Abstract
Toxoplasma gondii is an obligate intracellular parasite capable of infecting virtually all nucleated cell types in almost all warm-blooded animals. Interestingly, Toxoplasma has a relatively full repertoire of amino acid biosynthetic machinery, perhaps reflecting its broad host range and, consequently, its need to adapt to a wide array of amino acid resources. Although Toxoplasma has been shown to be auxotrophic for tryptophan and arginine, it has not previously been determined if Toxoplasma is also auxotrophic for tyrosine. Toxoplasma tachyzoites and bradyzoites were recently found to express an amino acid hydroxylase (AAH2) that is capable of synthesizing tyrosine and dihydroxyphenylalanine (DOPA) from phenylalanine; however, the role of AAH2 in tachyzoite and bradyzoite infection has not yet been identified. To determine if Toxoplasma requires exogenous tyrosine for growth, we performed growth assays on tachyzoites and bradyzoites in nutrient-rich media titrated with varying amounts of tyrosine. We found that Toxoplasma tachyzoites form significantly smaller plaques in tyrosine-limiting media in a dose-dependent manner and that this phenotype is not affected by deletion of TgAAH2. To determine if bradyzoites require exogenous tyrosine for growth, we induced differentiation from tachyzoites in vitro in tyrosine-limiting media and found that replication and vacuole number are all decreased in tyrosine-deficient media. Importantly, culture of confluent human fibroblasts in tyrosine-deficient media does not affect their viability, indicating that, at least in vitro, the need for tyrosine is at the level of Toxoplasma, not the host cell supporting its growth.
Keywords: metabolism, Apicomplexan, amino acid hydroxylase
Graphical abstract
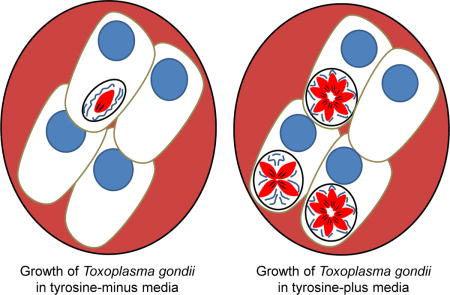
1. Introduction
Toxoplasma gondii is an obligate intracellular Apicomplexan parasite capable of infecting an extraordinarily wide range of cell types in almost all warm-blooded animals. The life cycle of Toxoplasma includes a sexual stage, which is only known to occur in the feline intestine, and an asexual stage, in which parasites replicate in various intermediate hosts, including humans. Upon infecting an intermediate host, Toxoplasma differentiates into tachyzoites that rapidly disseminate throughout host tissues. Although most tachyzoites are cleared by the immune system, some tachyzoites convert to bradyzoites, which form relatively quiescent tissue cysts in the brain and muscle [1].
Consistent with their parasitic nature, many Apicomplexan parasites have lost some amino acid biosynthetic capabilities; however, the Toxoplasma genome encodes a relatively full repertoire of biosynthetic machinery, perhaps reflecting the wide variety of intracellular environments in which it resides [2, 3]. Computational models of Toxoplasma metabolism have successfully predicted many of its metabolic capabilities, such as acetyl-CoA biosynthesis, but have made conflicting predictions on whether tachyzoites are auxotrophic for tyrosine [2, 4, 5]. Previous studies have also shown evidence for tachyzoites possessing an active shikimate pathway, which can synthesize tyrosine, tryptophan, and phenylalanine in bacteria; however, Toxoplasma does not appear to have orthologs for the enzymes required for de novo aromatic amino acid synthesis [6]. Moreover, previous studies have shown that depletion of tryptophan in the host cell upon IFN-γ stimulation inhibits growth of Toxoplasma tachyzoites, suggesting they are auxotrophic for tryptophan and therefore may not have retained this part of the shikimate pathway [7, 8].
Recently, two amino acid hydroxylases, AAH1 and AAH2, were found to be expressed at a low level in Toxoplasma tachyzoites and upregulated in bradyzoites [9]. These genes, which have 98% sequence similarity to each other, have homology to both phenylalanine hydroxylase and tyrosine hydroxylase and were shown to be able to convert phenylalanine to tyrosine and tyrosine to dihydroxyphenylalanine (DOPA) [10]. The inclusion of these two genes in computational modeling of Toxoplasma metabolism has led to the prediction that tachyzoites are not auxotrophic for tyrosine [2, 4]; however, previous reports have shown that AAH2 is not required for efficient growth in nutrient-rich media and no role for this gene in the tachyzoite stage or bradyzoite stage has been reported. Attempts to delete AAH1 using the highly efficient CRISPR-Cas9 have been unsuccessful to date and so its function is completely unexplored [9].
To determine if Toxoplasma requires exogenous tyrosine for growth, we performed growth assays on tachyzoites and bradyzoites in nutrient-rich media titrated with varying amounts of tyrosine. Using this approach, we show here that tachyzoites and bradyzoites are indeed auxotrophic for tyrosine but that AAH2 is apparently dispensable for tyrosine metabolism in these two developmental forms.
2. Methods and Materials
2.1 Parasite strains, culture, and growth
The Toxoplasma gondii PruΔku80Δhpt strain was used for this study. Toxoplasma tachyzoites were maintained by serial passage in human foreskin fibroblasts (HFFs) cultured in complete Dulbecco’s Modified Eagle Medium (cDMEM) supplemented with 10% heat-inactivated fetal bovine serum (FBS), 2 mM L-glutamine, 100 U/ml penicillin and 100 μg/ml streptomycin and grown at 37°C in 5% CO2. Infections included in this study were performed by scraping infected monolayers and lysing the host cells open using a 27 G needle. The released parasites were added to confluent HFFs at the multiplicity of infection (MOI) stated. To measure tachyzoite growth in tyrosine-titrated media, confluent HFFs were washed vigorously three times with phosphate-buffered saline (PBS) and cultured with high-glucose DMEM [11] lacking or titrated with varying concentrations of tyrosine and supplemented with 2 mM L-glutamine, 100 U/ml penicillin and 100 μg/ml streptomycin. The monolayers were immediately infected with tachyzoites in PBS. Plaques were scored by staining cultures with crystal violet after 14 days growth at 37°C. The number of parasites per vacuole was scored by fixing infected cultures at 36 hours post-infection with 4% formaldehyde for 15 min at room temperature (RT). The coverslips were mounted with Vectashield mounting media supplemented with 4,6-diamidino-2-phenylindole (DAPI) stain (Vector Laboratories) and visualized by wide-field fluorescence microscopy. The number of vacuoles and parasites per vacuole were quantified by cytosolic mCherry expression. Unless otherwise noted, only vacuoles with two or more parasites were quantified to be sure extracellular singlet parasites were not incorrectly scored as intracellular.
2.2 Generation of Δaah2 and “wild type” strain
The TgAAH2 open reading frame (TgME49_212740) was deleted from PruΔku80Δhpt and replaced with the hypoxanthine-xanthine-guanine phosphoribosyl transferase (HXGPRT or HPT) selectable marker by double homologous recombination using the pTKO2-AAH2 vector. To generate the pTKO2-AAH2 vector, ∼1.5 Kb and ∼1 Kb genomic sequence were amplified by PCR from the 5′- and 3′-regions flanking the TgAAH2 gene. The 5′-flanking region was amplified using 5′-GCGCGGTACCCACCACACGCAAGGCACTTTC-3′ and 5′-GCGCGAATTCGTGGCCTTATTTGAGCATATTCTG-3′ primer sequences and the resulting fragment was cloned into the KpnI and EcoRI restriction sites of pTKO2 [12]. The 3′-flanking region was amplified using 5′-GCGCAAGCTTCGTTGTGTTCACCGTCGCTAC-3′ and 5′-GCGCGCTAGCGGCGTTTTGACTCTTTTGTATGC-3′ primer sequences and was cloned into HindIII and NheI restriction sites. The pTKO2-AAH2 vector was linearized with NotI, and 30 μg of the linearized plasmid was transfected into PruΔku80Δhpt by electroporation, as previously described. The parasites were allowed to infect HFFs in 24-well plates for twenty-four hours, after which the media was changed to complete DMEM supplemented with 50 μg/ml mycophenolic acid (MPA) and 50 μg/ml xanthine (XAN) for HXGPRT selection. The parasites were passed twice into 24-well plates before being single cloned into 96-well plates by limiting dilution. Deletion of AAH2 in the Δaah2 strain was confirmed by PCR using the following primers: 5′-GCAACTTCTCGGGCTCGCGT-3′ and 5′-GATCTTGAGGGAGACAGGAGGCATATGTACAT-3′. The “wild type” control strain was generated by transfecting PruΔku80Δhpt with the pTKO2-AAH2 vector and selecting for stable integration using MPA/XAN, as described above. Stable integration of the vector outside of the AAH2 locus was confirmed by PCR.
2.3 Viability staining
The viability of human foreskin fibroblasts (HFFs) after continuous culture in tyrosine-free DMEM was determined using the live/dead viability/cytotoxicity kit for mammalian cells (Thermo Fisher). HFFs were cultured in cDMEM on glass coverslips in 24-well plates for a minimum of one week to reach confluency. The cells were then washed vigorously three times with PBS and cultured in DMEM lacking or containing 100 μg/ml tyrosine for 14 days. The cells were then simultaneously stained with green-fluorescent calcein-AM for intracellular esterase activity and red-fluorescent ethidium homodimer-1 for disrupted membrane integrity according to the protocol specified by the manufacturer. The coverslips were then mounted, sealed, and visualized by wide-field fluorescent microscopy using 494/517 nm excitation for live/dead visualization, respectively. HFFs cultured continuously in cDMEM were used as a control for viable cells, while HFFs treated with 70% methanol for twenty minutes were used as a control for nonviable cells.
2.4 Invasion assays
Tachyzoites were allowed to invade HFFs for one hour in DMEM containing or lacking tyrosine. The samples were then fixed with 4% formaldehyde and blocked for one hour at room temperature (RT) with 3% BSA. Extracellular parasites were quantified based on SAG1 surface staining. Specifically, extracellular SAG1 was stained without permeabilization using mouse DG52 anti-SAG1 antibody and 488-nm-fluorochrome-conjugated goat anti-mouse IgG. Total parasites were quantified by cytosolic mCherry expression.
2.5 Switch assays
Differentiation of tachyzoites to bradyzoites was induced in vitro by culturing infected cells at 37°C in alkaline, low-serum conditions at ambient CO2, as previously described [13]. Specifically, tachyzoites were allowed to infect HFF monolayers for 3 hours in cDMEM at 37°C, 5% CO2. The monolayers were washed three times with PBS and cultured in bradyzoite medium lacking or titrated with varying concentrations of tyrosine and supplemented with 1% dialyzed FBS, 10 mg/ml HEPES, 100 U/ml penicillin, and 100 μg/ml streptomycin (final pH 8.0). For quantification of parasites per cyst and vacuoles per field, infected monolayers cultured on glass coverslips were fixed at three days post-infection with methanol at −20°C for 20 min. The samples were then blocked with 3% bovine serum albumin (BSA) for one hour at RT and stained with mouse anti-SAG1 DG52 primary antibodies and fluorescein conjugated to dolichos biflorus agglutinin (DBA) overnight at 4°C. SAG1 was detected with 594-nm-fluorochrome-conjugated goat anti-mouse IgG. The number of vacuoles per field was quantified by counting the number of vacuoles with two or more parasites in a minimum of ten randomly chosen fields. The number of parasites per cyst was quantified by counting the number of parasites in DBA-positive vacuoles with two or more parasites.
3. Results
3.1 Tachyzoite Growth
To address tyrosine metabolism in Toxoplasma tachyzoites, we first set out to determine if they require exogenous tyrosine for growth. To do this, we infected human foreskin fibroblasts (HFFs) with tachyzoites in tyrosine-titrated media for 14 days and measured the size of the resulting plaques. The results (Fig. 1A) showed that plaques were dramatically smaller as tyrosine levels decreased and did not form in the absence of tyrosine. This indicates that tachyzoites are dependent on the addition of exogenous tyrosine for efficient growth.
Figure 1. Exogenous tyrosine is essential for Toxoplasma plaque formation independently of AAH2.
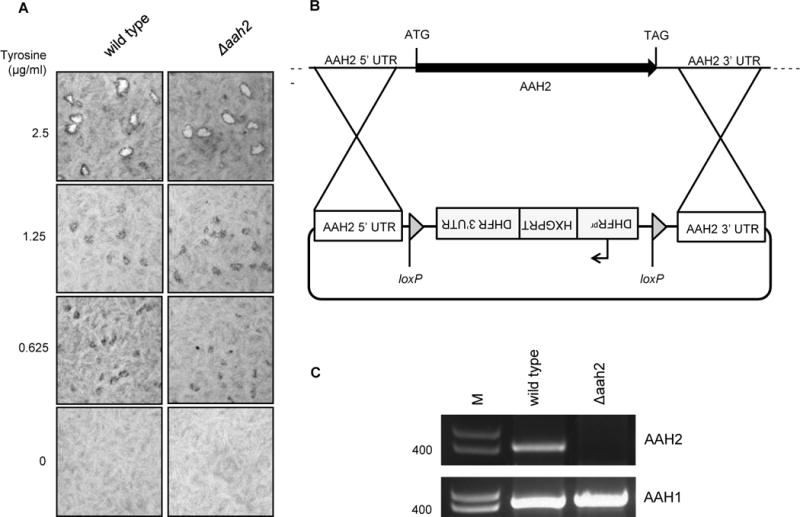
A. Absence of tyrosine results in a defect in plaque formation that is similar for wild type and Δaah2 parasites. Tachyzoites were cultured for 14 days on HFFs in DMEM titrated with varying concentrations of tyrosine. Plaques were fixed with methanol at 14 days post-infection, stained with crystal violet, and visualized by photomicroscopy. B. Schematic of the vector used to replace the AAH2 coding region with the hypoxanthine-xanthine-guanine phosphoribosyl transferase (HXGPRT) selectable marker via double homologous recombination. Arrow indicates promoter. UTR indicates untranslated region. C. Confirmation of AAH2 deletion. PCR was performed on genomic DNA from PruΔku80 wildtype and Δaah2 clones using primers specific for AAH2. Primers specific to AAH1 were used to confirm that AAH1 was not deleted. Size markers (M) are in base pairs.
The existence of two Toxoplasma genes predicted to encode aromatic amino acid hydroxylases, AAH1 and AAH2, suggested the possibility that some amount of the tyrosine levels needed for growth might also be generated by the action of one or both of these enzymes. To address this, we attempted to delete both genes individually and in combination. We did this using homologous recombination and constructs that would replace the genes with the selectable marker, hypoxanthine/xanthine/guanine phosphoribosyltransferase (HXGPRT). We were never able to obtain Δaah1 mutants by this approach (data not shown) but we were able to create Δaah2 mutants as shown in Fig. 1B, C.
To determine if AAH2 is involved in tyrosine metabolism, we performed the plaque assay in decreasing concentrations of tyrosine using the Δaah2 strain. The results (Fig. 1A) showed no difference in the plaque size relative to wild type (WT) tachyzoites when tyrosine was limiting. This argues that AAH2 does not provide significant amounts of tyrosine to the tachyzoites, at least as detectable by this assay.
Previous reports have shown that confluent fibroblasts, which were used in the assays described above, do not require exogenous tyrosine for viability [14, 15]. As such, the inability of tachyzoites to grow in the absence of tyrosine seems likely to be due to their own need for this amino acid rather than an indirect effect of tyrosine-deprivation on the host cells. Nevertheless, we tested HFF viability after continuous culture in tyrosine-deficient media using a more sensitive, fluorescence-based assay that measures death and viability simultaneously. We found that HFFs grown to confluency in nutrient-rich DMEM and then cultured for fourteen days in tyrosine-free media had similarly high levels of viability as HFFs cultured in tyrosine-rich media (Fig. 2). Although we cannot exclude the possibility that tachyzoite growth is affected indirectly by tyrosine deprivation in the host cell, these results suggest the effects observed here were most likely due to starvation for tyrosine within the parasites themselves.
Figure 2. Absence of tyrosine does not affect host cell viability.
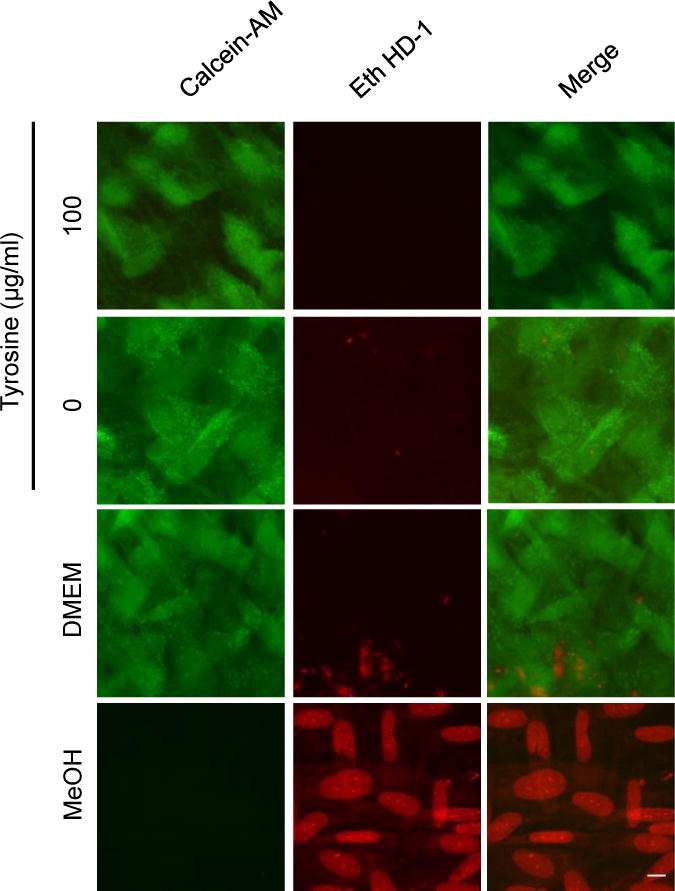
Confluent HFFs were cultured in tyrosine-rich or tyrosine-depleted DMEM for 14 days and stained with calcein-AM and ethidium homodimer-1 (Eth HD-1) to measure cell viability (green) and death (red), respectively. HFFs cultured in complete DMEM or treated with 70% methanol were used as controls. Scale bar indicates 5 μm.
Plaque size is a crude measure of growth and does not distinguish between defects in attachment, invasion, replication and egress. To further refine our analysis, therefore, we first looked 36 hours after infection for an effect of tyrosine on parasite growth, measured as numbers of parasites per vacuole. To be sure extracellular singlet parasites were not mistakenly counted as intracellular vacuoles, only vacuoles with two or more parasites were scored. The results showed that for both the WT and Δaah2 parasites there were significant decreases in this measure (Fig. 3A), reflecting a smaller average number of doublings (Fig. 3B), when exogenous tyrosine was absent.
Figure 3. Exogenous tyrosine is required for replication but not invasion of both wild type and Δaah2 tachyzoites.
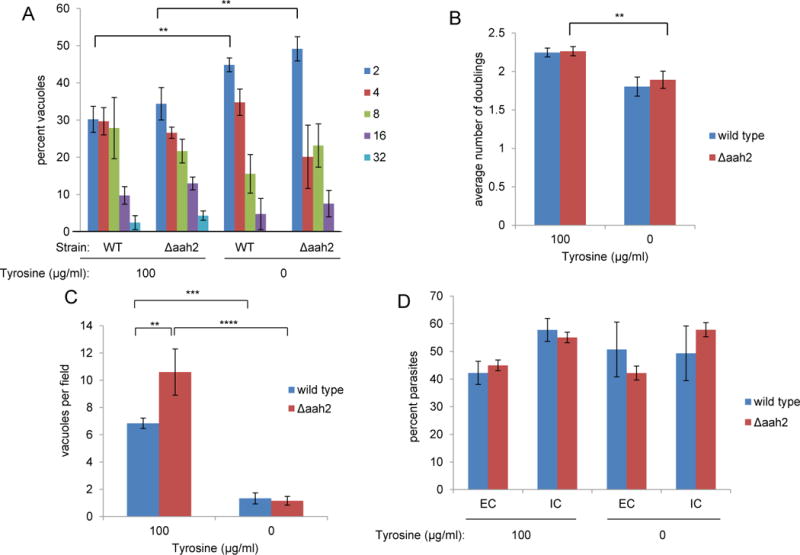
A. The absence of tyrosine results in fewer parasites per vacuole using both wild type and Δaah2 tachyzoites. The indicated tachyzoites were cultured on HFFs in DMEM containing or lacking tyrosine. Infected coverslips were fixed with formaldehyde at 36 hours post-infection. The number of tachyzoites per vacuole was quantified based on mCherry expression and counting parasites per vacuole in random fields at 100x magnification. Only vacuoles with two or more parasites were scored. B. The absence of tyrosine results in fewer doublings using both wild type and Δaah2 tachyzoites. The data on the average numbers of parasites per vacuole shown in 3A were converted to parasite doublings using a log base 2 scale. C. The absence of tyrosine results in fewer vacuoles per field using both wild type and Δaah2 tachyzoites. The number of vacuoles containing two or more parasites was scored on random fields as described above. D. The absence of tyrosine does not affect invasion efficiency of wild type and Δaah2 parasites. Tachyzoites were allowed to invade HFFs for one hour in DMEM containing or lacking tyrosine. The samples were then fixed with formaldehyde and extracellular parasites were stained with anti-SAG1 antibody and Alexa-488-conjugated secondary antibody without permeabilization. Total parasites were quantified by cytosolic mCherry expression. Extracellular parasites were quantified based on SAG1 surface staining. The results are plotted as the percentage of all parasites seen that are intracellular (IC) versus extracellular (EC). Data shown are the average values in one experiment that is representative of three or more repeated experiments. Error bars show standard deviation. Values with statistically significant differences are labeled by brackets and asterisks as follows: *, p < 0.05; **, p<0.01; ***, p<0.001; ****, p<0.0001. The averages shown were compared by two-way ANOVA and Tukey’s multiple comparisons test.
We next looked at the efficiency with which vacuoles are formed by counting the number of vacuoles present at 36 hours in the presence and absence of tyrosine. The results (Fig. 3C) showed that in the absence of tyrosine, the number of vacuoles with two or more parasites was reduced by about 80–90%. Interestingly, in the tyrosine-replete media there was a significantly higher number of vacuoles formed using the Δaah2 vs. wild type strains (Fig. 3C). The basis for this is not known and was not pursued further but, overall, these results show that tyrosine is needed for efficient formation of productive vacuoles in both strains.
To determine if the lower number of vacuoles at 36 hours is due to a defect in invasion, we infected HFFs with WT and Δaah2 strains in tyrosine-rich and tyrosine-depleted media for one hour and measured the percentage of intracellular and extracellular parasites. The results (Fig. 3D) showed that the percentage of intracellular parasites, be they WT or Δaah2, was not significantly different in the presence or absence of tyrosine. The number of extracellular parasites per field likewise did not vary significantly for either strain depending on tyrosine concentration, suggesting that there is also no defect in attachment (Fig. 3D and data not shown). Collectively, these results suggest that Toxoplasma tachyzoites require exogenous tyrosine for growth but not attachment or invasion, and that many of the invasion events in the absence of tyrosine are non-productive (hence the substantially lower number of vacuoles observed at 36 hours). They also argue that AAH2 is not contributing significantly to the intra-parasite pools of tyrosine since no further impairment was seen in tyrosine-deficient conditions when this gene was missing.
3.2 Bradyzoite Growth
Because AAH2 has been reported to be up-regulated in the bradyzoite stage [9, 16], we next asked whether WT and Δaah2 bradyzoites generated in vitro differ in their ability to grow and differentiate in limiting tyrosine. To do this, we used high pH media known to induce a differentiation (“switch”) from tachyzoites to bradyzoites in vitro [13]. We first measured the number of vacuoles and parasites per cyst in tyrosine-rich and tyrosine-depleted “switch” media at three days post-infection. As before, the number of vacuoles per field and average number of doublings were significantly less for parasites cultured in tyrosine-depleted switch media, but there was no significant difference between WT and Δaah2 strains (Fig 4A, B, C). We then assessed the percentage of vacuoles that contained differentiated parasites as indicated by the presence of a carbohydrate-rich tissue cyst wall detected with dolichos biflorus agglutinin (DBA). In agreement with previous work [9], WT and Δaah2 bradyzoites had similar switch efficiencies in tyrosine-replete alkaline media (Fig. 4D). The switch efficiency for both strains decreased somewhat in tyrosine-free “switch” media (Fig. 4D), but the difference was not statistically significant. Overall, these results suggest that bradyzoites also require exogenous tyrosine for efficient growth but that, as for tachyzoites, AAH2 does not appear to play a detectable role in tyrosine generation in this developmental stage.
Figure 4. Exogenous tyrosine is required for bradyzoite growth but not differentiation.
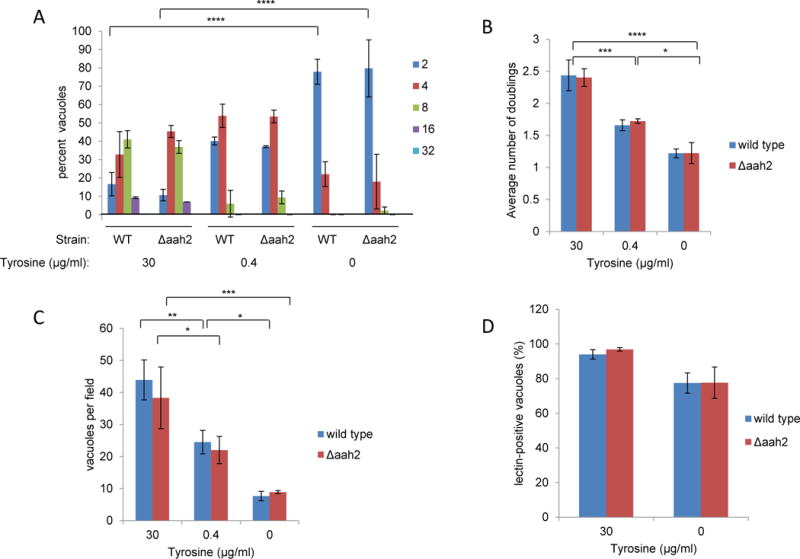
A. The absence of tyrosine results in fewer parasites per cyst for both wild type and Δaah2 bradyzoites. Tachyzoites were allowed to infect HFFs on coverslips in DMEM for 3.5 hours, after which the monolayers were washed three times with PBS and cultured in tyrosine-titrated, alkaline switch media. Infected monolayers were fixed with methanol at three days post-infection and stained with fluorescein-conjugated dolichos biflorus agglutinin (DBA), a cyst wall marker, and anti-SAG1 antibodies. DBA-positive vacuoles with two or more parasites were scored. Data shown are from one experiment that is representative of three or more repeated experiments. Error bars indicate standard deviation. B. The absence of tyrosine results in fewer doublings for both wild type and Δaah2 bradyzoites. Data for the average numbers of parasites per vacuole shown in (4A) were converted to parasite doublings using a log base 2 scale. Error bars indicate standard deviation. C. The absence of tyrosine results in fewer vacuoles per field using both wild type and Δaah2 strains. Wild type and Δaah2 tachyzoites were induced to switch and stained as described in (4A). The number of vacuoles containing two or more parasites was scored at 100× magnification on random fields. Data shown are averages from one experiment that is representative of three or more repeated experiments. Error bars indicate standard deviation. D. The absence of tyrosine does not significantly affect switch efficiency of wild type and Δaah2 strains. Tachyzoites were induced to switch in tyrosine-titrated, alkaline media, fixed with methanol at three days post-infection, and stained as described in (4A). Switch efficiency was measured by counting the number of vacuoles stained with DBA over the number of vacuoles staining with SAG1. Data shown are the combined averages of three independent experiments on triplicate coverslips using the same conditions. Error bars indicate standard error of the mean. Values with statistically significant differences are labeled by brackets and asterisks as follows: *, p<0.05; **, p<0.01; ***, p<0.001; ****, p<0.0001. The averages shown were compared by two-way ANOVA and Bonferroni’s or Tukey’s multiple comparisons test.
4. Discussion
In this study we have shown that Toxoplasma tachyzoites and bradyzoites require exogenous tyrosine for growth in vitro. Tachyzoites cultured in tyrosine-free media did not form visible plaques and showed a significant, dose-dependent decrease in the number of vacuoles per field as well as the average number of doublings at 36 hours post-infection. Importantly, tachyzoites did not show a defect in invasion or the number of parasites per field at one-hour post-infection, suggesting that these decreases result from parasite death and/or decreased replication after invasion. These results indicate that Toxoplasma tachyzoites are auxotrophic for tyrosine, which was not predicted by previous computational models of Toxoplasma metabolism [2, 4, 5]. The reason for this discrepancy is unclear, but it may be because Toxoplasma encodes AAH1 and AAH2, which are homologous to phenylalanine hydroxylase. Although recombinant AAH1 and AAH2 have been shown to be capable of converting phenylalanine to tyrosine when expressed as recombinant proteins and assayed biochemically, their expression levels during tachyzoite growth are exceedingly low [9]. This may explain, therefore, why tachyzoites are auxotrophic for tyrosine. Interestingly, the effect on tachyzoite growth appeared to differ at the level of individual infected cells, perhaps due to cell-to-cell differences in the tyrosine pools absent supplementation and/or the needs of individual parasites when they infect cells.
Tachyzoites induced to differentiate into bradyzoites in alkaline media likewise showed a dependence on exogenous tyrosine. The number of vacuoles per field and average number of doublings was significantly lower in tyrosine-free and tyrosine-limiting conditions. This decrease in the number of vacuoles positive for SAG1 or DBA may be due to parasite death or their inability to produce sufficient protein for replication or differentiation in limiting tyrosine.
Deletion of AAH2 did not affect tachyzoite or bradyzoite growth in tyrosine-rich or tyrosine-limiting media, suggesting that AAH2 is not required for growth or tyrosine metabolism. Although AAH2 is expressed in tachyzoites and upregulated in bradyzoites, no role has yet been discovered for AAH2 in either life cycle stage [9, 16]. The lack of a phenotype upon AAH2 deletion may be due to functional redundancy with AAH1, another aromatic amino acid hydroxylase with 98% sequence similarity to AAH2 [10]. We and others have attempted to delete AAH1 using double homologous recombination and the highly efficient CRISPR-Cas9 system, without success. This may be due to AAH1 essentiality or misannotation of its sequence due to its high similarity to AAH2. Alternatively, AAH2 may be functionally important in a different life cycle stage, such as in oocyst formation. RNASeq data shows substantial upregulation of AAH1 and AAH2 in oocysts; they may therefore be required for synthesizing tyrosine for tyrosine-rich protein production or for producing DOPA, which is a common component of cyst walls [17, 18].
Overall, this work shows that Toxoplasma tachyzoites and bradyzoites require exogenous tyrosine for growth in vitro. Although Toxoplasma appears to possess many biosynthetic pathways, this work reaffirms the need to validate computational metabolic models and consider protein expression levels. Future experiments will be needed to determine the role of AAH2, if any, in the tachyzoite and bradyzoite stages.
Highlights.
Toxoplasma tachyzoites require exogenous tyrosine for growth in vitro
Toxoplasma bradyzoites require exogenous tyrosine for growth but not differentiation in vitro
AAH2 appears to not be required for tyrosine metabolism in asexual growth of Toxoplasma gondii in vitro
Acknowledgments
This work was supported by NIH R21 AI112962 (JCB), NIH F31 AI120649 (NDM), and Stanford Graduate Fellowship (NDM). We thank Melanie Espiritu for help with tissue culture and all the members of the Boothroyd lab for thoughtful discussions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dubey JP. Advances in the life cycle of Toxoplasma gondii. Int J Parasitol. 1998;28(7):1019–24. doi: 10.1016/s0020-7519(98)00023-x. [DOI] [PubMed] [Google Scholar]
- 2.Chaudhary K, Roos DS. Protozoan genomics for drug discovery. Nat Biotechnol. 2005;23(9):1089–91. doi: 10.1038/nbt0905-1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Popp J, et al. Role of host cell-derived amino acids in nutrition of intracellular Salmonella enterica. Infect Immun. 2015;83(12):4466–75. doi: 10.1128/IAI.00624-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Song C, et al. Metabolic reconstruction identifies strain-specific regulation of virulence in Toxoplasma gondii. Mol Syst Biol. 2013;9:708. doi: 10.1038/msb.2013.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tymoshenko S, et al. Metabolic Needs and Capabilities of Toxoplasma gondii through Combined Computational and Experimental Analysis. PLoS Comput Biol. 2015;11(5):e1004261. doi: 10.1371/journal.pcbi.1004261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roberts F, et al. Evidence for the shikimate pathway in apicomplexan parasites. Nature. 1998;393(6687):801–5. doi: 10.1038/31723. [DOI] [PubMed] [Google Scholar]
- 7.Sibley LD, Messina M, Niesman IR. Stable DNA transformation in the obligate intracellular parasite Toxoplasma gondii by complementation of tryptophan auxotrophy. PNAS. 1994 doi: 10.1073/pnas.91.12.5508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pfefferkorn ER. Interferon gamma blocks the growth of Toxoplasma gondii in human fibroblasts by inducing the host cells to degrade tryptophan. Proc Natl Acad Sci U S A. 1984;81(3):908–12. doi: 10.1073/pnas.81.3.908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang ZT, et al. Reassessment of the role of aromatic amino acid hydroxylases and the effect of infection by Toxoplasma gondii on host dopamine. Infect Immun. 2015;83(3):1039–47. doi: 10.1128/IAI.02465-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaskell EA, et al. A unique dual activity amino acid hydroxylase in Toxoplasma gondii. PLoS One. 2009;4(3):e4801. doi: 10.1371/journal.pone.0004801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dulbecco R, Freeman G. Plaque production by the polyoma virus. Virology. 1959;8(3):396–7. doi: 10.1016/0042-6822(59)90043-1. [DOI] [PubMed] [Google Scholar]
- 12.Caffaro CE, et al. A nucleotide sugar transporter involved in glycosylation of the Toxoplasma tissue cyst wall is required for efficient persistence of bradyzoites. PLoS Pathog. 2013;9(5):e1003331. doi: 10.1371/journal.ppat.1003331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fux B, et al. Toxoplasma gondii strains defective in oral transmission are also defective in developmental stage differentiation. Infect Immun. 2007;75(5):2580–90. doi: 10.1128/IAI.00085-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choo KH, Cotton RG. Genetics of the mammalian phenylalanine hydroxylase system: I. Isolation of phenylalanine hydroxylase-deficient tyrosine auxotrophs from rat hepatoma cells. Somatic Cell Genet. 1977;3(5):457–70. doi: 10.1007/BF01539118. [DOI] [PubMed] [Google Scholar]
- 15.Choo KH, Cotton RG, Danks DM. Phenylalanine hydroxylation and tyrosine requirement of cultured cells. Evidence of phenylalanine hydroxylation in mastocytoma cells in culture. Exp Cell Res. 1976;101(2):370–82. doi: 10.1016/0014-4827(76)90389-x. [DOI] [PubMed] [Google Scholar]
- 16.Prandovszky E, et al. The neurotropic parasite Toxoplasma gondii increases dopamine metabolism. PLoS One. 2011;6(9):e23866. doi: 10.1371/journal.pone.0023866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Belli SI, et al. Roles of tyrosine-rich precursor glycoproteins and dityrosine- and 3–4-dihydroxyphenylalanine-mediated protein cross-linking in development of the oocyst wall in the coccidian parasite Eimeria maxima. Eukaryot Cell. 2003;2(3):456–64. doi: 10.1128/EC.2.3.456-464.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Possenti A, et al. Molecular characterisation of a novel family of cysteine-rich proteins of Toxoplasma gondii and ultrastructural evidence of oocyst wall localisation. Int J Parasitol. 2010;40(14):1639–49. doi: 10.1016/j.ijpara.2010.06.009. [DOI] [PubMed] [Google Scholar]


