) robot invented by Guerrouad and Vidal in 1989.[10] Since then, researchers around the world have proposed new robotic platforms for vitreoretinal eye surgery. Table 1 summarizes the focus and location of research on robotic eye surgery.[8,10,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40] It is to be noted that because of the size and delicacy of the eye, available general purpose robotic systems are not suitable solutions for vitreoretinal surgery applications, as they are bulky, are not compatible with the size of ophthalmic operating rooms, and lack the required 3-D precision inherent to eye surgery.[9]
Table 1.
Eye surgery robots developed since 1989
Ophthalmic robots can be classified into three main categories: assistive hand-held instruments, co-manipulation platforms, and tele-manipulation systems. In addition, some special eye surgery robots such as Octomag and Microhand have been introduced for special applications.[41,42]
A hand-held instrument is a special tool that aims to negate the tremor and unintentional movements of the surgeon's hand.[43] As depicted in Figure 2, the surgical instrument is mounted on the hand-held device, and the robotic system stabilizes and cancels out the unwanted fine motions of the surgeon's hand. In one special design, the hand-held instruments provide a magnified sense of the forces at the level of the eye structures during surgery.[44]
Figure 2.

Micron, a hand held robotic instrument, developed at Carnegie Mellon University (CMU) is designed to remove unintentional hand movement and compensating for hand tremor. Permitted by Cameron N. Riviere, Ph.D. Research Professor and Director of Surgical Mechatronics Laboratory, The Robotics Institute, Carnegie Mellon University, Pennsylvania, USA.
Co-operative systems [Figure 3] also cancel the unwanted motions of the surgeon's hand; they use a mechanism that holds the incision point fixed and provides precise, tremor-free motion control.[45] It can also be equipped with a force magnifying system to provide the surgeon with a perception of the tool-tissue interaction force. In a comparison of the hand-held and co-operative devices, it should be mentioned that the co-operative device is more bulky and the surgeon experiences more inertial force while using it. However, it has advantages, including the capacity to stabilize the motion of the surgeon's hand at the entry point, which minimizes damage to the sclera.
Figure 3.
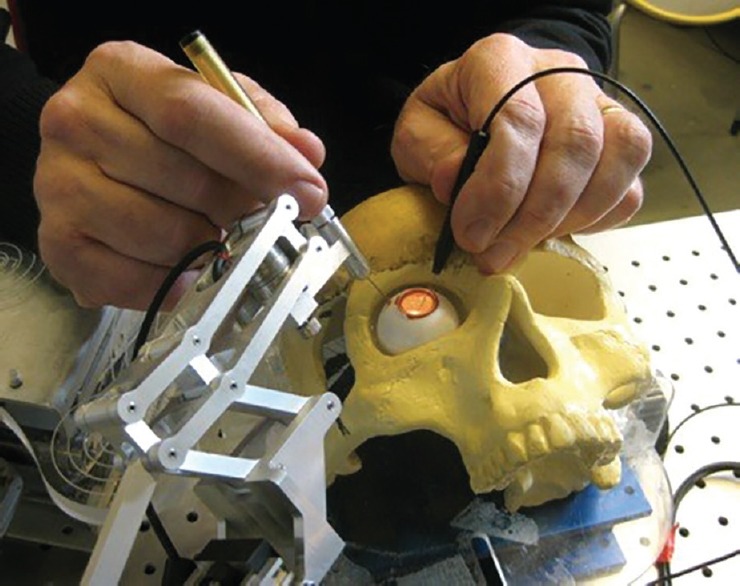
The steady hand of John Hopkins University is a cooperatively controlled microsurgical system where the surgeon and the actively controlled robotic arm move the surgical instrument simultaneously. Permission by Iulian IORDACHITA, Ph.D. Associate Research Professor, Johns Hopkins University, WSE, ME/LCSR. https://amiro.lcsr.jhu.edu/IulianIordachita.
In tele-operated systems, the surgeon controls the surgical robot (slave robot) using a joystick (master robot) through a computerized control system. Using the benefits of tele-operation, the surgeon can make full use of the functionalities provided by a robotic system: a precise overview and controlled movement within the surgical site visible on a monitor. Larger movements of the surgeon's hand within the surgical field are mapped to much more delicate motions at the surgical site, which increases the accuracy of the surgical task. A magnified view limited to the surgical site can be used for precise manipulations, as all movements of the robot can be confined to a particular three dimensional space, as required. Stand-by functionality, positional stability and memorized location are other functions that are possible in tele-operated systems. The surgeon can also perform the operation from remote locations while performing surgery from an ergonomic position. Thus, highly qualified surgeons are able to perform complicated surgeries for patients in remote areas with minimal stress.
The surgical robotic arms (master robots) available for tele-surgery are inspired by the motion of surgical instruments in MIS. In a typical MIS, the incision point of the surgical instrument is fixed and the surgeon orients the surgical tool about the entry point. The fixed entry port, or virtual fixture, is an important requirement for any clinically acceptable robot used for vitreoretinal surgery. For safety reasons, the fixed entry port of the surgical instrument is provided mechanically using a remote center of motion (RCM) mechanism, which holds the incision point fixed regardless of the motion of the robot linkages. The eye surgery robots should also be compact enough to become head mountable, and their workspace must cover the required angular region for vitreoretinal surgeries. Figure 4 illustrates several available RCM mechanisms that have been introduced for vitreoretinal surgeries. While tele-operated systems may allow the surgeon to sit at a distance from the patient, there are many circumstances, where he may prefer to use the system for specific dedicated tasks. Hence, the ideal configuration of a mount to which the micromanipulator is fixed should be non-obtrusive. It should allow the surgeon to easily switch between manual and robotic surgery.
Figure 4.
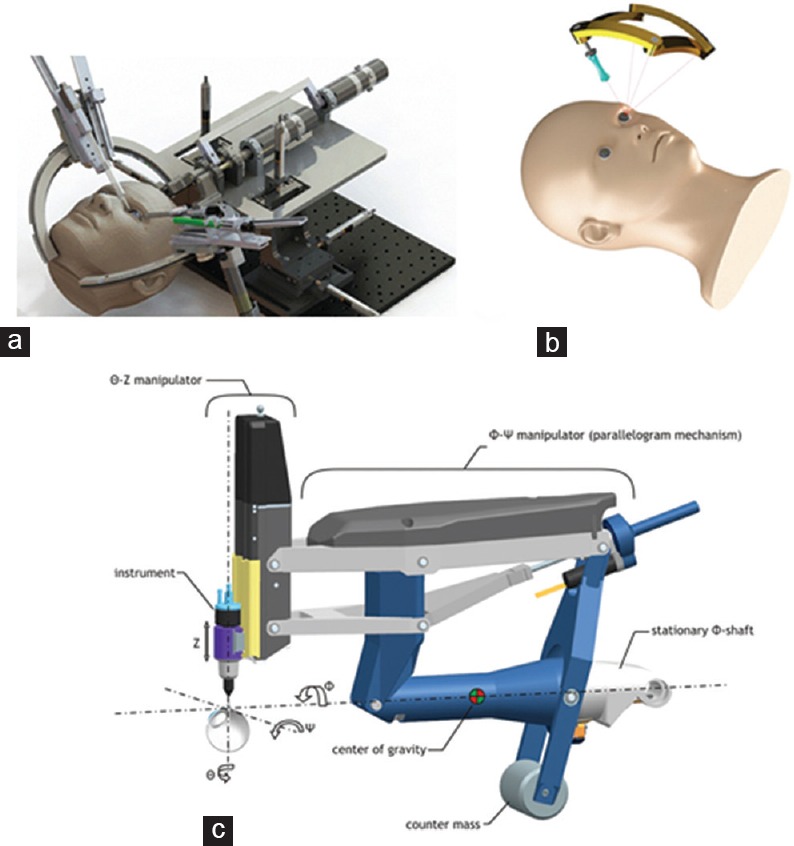
IRISS; an isotropic spherical serial mechanism having RCM point developed at UCLA mechatronics and control lab (a) DIAMOND; a spherical compact size mechanism having singularity free workspace with RCM point, developed at K.N Toosi University[38] (b) Eye-Rhas; a double parallelogram based gravity balanced RCM mechanism, developed at Eindhoven University of Technology. “Courtesy of Preceyes b.v.”[32] (c).
Potential Applications of Robotics for Intraocular Applications
Retinal surgery
Vitreoretinal surgeries address serious sight-threatening conditions such as retinal detachment, macular pucker, macular holes, vitreous hemorrhage, and diabetic retinopathy.[46] Retinal surgeries demand a high degree of accuracy, which is at the limit of human physiological ability. Additionally, the long surgical times are uncomfortable for both the surgeon and the patient. Increasing precision and fidelity while decreasing duration of surgery has led to a breakthrough in vitreoretinal surgery, which is accessible through the use of robotic systems.
Retinal vein cannulation
In retinal vein cannulation, a clot-dissolving drug is injected into the occluded vein, but the limited positioning, precision, and force perception make the procedure challenging and risky.[47] Veins in the eye also have a tendency to collapse when a cannulation is attempted, just as in the peripheral venous system. Since a tourniquet cannot be placed on the retinal vessel without damaging neighboring structure, cannulation requires a very precise piercing motion to avoid penetrating the outer vessel wall. Thus, it is not yet a common practice in intraocular surgeries. However, robotic systems have the potential to enable the surgeon to safely administer injections into the micron-sized vein of the retina.
Implant surgery
Retinal implant surgery allows surgeons to partially restore the sight of a patient suffering from macular degeneration.[48] These devices transfer an electrical signal through a multitude of electrodes which are preferably imbedded at a specific depth inside the retinal tissue. An intraocular robotic system can provide the surgeon with a manipulation mechanism capable of carefully orienting the implant and inserting it to the appropriate depth inside the retina. The depth of implantation can for example be controlled by intraoperative OCT.
Drug delivery
Drug delivery refers to any method used to transport drugs and compounds to specific sites within the body.[49] The ability to inject a precise amount of drug under the retina or at a precise location within the vitreous cavity will provide high precision drug delivery to any location within the eye.
Gene therapy
Gene therapy has been gaining more and more prominence in medicine. The eye is considered one of sites most likely to benefit from advances in gene therapy. It is a unique target, due to its accessibility and immunologically privileged condition.[50] However, immune privilege is relative and depends on respecting ocular structures, particularly the choroid which should not be penetrated. Robotic systems provide a highly reliable method to deliver gene therapy constructs at a specific site inside the eye with high precision and speed.
Training surgeons
Robotically assisted eye surgery provides an intuitive platform for educational purposes. By integrating the method in a virtual reality system, trainees can benefit from a safe, controlled, and feedback-oriented learning environment. In many training centers today, beginner surgeons are required to first train on a simulator before moving on to patients. A transition to robotic surgery would in such circumstance be facilitated, and also allow surgeons to simulate difficult surgeries prior to carrying out the procedure. Technologies and training strategies used to train and maintain skills in commercial airlines may be applicable to future ophthalmic surgeons.
Tele-surgery
Robotic systems provide the surgeon with the ability to perform surgery over long distances while sitting in an ergonomic position. In the near future, ocular tele-surgery will be a feasible way to bring emergency eye care to remote locations.
Bio-printing inside the eye
One of the major advances we may expect in the near future in medicine will be the use of bio-printing of living tissues and organs.[51] Robotics will provide the surgeon with a suitable tool to bio-print living cells into the RPE and choriocapillaris and possibly even allow damaged cells to be replaced in the retina.
Barriers and Challenges to Development of Eye Surgery Robots
Despite widespread usage of robotic surgery, robotically assisted eye surgery is still in its infancy. One important hurdle was the downscaling of systems to a level that allows for ophthalmic microsurgery. Another, is to provide the surgeon with an intuitive work environment in which the micromanipulator becomes an extension of his own hands. An environment in which he does not need to worry about the speed of approach to the retina, or the risk of moving too close to critical structures. Scaling of motion can be adjusted for distance from the retina for example without the surgeon being aware that the scaling is variable – similar to the motion of a computer mouse. Development of a tele-operated system needs an appropriately tested and validated software. In a co-manipulator, it has to be built into the design. Whatever is being designed and optimized, it must take into account the fragility of the eye, the needed precision of specific surgical tasks, even the risk of inadvertent movement by the patient's head.
In summary, major advances in intraocular surgeries are owed to a large extent to engineering developments, including small-gauge instrumentation, high-speed cutters, panoramic visualization systems, and wide-field illumination probes. Similarly, robotically assisted surgery offers profound advantages for vitreoretinal surgeries compared to unaided human hands. These benefits comprise highly precise positioning, scaled force feedback, tremor and unwanted hand motion cancellation, and the possibility of performing surgery at a distance. In the near future robots will be the second hands of the surgeons, helping them to overcome the current difficulties of conventional vitreoretinal surgeries. The barriers to adaptation of eye surgery robots are the same ones once encountered by proponents of minimally invasive laparoscopic surgery. However, laparoscopic robotic surgeries evolved through the years and now laparoscopic surgery is a widely accepted technique. Evidence of the past three decades implies that robotic systems might be useful to improve health care. Ongoing developments in eye surgery robots could make them useful tools for eye surgeons.
Financial Support and Sponsorship
Nil.
Conflicts of Interest
There are no conflicts of interest.
REFERENCES
- 1.Lanfranco AR, Castellanos AE, Desai JP, Meyers WC. Robotic surgery: A current perspective. Ann Surg. 2004;239:14–21. doi: 10.1097/01.sla.0000103020.19595.7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haggag AA. Robotic surgery: When technology meets surgical precision. Internet J Health. 2006;5:3. [Google Scholar]
- 3.Macherner R. The development of pars plana vitrectomy: A personal account. Graefes Arch Clin Exp Ophthalmol. 1995;233:453–468. doi: 10.1007/BF00183425. [DOI] [PubMed] [Google Scholar]
- 4.Riviere CN, Ang WT, Khosla PK. Toward active tremor canceling in handheld microsurgical instruments. IEEE Trans Rob Autom. 2003;19:793–800. [Google Scholar]
- 5.Singhy SP, Riviere CN. Physiological tremor amplitude during retinal microsurgery. InBioengineering Conference, 2002. Proceedings of the IEEE 28th Annual Northeast 2002 (pp. 171.172). IEEE. [Google Scholar]
- 6.Wallace RB., III The 45 degree tilt: Improvement in surgical ergonomics. J Cataract Refract Surg. 1999;25:174–176. doi: 10.1016/s0886-3350(99)80122-9. [DOI] [PubMed] [Google Scholar]
- 7.Yu D, Sackllah M, Woolley C, Kasten S, Armstrong T. Quantitative posture analysis of 2D, 3D, and optical microscope visualization methods for microsurgery tasks. Work. 2012;41(Suppl 1):1944–1947. doi: 10.3233/WOR-2012-0412-1944. [DOI] [PubMed] [Google Scholar]
- 8.Nakano T, Sugita N, Ueta T, Tamaki Y, Mitsuishi M. A parallel robot to assist vitreoretinal surgery. Int J Comput Assist Radiol Surg. 2009;4:517–526. doi: 10.1007/s11548-009-0374-2. [DOI] [PubMed] [Google Scholar]
- 9.Hubschman JP, Tsirbas A, Schwartz SD. Robotic Surgery in Ophthalmology. Retina Today. 2008:81–84. [Google Scholar]
- 10.Guerrouad A, Vidal P. SMOS: Stereotaxical microtelemanipulator for ocular surgery. InEngineering in Medicine and Biology Society, 1989. Images of the Twenty-First Century. Proceedings of the Annual International Conference of the IEEE Engineering in 1989 Nov 9 (pp. 879.880). IEEE. [Google Scholar]
- 11.de Smet MD, Meenink TC, Janssens T, Vanheukelom V, Naus GJ, Beelen MJ, et al. Robotic Assisted Cannulation of Occluded Retinal Veins. PloS One. 2016;11:e0162037. doi: 10.1371/journal.pone.0162037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Smet MD, Stassen JM, Meenink TC, Janssens T, Vanheukelom V, Naus GJ, et al. Release of experimental retinal vein occlusions by direct intraluminal injection of ocriplasmin. Br J Ophthalmol. 2016;100:1742–1746. doi: 10.1136/bjophthalmol-2016-309190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gonenc B, Tran N, Gehlbach P, Taylor RH, Iordachita I. Robot-assisted retinal vein cannulation with force-based puncture detection: Micron vs. the steady-hand eye robot. Engineering in Medicine and Biology Society (EMBC), 2016 IEEE 38th Annual International Conference of the 2016 Oct 18; IEEE; pp. 5107–5111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robert MacLaren. World first for robot eye operation. [Last accessed on 2016 Sep 12]. Available from: http://www.ox.ac.uk/news/2016-09-12-world-first-robot-eye-operation .
- 15.Guerrouad A, Jolly D. Automatic analysis of weariness during a micromanipulation task by SMOS. In: Engineering in Medicine and Biology Society, 1989. Images of the Twenty-First Century. Proceedings of the Annual International Conference of the IEEE Engineering in 1989 Nov 9 (pp. 906-907). IEEE. [Google Scholar]
- 16.Pournaras CJ, Shonat RD, Munoz JL, Petrig BL. New ocular micromanipulator for measurements of retinal and vitreous physiologic parameters in the mammalian eye. Exp Eye Res. 199;;3:723–727. doi: 10.1016/0014-4835(91)90107-p. [DOI] [PubMed] [Google Scholar]
- 17.Grace KW, Colgate JE, Glucksberg MR, Chun JH. A six degree of freedom micromanipulator for ophthalmic surgery. Robotics and Automation, 1993. Proceedings. 1993 IEEE International Conference on 1993 May 2; IEEE; pp. 630–635. [Google Scholar]
- 18.Jensen PS, Grace KW, Attariwala R, Colgate JE, Glucksberg MR. Toward robot-assisted vascular microsurgery in the retina. Graefes Arch Clin Exp Ophthalmol. 1997;235:696–701. doi: 10.1007/BF01880668. [DOI] [PubMed] [Google Scholar]
- 19.Hunter IW, Doukoglou TD, Lafontaine SR, Charette PG, Jones LA, Sagar MA, et al. A teleoperated microsurgical robot and associated virtual environment for eye surgery. Presence-Teleop Virt. 1993;2:265–280. [Google Scholar]
- 20.Charles S, Das H, Ohm T, Boswell C, Rodriguez G, Steele R, et al. Dexterity-enhanced telerobotic microsurgery. Advanced Robotics, 1997. ICAR'97. Proceedings, 8th International Conference on 1997 Jul 7; IEEE; pp. 5–10. [Google Scholar]
- 21.Schenker PS, Das H, Ohm TR. Development of a new high-dexterity manipulator for robot-assisted microsurgery. Photonics for Industrial Applications. 1995;2351:191–198. [Google Scholar]
- 22.Yu DY, Cringle SJ, Constable IJ. Robotic ocular ultramicrosurgery. Aust N Z J Ophthalmol. 1998;26:S6–S8. doi: 10.1111/j.1442-9071.1998.tb01375.x. [DOI] [PubMed] [Google Scholar]
- 23.Taylor R, Jensen P, Whitcomb L, Barnes A, Kumar R, Stoianovici D, et al. A steady-hand robotic system for microsurgical augmentation. Int J Rob Res. 1999;18:1201–1210. [Google Scholar]
- 24.Üneri A, Balicki MA, Handa J, Gehlbach P, Taylor RH, Iordachita I. New steady-hand eye robot with micro-force sensing for vitreoretinal surgery. Biomedical Robotics and Biomechatronics (BioRob), 2010 3rd IEEE RAS and EMBS International Conference on 2010 Sep 26; IEEE; pp. 814–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ang WT, Riviere CN, Khosla PK. Design and implementation of active error canceling in hand-held microsurgical instrument. Intelligent Robots and Systems, 2001. Proceedings. 2001 IEEE/ RSJ International Conference on 2001; IEEE; pp. 1106–1111. [Google Scholar]
- 26.MacLachlan RA, Becker BC, Tabarés JC, Podnar GW, Lobes LA, Jr, Riviere CN. Micron: An actively stabilized handheld tool for microsurgery. IEEE Trans Robot. 2012;28:195–212. doi: 10.1109/TRO.2011.2169634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wei W, Goldman R, Simaan N, Fine H, Chang S. Design and theoretical evaluation of micro-surgical manipulators for orbital manipulation and intraocular dexterity. Proceedings 2007 IEEE International Conference on Robotics and Automation; 2007 Apr 10; IEEE; pp. 3389–3395. [Google Scholar]
- 28.Wei W, Goldman RE, Fine HF, Chang S, Simaan N. Performance evaluation for multi-arm manipulation of hollow suspended organs. IEEE Trans Robot. 2009;25:147–157. [Google Scholar]
- 29.Mulgaonkar AP, Hubschman JP, Bourges JL, Jordan BL, Cham C, Wilson JT, et al. A prototype surgical manipulator for robotic intraocular micro surgery. Stud Health Technol Inform. 2009;142:215–217. [PubMed] [Google Scholar]
- 30.Rahimy E, Wilson J, Tsao TC, Schwartz S, Hubschman JP. Robot-assisted intraocular surgery: Development of the IRISS and feasibility studies in an animal model. Eye. 2013;27:972–978. doi: 10.1038/eye.2013.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakano T, Sugita N, Ueta T, Tamaki Y, Mitsuishi M. A parallel robot to assist vitreoretinal surgery. Int J Comput Assist Radiol Surg. 2009;4:517–526. doi: 10.1007/s11548-009-0374-2. [DOI] [PubMed] [Google Scholar]
- 32.Meenink HT. Vitreo-retinal eye surgery robot: Sustainable precision (Doctoral dissertation, Technische Universiteit Eindhoven)
- 33.Meenink HC, Hendrix R, Rosielle PC, Steinbuch M, de Smet M. A master-slave robot for vitreo-retinal eye surgery. Proc. Of the 10th Int. Conf. of European Society for Precision Engineering and Nanotechnology 2010. [Google Scholar]
- 34.Caers P, Gijbels A, De Volder M, Gorissen B, Stalmans P, Reynaerts D, et al. Precision experiments on a comanipulated robotic system for use in retinal surgery. Proceedings of the 2011 SCATh Joint Workshop on New Technologies for Computer/Robot Assisted Surgery; 2011 Jul 11; Graz, Austria. pp. 1–7. [Google Scholar]
- 35.Gijbels A, Vander Poorten EB, Stalmans P, Van Brussel H, Reynaerts D. Design of a teleoperated robotic system for retinal surgery. 2014 IEEE International Conference on Robotics and Automation (ICRA); 2014 May 31; IEEE; pp. 2357–2363. [Google Scholar]
- 36.Nasseri MA, Eder M, Nair S, Dean EC, Maier M, Zapp D, et al. The introduction of a new robot for assistance in ophthalmic surgery. 2013 35th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC); 2013 Jul 3; IEEE; pp. 5682–5685. [DOI] [PubMed] [Google Scholar]
- 37.Nasseri MA, Eder M, Eberts D, Nair S, Maier M, Zapp D, et al. Kinematics and dynamics analysis of a hybrid parallel-serial micromanipulator designed for biomedical applications. 2013 IEEE/ASME International Conference on Advanced Intelligent Mechatronics; 2013 Jul 9; IEEE; pp. 293–299. [Google Scholar]
- 38.Molaei A, Abedloo E, Taghirad HD, Marvi Z. Kinematic and workspace analysis of diamond: An innovative eye surgery robot. 2015 23rd Iranian Conference on Electrical Engineering; 2015 May 10; IEEE; pp. 882–887. [Google Scholar]
- 39.Nambi M, Bernstein PS, Abbott JJ. A compact retinal-surgery telemanipulator that uses disposable instruments. International Conference on Medical Image Computing and Computer-Assisted Intervention; 2015 Oct 5; Springer International Publishing; pp. 258–265. [Google Scholar]
- 40.Nambi M, Bernstein PS, Abbott JJ. A Compact Telemanipulated Retinal-Surgery System that Uses Commercially Available Instruments with a Quick-Change Adapter. J Med Robot Res. 2016;1:1630001. [Google Scholar]
- 41.Kummer MP, Abbott JJ, Kratochvil BE, Borer R, Sengul A, Nelslon BJ. OctoMag: An electromagnetic system for 5-DOF wireless micromanipulation. IEEE Trans Robot. 2010;26:1006–1017. [Google Scholar]
- 42.Hubschman JP, Bourges JL, Choi W, Mozayan A, Tsirbas A, Kim CJ, Schwartz SD. 'The Microhand': A new concept of micro-forceps for ocular robotic surgery. Eye. 2010;24:364–367. doi: 10.1038/eye.2009.47. [DOI] [PubMed] [Google Scholar]
- 43.Becker BC, Yang S, MacLachlan RA, Riviere CN. Towards vision-based control of a handheld micromanipulator for retinal cannulation in an eyeball phantom. 2012 4th IEEE RAS & EMBS International Conference on Biomedical Robotics and Biomechatronics (BioRob); 2012 Jun 24; IEEE; pp. 44–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stetten G, Wu B, Klatzky R, Galeotti J, Siegel M, Lee R, et al. Hand-held force magnifier for surgical instruments. International Conference on Information Processing in Computer-Assisted Interventions; 2011 Jun 22; Springer Berlin Heidelberg; pp. 90–100. [Google Scholar]
- 45.Gonenc B, Handa J, Gehlbach P, Taylor RH, Iordachita I. A comparative study for robot assisted vitreoretinal surgery: Micron vs. the Steady-Hand Robot. Robotics and Automation (ICRA), 2013 IEEE International Conference on 2013 May 6; IEEE; pp. 4832–4837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fleming I, Balicki M, Koo J, Iordachita I, Mitchell B, Handa J, et al. Cooperative robot assistant for retinal microsurgery. International conference on medical image computing and computer-assisted intervention; 2008 Sep 6; Springer Berlin Heidelberg; pp. 543–550. [DOI] [PubMed] [Google Scholar]
- 47.Tameesh MK, Lakhanpal RR, Fujii GY, Javaheri M, Shelley TH, D'anna S, et al. Retinal vein cannulation with prolonged infusion of tissue plasminogen activator (t-PA) for the treatment of experimental retinal vein occlusion in dogs. Am J Ophthalmol. 2004;138:829–839. doi: 10.1016/j.ajo.2004.06.083. [DOI] [PubMed] [Google Scholar]
- 48.Maghami MH, Sodagar AM, Lashay A, Riazi-Esfahani H, Riazi-Esfahani M. Visual prostheses: The enabling technology to give sight to the blind. J Ophthalmic Vis Res. 2014;9:494. doi: 10.4103/2008-322X.150830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gaudana R, Ananthula HK, Parenky A, Mitra AK. Ocular drug delivery. AAPS J. 2010;12:348–360. doi: 10.1208/s12248-010-9183-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bennett J, Maguire AM. Gene therapy for ocular disease. Mol Ther. 2000;1:501. doi: 10.1006/mthe.2000.0080. [DOI] [PubMed] [Google Scholar]
- 51.Murphy SV, Atala A. 3D bioprinting of tissues and organs. Nat Biotechnol. 2014;32:773–785. doi: 10.1038/nbt.2958. [DOI] [PubMed] [Google Scholar]


