Abstract
Scavenger receptor MARCO promotes protective innate immunity against bacterial and parasitic infections; however, its role in host immunity against fungal pathogens, including the major human opportunistic fungal pathogen Cryptococcus neoformans, remains unknown. Using a mouse model of C. neoformans infection we demonstrated that MARCO deficiency leads to impaired fungal control during the afferent phase of cryptococcal infection. Diminished fungal containment in MARCO-/- mice was accompanied by impaired recruitment of Ly6Chigh monocytes and monocyte-derived dendritic cells (moDC) and lower moDC co-stimulatory maturation. The reduced recruitment and activation of mononuclear phagocytes in MARCO-/- mice was linked to diminished early expression of INF-γ along with profound suppression of CCL2 and CCL7 chemokines, providing evidence for roles of MARCO in activation of the CCR2-axis during C. neoformans infection. Lastly, we found that MARCO was involved in C. neoformans phagocytosis by resident pulmonary macrophages and DC. We conclude that MARCO facilitates early interactions between C. neoformans and lung resident cells and promotes the production of CCR2-ligands. In turn, this contributes to a more robust recruitment and activation of moDC that opposes rapid fungal expansion during the afferent phase of cryptococcal infection.
Introduction
Cryptococcus neoformans is a ubiquitous opportunistic fungal pathogen that causes substantial morbidity and mortality amongst immunocompromised patients (1, 2). Spores or desiccated yeast cells of C. neoformans enter the host through the respiratory tract, from which uncontrolled infection disseminates to the central nervous system and causes fatal meningitis (1-4). However, the existing treatments for cryptococcosis are generally ineffective in immune-compromised patients with impaired adaptive immunity. The innate immune response, which is critical for eliciting immediate defense towards C. neoformans, may be the only functional arm against the expansion of pathogens in patients with severe T cell deficiencies (5). Thus, understanding mechanisms of innate anti-cryptococcal defenses could aid the development of supportive immunotherapies for the treatment of cryptococcosis in immunocompromised patients.
Innate control of cryptococcal infection requires cells of the monocyte lineage including macrophages and dendritic cells (DC), and decreased recruitment of these cells, observed for example in CCR2 deficient mice, leads to severely impaired fungal clearance (6-8). Macrophages and DC can polarize to classical or alternative activation phenotypes in response to specific pathogen stimuli or their cytokine environment (9). Classically skewed mononuclear phagocytes show robust expression the enzyme inducible nitric oxide synthase (iNOS) which is needed to generate the major fungicidal molecule, nitric oxide. Consistently, the onset of cryptococcal clearance in mice coincides with peak accumulation of monocyte progenitor cells and corresponding up-regulation of iNOS (10). While classically activated macrophages and DC are highly efficient killers of C. neoformans, alternatively skewed cells are non-protective. These cells show diminished expression of co-stimulatory molecules and iNOS, but robust expression of alternative activation markers including arginase-1 (Arg1) and CD206 (11).
Though under extensive investigation, the mechanisms and factors that foster effective defense during the afferent phase of cryptococcosis (defined in mouse models as 0-14 days post infection) are not well understood. Early production of iNOS and IFN-γ is associated with improved control of C. neoformans growth (12, 13). However, pathways that initiate these early responses are unclear. Previous studies showed innate receptors, such as TLR9 and mannose receptor, are critical for development of protective immunity against C. neoformans but affect fungal control at the efferent stage (>14 dpi) of infection (14-16). Thus, studies of other components and signaling cascades that initiate and promote protective afferent responses to C. neoformans infection are needed.
Macrophage receptor with collagenous structure (MARCO) is a scavenger receptor that facilitates pro-inflammatory cytokine production and mononuclear cell recruitment in response to bacterial infection (17-19). MARCO is mainly expressed on macrophages, DCs and inflammatory monocytes and its expression can also be up regulated during bacterial infections, suggesting that MARCO is important for innate immune defense (20-23). MARCO also participates in the phagocytosis of fungal pathogens by host cells (24, 25). However, whether MARCO modulates cell recruitment and pro-inflammatory cytokine production during fungal immunity remains unknown.
In this study we found that MARCO contributes to fungal containment during the afferent stage of C. neoformans infection. MARCO-/- mice had impaired cellular recruitment of mononuclear phagocytes and diminished pro-inflammatory cytokine production during cryptococcal infection. MARCO expression enhanced uptake of fungal pathogen by lung macrophages/DCs. Thus, MARCO plays critical roles in modulating afferent anti-cryptococcal defenses.
Materials and Methods
Mice
C57BL/6 mice were obtained from Jackson Laboratories (Bar Harbor, ME). MARCO-/- mice were bred and housed under specific pathogen-free conditions in the Animal Care Facility at the VA Ann Arbor Healthcare System. Mice were aged to 8-10 wk at the time of infection, and were humanely euthanized by CO2 inhalation at the time of data collection. All experiments were approved by the University Committee on the Use and Care of Animals and the Veterans Administration Institutional Animal Care and Use Committee.
C. neoformans
C. neoformans strain 52D and K99 were recovered from 10% glycerol frozen stocks stored at −80°C and grown to stationary phase at 37°C using Sabouraud dextrose broth (1% Neopeptone, 2% dextrose; Difco, Detroit, MI) on a shaker. The cultures were then centrifuged and the pellets were washed with non-pyrogenic saline (Travenol, Deerfield, IL). Cells were counted via hemocytometer, and diluted to 3.3 × 105 yeast/mL in sterile non-pyrogenic saline. All experiments were performed with C. neoformans strain 52D unless otherwise indicated in the text.
Intratracheal inoculation of C. neoformans
Mice were anesthetized via intraperitoneal (i.p.) injection of ketamine (100 mg/kg body weight) plus xylazine (6.8 mg/kg) and were restrained on a foam plate. A small incision was made through the skin covering the trachea. The underlying salivary glands and muscles were separated. A 30-gauge needle was attached to a 1 mL tuberculin syringe with C. neoformans suspension (3.3 × 105 yeast/ml) and infection was performed by intratracheally injecting 30 μL (104 CFU) of inoculum into the lungs. After inoculation, the skin was closed with cyanoacrylate adhesive and the mice were monitored during recovery from the anesthesia.
Lung leukocytes isolation
The lungs from each mouse were excised, washed in RPMI 1640 and digested enzymatically as previously described (26). In brief, lungs were minced with scissors followed by gentle MACS homogenization and incubated at 37°C for 35 min in 5 ml/mouse digestion buffer (RPMI 1640, 5% FBS, penicillin and streptomycin [Invitrogen, Grand Island, NY]; 1 mg/mL collagenase A [Roche Diagnostics, Indianapolis, IN]; and 30 μg/ml DNase I [Sigma, St. Louis, MO]). The cell suspension and tissue fragments were further dispersed by gentle MACS homogenization and were centrifuged. Erythrocytes in the cell pellets were lysed by addition of 3 mL NH4Cl buffer (0.829% NH4Cl, 0.1% KHCO3, and 0.0372% Na2EDTA, pH 7.4) for 3 min followed by a 3-fold excess of RPMI 1640. Cells were re-suspended and subjected to syringe dispersion and filtered through a sterile 100-μm nylon screen (Nitex, Kansas City, MO). The filtrate was centrifuged for 30 min at 1500 g with no brake in the presence of 20% Percoll (Sigma) to separate leukocytes from cell debris and epithelial cells. Leukocyte pellets were re-suspended in complete RPMI 1640 media and enumerated on a hemocytometer after dilution in trypan blue (Sigma).
Lung CFU assay
For determination of fungal burden in the lungs, small aliquots of digested lungs were collected. Series of 10-fold dilutions of the samples were plated on Sabouraud dextrose agar plates in duplicate 10-μl aliquots and incubated at room temperature. C. neoformans colonies were counted two days later and the number of CFUs was calculated on a per-organ basis.
RT-qPCR
Total RNA from lung leukocytes was prepared using TRIzol reagent (Invitrogen), and first-strand cDNA was synthesized using Reverse Transcription Kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions. Relative gene expression was quantified with SYBR green–based detection using an light cycler96 system (Qiagen) according to the manufacturer's protocols. Forty-five cycles of PCR (95°C for 15 s followed by 60°C for 30 s) were performed on a cDNA template. The data were normalized to 18S mRNA, compared with baseline expression in corresponding samples from uninfected mice and expressed as fold induction using the 2-ΔΔCt method.
Abs and flow cytometric analysis
Ab cell staining was performed as previously described (27). Data were collected on a FACS LSR2 flow cytometer using FACSDiva software (Becton Dickinson Immunocytometry Systems, Mountain View, CA) and analyzed using FlowJo software (Tree Star, San Carlos, CA). The following gating strategy was used to identify leukocyte subsets in the lungs: First, consecutive gates identified singlets, live cells and CD45+ leukocytes. Next, a series of selective gates were used to identify lymphocytes (FSClow cells expressing CD3 or CD19); neutrophils (CD11b+Ly6G+); eosinophils (SSChighCD11clow/SiglecF+); Alveolar macrophages (CD11chigh/SiglecF+); monocytes (CD11c-/CD11b+/Ly6Chigh); and DC were then identified within the remaining cell populations as cells expressing both CD11c and high levels of MHCII. DC subsets of moDC, CD11b+ DC and CD103+ DC were identified as follows: moDC were gated as CD11c+MHCII+CD64+ cells, then remaining CD11c+MHCII+CD64- cells were further divided into CD11b+; DC and CD103+ DC based on the expression of SIRPα and XCR1, respectively (28). Total numbers of each cell population were calculated by multiplying the frequency of the population by the total number of leukocytes (the original hemocytometer count of total cells). Isotype control antibodies were used to set gates for positive events in all flow cytometric analyses.
Bone marrow–derived macrophage and DC culture
Bone marrow was isolated as previously described (9). To obtain BMM and BMDC, we cultured bone marrow cells from uninfected MARCO+/+ or MARCO−/− mice in Dulbecco's minimal essential medium (DMEM, from Life Technologies, Inc., Gaithersburg, MD) containing 20% fetal calf serum, GlutaMAX, MEM-nonessential amino acids, sodium pyruvate, penicillin, and streptomycin. 50 ng/mL G-CSF and 20 ng/mL GM-CSF (PeproTech, Rocky Hill, NJ) were added for BMM and BMDC culture, respectively. Cells were harvested after 7 days of culture. For subsequent experiments, BMM/BMDC were removed from differentiation dishes using cold sterile pyrogen-free PBS and subsequently cultured in RPMI 1640 containing 10% fetal calf serum, GlutaMAX, MEM-nonessential amino acids, sodium pyruvate. To evaluate fungicidal activity of BMDC and BMM, 1 × 105 cells were seeded on 96-well plate and infected with C. neoformans yeast (1 ×104) with or without IFN-γ (100 ng/mL). After 24 hrs incubation, the yeast were collected by lysing BMM/BMDC and enumerated by plating onto Sabouraud dextrose agar plates. Colony values in individual treatment groups were compared to culture wells without BMDC/BMM and expressed as percent of growth inhibition.
C. neoformans phagocytosis by lung leukocytes
To determine the phagocytosis of C. neoformans by lung leukocytes, the fungi were first labeled with 1% Uvitex 2B in PBS buffer for 10 min. The fungi were then washed and collected at 1500 rpm for 5 min. Uvitex 2B-labeled C. neoformans were incubated with isolated lung leukocytes from uninfected mice for 2 hrs at 37 °C. Cells were harvested and stained. Flow cytometric analysis was then performed on the samples.
Calculations and statistics
All values are reported as mean ± SEM. Student t test or two-way ANOVA with a Bonferroni post-hoc test were used for comparisons of individual means. Statistical calculations were performed using GraphPad Prism version 6.00 (GraphPad Software, San Diego, CA). Means with p values <0.05 were considered significantly different.
Results
MARCO expression contributes to control of pulmonary fungal growth during afferent phase of the response to C. neoformans infection
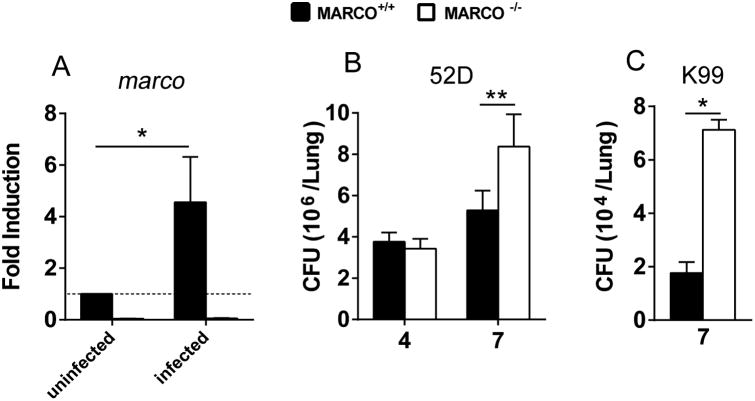
The receptor MARCO contributes to innate response to bacterial and viral pathogens (18, 20, 29) but its role in anti-cryptococcal defenses remains unknown. Our first goal was to determine if MARCO is required for anti-cryptococcal host defenses. MARCO+/+ and MARCO-/- mice were infected intratracheally with 104 CFU C. neoformans 52D and expression of the marco gene was analyzed in leukocytes isolated from uninfected and infected lungs. We found that C. neoformans-infection resulted in significant up regulation of the marco gene in pulmonary leukocytes relative to that in uninfected controls as early as 4 dpi (Fig. 1A). As expected, no transcription of the marco gene was detected in the MARCO-/- mice (Fig. 1A). Next, we evaluated the effect of MARCO deletion on the pulmonary control of C. neoformans growth. MARCO deletion did not affect the pulmonary fungal growth at 4 dpi in the infected mice. However, a significant increase in fungal load was noted at 7 dpi in the lungs of MARCO-/- mice compared to infected MARCO+/+ mice (Fig. 1B). Interestingly, we did not observe significantly higher fungal burden in MARCO-/- mice at 21 dpi (not shown), suggesting that the effects of MARCO are most important during the afferent phase of host defense against cryptococcosis (30-32). Finally, to determine if the beneficial effect of MARCO receptor was applicable to other serotype strains of C. neoformans, we analyzed fungal burdens in mice infected with serotype A strain K99. We found similarly impaired fungal control in MARCO deficient mice infected with strain K99 at 7 dpi (Fig. 1C). Thus, expression of MARCO contributes to anti-cryptococcal defenses during the afferent phase of the immune response.
Fig. 1. MARCO expression contributes to control of pulmonary C. neoformans growth during afferent phase of the immune response.
MARCO+/+ and MARCO-/- mice were infected intratracheally with 104 C. neoformans 52D (A, B) or C. neoformans K99 (C). Expression of MARCO mRNA and pulmonary fungal burdens were quantified. MARCO gene expression was significantly up-regulated in lung leukocytes of C. neoformans infected mice relative to uninfected control at 4 dpi (A). MARCO deficiency significantly impaired pulmonary fungal control in mice infected with C. neoformans strains 52D (B) and K99 (C) at 7 dpi. Results represent mean ± SEM from one of at least two separate matched experiments (n = 5 mice for each time point). * p < 0.05, ** p < 0.01.
MARCO expression contributes to the early accumulation of monocytes in C. neoformans infected lungs
Having determined that MARCO expression contributed to early anti-cryptococcal defenses, we explored whether MARCO had a role in early inflammatory responses to C. neoformans infection. We quantified leukocyte populations in the lungs of MARCO+/+ and MARCO-/- mice using flow cytometric analysis at 4 and 7 dpi. Both MARCO+/+ and MARCO-/- infected mice progressively accumulated CD45+ leukocytes at both time points in similar numbers (Fig. 2A), indicating that MARCO had no effect on the overall magnitude of the inflammatory responses during early inflammation. Total numbers of individual leukocyte subsets including lung alveolar macrophages (CD11c+SiglecF+, Fig. 2B), eosinophils (CD11clowSiglecF+, Fig. 2C) and neutrophils (CD11b+Ly6G+, Fig. 2D) were similar between infected MARCO+/+ and MARCO−/− mice at both 4 and 7 dpi. However, early accumulation of monocytes (CD11b+Ly6Chigh) was significantly reduced at 4 dpi in MARCO−/− mice compared with MARCO+/+ mice (Fig. 2E). MARCO−/− mice also showed diminishing trend in accumulation of monocytes at 7 dpi (p = 0.08) and DC at 4 dpi (p = 0.07) (Fig. 2F). Thus, MARCO expression contributes to early pulmonary recruitment of innate mononuclear cells during afferent phase of response to C. neoformans infection.
Fig. 2. MARCO expression promotes afferent phase pulmonary monocytes accumulation during C. neoformans infection.
Lung leukocytes from infected MARCO+/+ and MARCO-/- mice were isolated and analyzed by flow cytometry as per Material and Methods. Total numbers of leukocytes (A), macrophage (B), eosinophil (C), neutrophil (D), monocytes (E) and DC (F) are shown. Dashed lines represent initial levels in uninfected mice. Note MARCO-/- mice exhibited less monocytes and DC relative to WT control mice. Results represent mean ± SEM (n = 5 mice for each time point). * p < 0.05 in comparison between MARCO+/+ and MARCO-/- mice.
MARCO expression promotes pulmonary accumulation of monocyte-derived DC but not conventional DC during the afferent response to C. neoformans
We further explored whether MARCO affected the accumulation of specific lung conventional DC subsets and inflammatory moDC known to be involved in clearance of fungal pathogens (8, 11). Using defined gating strategies, we separately analyzed moDC (CD11c+MHCII+CD64+) and two conventional DC (cDC) subsets, CD11b+ cDC (CD11c+MHCII+CD64-SIRPα+) and CD103+ cDC (CD11c+MHCII+CD64-XCR1+) (28, 33). We found that C. neoformans infection triggered significant accumulation of both moDC and CD11b+ cDC, but not CD103+ cDC in the infected lungs (Fig. 3). However, MARCO expression selectively enhanced accumulation of pulmonary moDC, without affecting pulmonary CD11b+ cDC and CD103+ cDC populations during cryptococcal infection (Fig. 3A-C). This conclusion was demonstrated by greater frequencies and total numbers of moDC at 4 and 7dpi (Fig. 3A and 3C) in infected MARCO+/+ mice, but no difference in frequencies and numbers of either CD11b+ cDC or CD103+ cDC between MARCO+/+ and MARCO-/- mice at these time points (Fig 3B and 3C). Taken together, the data demonstrate that MARCO selectively enhances accumulation of moDC, but not CD11b+ cDC or CD103+ cDC, in the lungs during afferent phase of C. neoformans infection.
Fig. 3. MARCO expression enhances pulmonary recruitment of moDC throughout the afferent immune response to C. neoformans infection.
Lung leukocytes were isolated from infected MARCO+/+ and MARCO-/- mice. (A) Frequency of moDC (CD11c+MHCII+CD64+) was significantly lower in MARCO-/- mice than MARCO+/+ mice. (B) MARCO has no effect on the frequencies of CD11b+ cDC (CD11c+MHCII+CD64-SIRPα+) and CD103+ cDC (CD11c+MHCII+CD64-XCR1+). (C) MARCO-/- mice had significantly lower total number of moDC but not CD11b+cDC and CD103+ cDC in the infected lungs relative to WT control. Results represent mean ± SEM (n = 5 mice for each time point). * p<0.05 in comparison between MARCO+/+ and MARCO-/- mice.
MARCO expression promotes early induction of chemokines responsible for monocyte recruitment in the C. neoformans-infected lungs
Since monocyte and moDC accumulation depends on CCR2 signaling (7, 14), we next tested whether MARCO expression promoted pulmonary expression of CCR2 ligands. We analyzed the mRNA expression of CCR2-axis chemokines ccl2, ccl7 and ccl12 by the pulmonary leukocytes in infected mice. The mRNA expression of all three chemokines was significantly up regulated post-C. neoformans infection (Fig. 4 A-4C) as previously reported (14). However, MARCO deficiency substantially impaired the expression of ccl2 and ccl7, but not ccl12 in lung leukocytes (Fig. 4A-4C). Consistent with suppressed mRNA expression, CCL2 protein production in the infected lungs was reduced in MARCO-/- mice compared to MARCO+/+ mice at 7 dpi (Fig. 4D). However, MARCO deficiency had no effect on ccl2/ccl7 expression by bone marrow-derived macrophages (BMM) and DCs (BMDC) stimulated directly with C. neoformans in vitro (not shown). While these cells typically represent monocyte-derived, nonresident mononuclear cells, these results suggest either that MARCO enhanced chemokine production by lung resident cells, or that its effect on CCR2 ligands expression by recruited cells in the infected lungs was indirect. Together, we found that MARCO expression contributed to induction of two major CCR2 chemokines ligands in the lungs, which could explain its role in pulmonary accumulation of monocytes and moDC in C. neoformans-infected lungs.
Fig. 4. MARCO promotes the expression of ccl2 and ccl7 but not ccl12 chemokines in C. neoformans infected lungs.
Total RNA of lung leukocytes from MARCO-/- and MARCO+/+ mice were isolated. Gene expression of ccl2 (A), ccl7 (B) and ccl12 (C) at 4 and 7 dpi was evaluated by RT-qPCR. Note that the expressions of ccl2 and ccl7 in pulmonary leukocytes were significantly reduced by MARCO deletion. (D) Lungs of MARCO+/+ and MARCO-/- mice were homogenized and CCL2 protein level was evaluated by ELISA at 7 dpi. Dotted line shows CCL2 level in uninfected lungs. Note that MARCO deletion resulted in reduced CCL2 protein production in C. neoformans infected lungs, mirroring CCL2 gene expression data. Results represent mean ± SEM (n = 5 mice for each time point). *p < 0.05, ** p<0.01 compared with control mice;
MARCO expression promotes early induction of major protective cytokines in C. neoformans-infected lungs
Robust early expression of pro-inflammatory cytokines, such as IFNγ, is important for the innate control of C. neoformans growth in the infected lungs (13, 34). Thus, we further compared the cytokine production by leukocytes obtained from the infected lungs of MARCO+/+ and MARCO-/- mice. Gene expression of beneficial (IFN-γ, IL-12b and IL-17A) and detrimental (IL-4, IL-5 and IL-13) cytokines by pulmonary leukocytes was evaluated by RT-qPCR (5, 35, 36). Consistent with better fungal control, up-regulation of protective cytokines IFN-γ, IL-12b and IL-17A was significantly greater in infected MARCO+/+ compared to MARCO-/- mice at 4 or 7 dpi (Fig. 5A-5C). However, the early expression of non-protective cytokines was not affected by MARCO deletion (Fig. 5D-5E). Thus, MARCO is required for the optimal expression of protective cytokines during the afferent anti-cryptococcal responses.
Fig. 5. MARCO promotes early protective cytokine expression in leukocytes from C. neoformans infected lungs.
Gene expression of protective cytokines IFN-γ (A), IL-12b (B) and IL-17A (C) as well as non-protective cytokines IL-4 (D), IL-5 (E) and IL-13 (F) by pulmonary leukocytes at 4 and 7 dpi was evaluated by RT-qPCR. Note that the expression of protective cytokines was significantly lower in infected MARCO-/- mice than WT mice at 4 or 7 dpi. Results represent mean ± SEM (n = 5 mice for each time point). *p < 0.05 compared with control mice.
MARCO expression promotes moDC classical activation during the afferent response to C. neoformans infection
The environmental cytokines influence the classical versus alternative activation of effector phagocytic cells, which in turn determines their fungicidal capacity and the outcome of cryptococcal infection (1, 37). Thus, we evaluated whether MARCO participates in the classical versus alternative activation of macrophage and DC. We found that MARCO deletion resulted in diminished numbers of CD80hi moDC (Fig. 6A, 6B) and increased frequency of CD206hi moDC (Fig. 6C, 6D), suggesting that MARCO expression contributed to more classical activation profile of moDC. Interestingly, MARCO deletion had no effect on the activation of alveolar macrophage, CD11b+cDC and CD103+cDC (not shown), consistent with MARCO being less important for the activation status of these resident subsets.
Fig. 6. MARCO expression modulates moDC DC1/DC2 activation profile during the afferent immune response to C. neoformans infection.
Lung leukocytes isolated from infected MARCO+/+ and MARCO-/- mice were analyzed using flow cytometry as per Material and Methods. Frequency and total number of moDC that express CD80 (A and B) and CD206 (C and D) at 7 dpi were assessed. Note the total number of moDC expressing CD80 and the frequency of moDC expressing CD206 were affected by MARCO expression. Gene expression of iNOS (E) and Arg1 (F) by pulmonary leukocytes at 4 and 7 dpi was evaluated by RT-qPCR. Note that the expression of iNOS was affected by MARCO deletion, but the expression of Arg1 were similar between MARCO+/+ and MARCO-/- groups. Results represent mean ± SEM (n = 5 mice for each time point). * p < 0.05 in comparison between MARCO+/+ and MARCO-/- mice.
Highly induced expression of iNOS is a hallmark of classically activated effector phagocytes, while alternative activation is marked by expression of Arg1 (37-39). To further explore the role of MARCO in expression of classical versus alternative activation genes, we analyzed mRNA expressions of iNOS and Arg1 in pulmonary leukocytes by RT-qPCR. We found that MARCO deletion caused a significant reduction in iNOS expression by pulmonary leukocytes at 4 and 7 dpi (Fig. 6E). However, the expression of Arg1 was similar in infected MARCO-/- and MARCO+/+ mice (Fig. 6F). Taken together, our data suggests that MARCO expression contributes to improved classical activation of mononuclear phagocytes during cryptococcal infection.
MARCO expression was not directly involved in the fungicidal activity of BMM and BMDC
To evaluate whether MARCO-expression contributed to fungicidal capacity of inflammatory effector cells, we examined the microbial growth inhibition by BMM and BMDC. BMM/BMDC from MARCO+/+ and MARCO-/- mice were cultured with C. neoformans in the presence or absence of IFN-γ stimulation. Absence of MARCO expression had no direct effect on the in vitro fungicidal ability of BMM and BMDC, demonstrating that MARCO deletion did not result in an intrinsic killing defect in macrophages or DCs (Fig. 7). In contrast, we found that cell-extrinsic factors such as IFN-γ whose expression in the lungs was promoted by MARCO facilitated the killing activity of BMM and BMDC in vitro (Fig. 7). These results strongly suggest that MARCO expression affected fungicidal function of pulmonary macrophages and DCs indirectly, via modulation of cytokine environment, rather than by intrinsically modulating their fungicidal machinery.
Fig. 7. MARCO has no direct role in fungicidal activity of BMDC and BMM.
Fungicidal activities of inflammatory DC (A) and macrophages (B) were evaluated in cells derived from bone marrow of MARCO+/+ and MARCO-/- mice. C. neoformans growth inhibition was evaluated in 24h co-incubation assay in the presence or absence of IFN-γ (100 ng/mL). Note that fungicidal potential of BMDC and BMM was not affected by MARCO expression and was equally enhanced by IFN-γ stimulation. Results represent mean ± SEM from three separate matched experiments. * p < 0.05, NS, no significant difference between MARCO+/+ and MARCO-/- mice.
MARCO expression contributes to the interaction between C. neoformans and mononuclear phagocytes
Lastly, to better understand how MARCO contributed to the induction of early protective events during immune response to C. neoformans infection, we explored the roles of MARCO in binding/uptake of C. neoformans by pulmonary phagocytes. To test this, total lung leukocytes from uninfected MARCO+/+ and MARCO-/- mice were harvested and co-cultured with Uvitex 2B-labelled C. neoformans. Cell association between distinct leukocyte subsets and C. neoformans was determined by flow cytometry. We found that macrophages and DC from MARCO-/- mice showed decreased interaction with Uvitex 2B-labeled C. neoformans compared to cells from MARCO+/+ mice, indicating that MARCO is required for optimal phagocytosis of C. neoformans by mononuclear phagocyte subsets (Fig. 8). In contrast, MARCO had no effect on the uptake of C. neoformans by neutrophils and eosinophils (not shown). Thus MARCO is directly involved in the interaction between C. neoformans and mononuclear phagocytes.
Fig. 8. MARCO selectively promotes phagocytosis of C. neoformans by lung resident mononuclear phagocytes.
Lung leukocytes were isolated from uninfected MARCO+/+ or MARCO-/- mice as per Materials and Methods. (A) Lung leukocytes were co-cultured with Uvitex 2B-labeled C. neoformans. Cell association between different cell subsets and C. neoformans were measured by flow cytometry. (B) Bar graphs represent the percentage of cells associating with C. neoformans. Note that alveolar macrophage and DC from MARCO+/+ can interact with C. neoformans more efficiently than those from MARCO-/- mice. Results represent mean ± SEM from one of three matched experiments. *p < 0.05, ** p < 0.01, ***p < 0.001
Discussion
Because many patients with cryptococcosis have impaired T cell defenses, an improved understanding of factors that enhance innate immune defenses against C. neoformans may yield novel immunotherapies to supplement the standard antifungal treatment in these patients. In this study, we present novel evidence that MARCO, a class A scavenger receptor, exerts protective anti-cryptococcal effects that contribute to fungal control during afferent phase of response to C. neoformans infection. Specifically, we demonstrate that 1) MARCO contributes to early control of fungal growth in the lungs at 7 dpi; 2) MARCO expression facilitates the cellular recruitment and activation of monocytes/moDC; 3) MARCO enhances the early expression of chemokines and cytokines, including CCL2, CCL7 and IFN-γ, which affect the influx and activation of monocytes/moDC during C. neoformans infection; 4) MARCO promotes phagocytosis of C. neoformans by pulmonary macrophages and DC. Collectively, we demonstrate that MARCO is a critical factor that promotes the protective host defenses during the afferent phase of response to cryptococcal infection.
MARCO has been implicated in the development of protective immune response to viral, bacterial and parasitic infections (18, 20, 21, 40), however its role in host control of fungal pathogens, including C. neoformans, was previously unknown. Using an established murine cryptococcosis model we demonstrate that MARCO deletion impairs early fungal control in mice infected with C. neoformans strain 52D at the peak of afferent phase of the host immune response (Fig. 1B). These results were reproduced in infection with serotype A strain K99 (Fig. 1C), demonstrating that MARCO expression is required for the fungal containment of C. neoformans strains of serotype A and D. Thus, these results extend prior studies on viruses and bacteria and identify MARCO as an important component for the early defense against invasive fungal infections.
Our findings that fungal burden in MARCO-/- mice was not different at 4 dpi but increased at 7 dpi in comparison with MARCO+/+ mice suggested that MARCO expression promoted defenses mediated by recruited leukocytes rather than resident immune cells. Using a recently defined gating strategy (28, 33), we found that MARCO expression specifically promoted accumulation of monocytes and moDC, which have become increasingly recognized as microbicidal effectors cells during fungal infections including invasive aspergillos is and cryptococcosis (6-8, 41-43). In support of our study, a recent report showed that MARCO-/- mice had impaired cellular recruitment of monocytes in Streptococcus pneumoniae infection (19). Together with our findings, these results demonstrate a conserved role of MARCO in promoting monocyte and moDC recruitment to the sites of infection during bacterial and fungal infections.
We further showed that MARCO expression is required for the induction of two major CCR2-axis chemokines, CCL2 and CCL7, in the infected lungs, providing a mechanistic link between MARCO expression and improved monocyte/moDC recruitment. While we have not identified the cellular sources of CCL2 and CCL7 in the infected lungs, our data does not favor that MARCO expression directly increases the production of CCL2 and CCL7 by monocyte-derived inflammatory macrophages and DCs. The enhanced expression of CCR2 ligands during C. neoformans infection could be attributed to a) MARCO directly enhancing production of these chemokines by lung resident cells other than recruited monocyte-derived cells; or b) chemokine expression being indirectly affected by other changes in the inflammatory mediator network downstream of MARCO.
In addition to promoting monocyte and moDC accumulation, MARCO expression also contributes to their classical activation in a manner consistent with enhanced fungicidal activity. Specifically, we observed that MARCO was associated with increased expression of CD80 and iNOS, and decreased expression of CD206. We believe at least two mechanisms could explain the enhanced classical activation profile observed in MARCO-expressing mice. First, recruited monocyte-derived phagocytes show greater propensity for classical activation (expression of iNOS) along with improved killing ability superior to that of resident alveolar macrophages (10). Second, robust CCL7 expression in C. neoformans-infected lungs is also linked to enhanced early IFN-γ induction, which in turn is the major factor that promotes classical activation (14, 37). The effect of MARCO favoring induction of cell-extrinsic factors such as IFN-γ rather than directly activating fungicidal machinery in the effector myeloid cells is supported by the fact that MARCO deletion did not affect fungicidal potential of macrophages and DCs in vitro (Fig. 7). Taken together, our findings favor the notion that the protective effect of MARCO is primarily indirect, acting via improved recruitment of monocytes/moDC and their subsequent activation by improved induction of protective cytokines within the pulmonary microenvironment during C. neoformans infection (Fig. 9). However, addressing the effect of MARCO on cytokine production by individual cell subsets is still needed in future studies to fully understand the exact mechanism by which MARCO modulates the early inflammatory network during innate antifungal defenses in the lungs.
Fig. 9. Model of MARCO orchestrating innate anti-fungal immunity.
MARCO promotes the expression of CCL2 and CCL7 which improves the CCR2-mediated recruitment of monocytes and moDC. MARCO expression also improves early induction of protective cytokines which promote stronger classical activation of recruited mononuclear effector cells. Together these effects support more efficient killing of C. neoformans in the infected lungs.
When it comes to understanding roles of different scavenger receptors, it is interesting that MARCO and SR-A, which both belong to the class A scavenger receptor family and possess very similar structures, have very different effects on fungal control during cryptococcal infection. In this study, we show that MARCO enhances early production of pro-inflammatory cytokines by lung leukocytes. In contrast, we and others have previously demonstrated that SR-A impairs fungal clearance through mechanisms involving dampened production of the pro-inflammatory cytokines (IFN-γ, TNF-α and IL-12) and increased alternative activation of mononuclear cells during C. neoformans and Pneumocystis carinii infection (27, 44). Furthermore, MARCO expression facilitates phagocytosis of C. neoformans by lung macrophages and DCs, while SR-A appears to have no effect on the uptake of C. neoformans by phagocytic cells (27). Thus, in contrast to SR-A which suppresses host defenses against fungal pathogens, MARCO enhances critical early effector pathways in response to cryptococcal lung infection.
The impact of MARCO on the early, afferent response to cryptococcosis was unique relative to the effects conferred by other innate receptors and signaling pathways studied in this model system. Here we observed that MARCO enhanced immune effector mechanisms and promoted fungal clearance at the times post-infection previously shown to be the peak of the afferent response to cryptococcal infection (30, 31). This immune-modulatory effect of MARCO contrasts with previously reported effects of other factors that mostly modulate the adaptive immune responses without affecting the afferent control of fungal growth during cryptococcal infection. For example, TLR9 signaling and early induction of IL-12 and TNF-α, have no impact on fungal clearance during the afferent phase, but rather promote the development of protective Th1 immunity, M1 polarization and enhance fungal clearance during the efferent phase of C. neoformans infection (14, 15, 45, 46). This unique effect of MARCO on early defenses suggests that MARCO and/or downstream signaling pathways might be targeted for the development of immunotherapies to treat cryptococcosis in patients with severe T cell deficiencies. Such therapeutic strategies could help to improve fungal containment in these patients either prior to the immune reconstitution, or in situations where immune reconstitution is not attainable or inadvisable.
In summary, this study has identified that MARCO modulates inflammatory responses against C. neoformans infection and contributes to fungal containment during the afferent stage of cryptococcal infection. These effects are associated with improved pulmonary monocyte recruitment, increased inflammatory cytokine production and classical myeloid cell activation. Our study unlocks the potential for manipulation of MARCO signaling to support the treatment of fungal diseases.
Acknowledgments
The authors wish to acknowledge technical assistance of Guolei Zhao, Xueli Gao, Enze Xing, Jay Akolkar and Michael Ivey.
Michal A. Olszewski, John J. Osterholzer and Jeffrey L. Curtis were supported by VA Merit grants (1I01BX000656, BX002120-01 and I01CX000911, respectively). Bethany B. Moore was supported by NIH grant AI117229, HL127805 and HL119682. Lori M. Neal was supported by T-32 research training grant T32HL07749. Alison J Eastman was supported by T-32 training grant T-32 AI007413.
References
- 1.Olszewski MA, Zhang Y, Huffnagle GB. Mechanisms of cryptococcal virulence and persistence. Future Microbiol. 2010;5:1269–1288. doi: 10.2217/fmb.10.93. [DOI] [PubMed] [Google Scholar]
- 2.Rohatgi S, Pirofski LA. Host immunity to Cryptococcus neoformans. Future Microbiol. 2015;10:565–581. doi: 10.2217/fmb.14.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gibson JF, Johnston SA. Immunity to Cryptococcus neoformans and C. gattii during cryptococcosis. Fungal Genet Biol. 2015;78:76–86. doi: 10.1016/j.fgb.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wager CML, Wormley FL., Jr Is Development of a Vaccine against Cryptococcus neoformans Feasible? PLoS Pathog. 2015;11:e1004843. doi: 10.1371/journal.ppat.1004843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hole C, Wormley FL., Jr Innate host defenses against Cryptococcus neoformans. Journal of Microbiology. 2016;54:202–211. doi: 10.1007/s12275-016-5625-7. [DOI] [PubMed] [Google Scholar]
- 6.Osterholzer JJ, Chen GH, Olszewski MA, Curtis JL, Huffnagle GB, Toews GB. Accumulation of CD11b+ lung dendritic cells in response to fungal infection results from the CCR2-mediated recruitment and differentiation of Ly-6Chigh monocytes. J Immunol. 2009;183:8044–8053. doi: 10.4049/jimmunol.0902823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Osterholzer JJ, Curtis JL, Polak T, Ames T, Chen GH, McDonald R, Huffnagle GB, Toews GB. CCR2 mediates conventional dendritic cell recruitment and the formation of bronchovascular mononuclear cell infiltrates in the lungs of mice infected with Cryptococcus neoformans. J Immunol. 2008;181:610–620. doi: 10.4049/jimmunol.181.1.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Espinosa V, Jhingran A, Dutta O, Kasahara S, Donnelly R, Du P, Rosenfeld J, Leiner I, Chen CC, Ron Y. Inflammatory monocytes orchestrate innate antifungal immunity in the lung. PLoS Pathog. 2014;10:e1003940. doi: 10.1371/journal.ppat.1003940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arora S, Olszewski MA, Tsang TM, McDonald RA, Toews GB, Huffnagle GB. Effect of cytokine interplay on macrophage polarization during chronic pulmonary infection with Cryptococcus neoformans. Infect Immun. 2011;79:1915–1926. doi: 10.1128/IAI.01270-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Osterholzer JJ, Chen GH, Olszewski MA, Zhang YM, Curtis JL, Huffnagle GB, Toews GB. Chemokine receptor 2-mediated accumulation of fungicidal exudate macrophages in mice that clear cryptococcal lung infection. Am J Pathol. 2011;178:198–211. doi: 10.1016/j.ajpath.2010.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eastman AJ, Osterholzer JJ, Olszewski MA. Role of dendritic cell-pathogen interactions in the immune response to pulmonary cryptococcal infection. Future Microbiol. 2015;10:1837–1857. doi: 10.2217/fmb.15.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamamoto H, Nakamura Y, Sato K, Takahashi Y, Nomura T, Miyasaka T, Ishii K, Hara H, Yamamoto N, Kanno E, Iwakura Y, Kawakami K. Defect of CARD9 leads to impaired accumulation of gamma interferon-producing memory phenotype T cells in lungs and increased susceptibility to pulmonary infection with Cryptococcus neoformans. Infect Immun. 2014;82:1606–1615. doi: 10.1128/IAI.01089-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wormley FL, Jr, Perfect JR, Steele C, Cox GM. Protection against cryptococcosis by using a murine gamma interferon-producing Cryptococcus neoformans strain. Infect Immun. 2007;75:1453–1462. doi: 10.1128/IAI.00274-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qiu Y, Zeltzer S, Zhang Y, Wang F, Chen GH, Dayrit J, Murdock BJ, Bhan U, Toews GB, Osterholzer JJ, Standiford TJ, Olszewski MA. Early induction of CCL7 downstream of TLR9 signaling promotes the development of robust immunity to cryptococcal infection. J Immunol. 2012;188:3940–3948. doi: 10.4049/jimmunol.1103053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Y, Wang F, Bhan U, Huffnagle GB, Toews GB, Standiford TJ, Olszewski MA. TLR9 signaling is required for generation of the adaptive immune protection in Cryptococcus neoformans-infected lungs. Am J Pathol. 2010;177:754–765. doi: 10.2353/ajpath.2010.091104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dan JM, Kelly RM, Lee CK, Levitz SM. Role of the mannose receptor in a murine model of Cryptococcus neoformans infection. Infect immun. 2008;76:2362–2367. doi: 10.1128/IAI.00095-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Areschoug T, Gordon S. Scavenger receptors: role in innate immunity and microbial pathogenesis. Cellular microbiology. 2009;11:1160–1169. doi: 10.1111/j.1462-5822.2009.01326.x. [DOI] [PubMed] [Google Scholar]
- 18.Bowdish DM, Sakamoto K, Kim MJ, Kroos M, Mukhopadhyay S, Leifer CA, Tryggvason K, Gordon S, Russell DG. MARCO, TLR2, and CD14 are required for macrophage cytokine responses to mycobacterial trehalose dimycolate and Mycobacterium tuberculosis. PLoS Pathog. 2009;5:e1000474. doi: 10.1371/journal.ppat.1000474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dorrington MG, Roche AM, Chauvin SE, Tu Z, Mossman KL, Weiser JN, Bowdish DM. MARCO is required for TLR2-and Nod2-mediated responses to Streptococcus pneumoniae and clearance of pneumococcal colonization in the murine nasopharynx. J Immunol. 2013;190:250–258. doi: 10.4049/jimmunol.1202113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arredouani M, Yang Z, Ning Y, Qin G, Soininen R, Tryggvason K, Kobzik L. The scavenger receptor MARCO is required for lung defense against pneumococcal pneumonia and inhaled particles. J Exp Med. 2004;200:267–272. doi: 10.1084/jem.20040731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gomes IN, Palma LC, Campos GO, Lima JGB, De Almeida TF, De Menezes JP, Ferreira CAG, Santos RD, Buck GA, Manque PA. The scavenger receptor MARCO is involved in Leishmania major infection by CBA/J macrophages. Parasite immunology. 2009;31:188–198. doi: 10.1111/j.1365-3024.2009.01093.x. [DOI] [PubMed] [Google Scholar]
- 22.Gren ST, Rasmussen TB, Janciauskiene S, Håkansson K, Gerwien JG, Grip O. A single-cell gene-expression profile reveals inter-cellular heterogeneity within human monocyte subsets. PLoS One. 2015;10:e0144351. doi: 10.1371/journal.pone.0144351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Getts DR, Terry RL, Getts MT, Deffrasnes C, Müller M, van Vreden C, Ashhurst TM, Chami B, McCarthy D, Wu H. Therapeutic inflammatory monocyte modulation using immune-modifying microparticles. Science translational medicine. 2014;6:217–219. doi: 10.1126/scitranslmed.3007563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Józefowski S, Yang Z, Marcinkiewicz J, Kobzik L. Scavenger receptors and β-glucan receptors participate in the recognition of yeasts by murine macrophages. Inflammation Research. 2012;61:113–126. doi: 10.1007/s00011-011-0395-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bin LH, Nielson LD, Liu X, Mason RJ, Shu HB. Identification of uteroglobin-related protein 1 and macrophage scavenger receptor with collagenous structure as a lung-specific ligand-receptor pair. J Immunol. 2003;171:924–930. doi: 10.4049/jimmunol.171.2.924. [DOI] [PubMed] [Google Scholar]
- 26.Olszewski MA, Huffnagle GB, McDonald RA, Lindell DM, Moore BB, Cook DN, Toews GB. The role of macrophage inflammatory protein-1 alpha/CCL3 in regulation of T cell-mediated immunity to Cryptococcus neoformans infection. J Immunol. 2000;165:6429–6436. doi: 10.4049/jimmunol.165.11.6429. [DOI] [PubMed] [Google Scholar]
- 27.Qiu Y, Dayrit JK, Davis MJ, Carolan JF, Osterholzer JJ, Curtis JL, Olszewski MA. Scavenger receptor A modulates the immune response to pulmonary Cryptococcus neoformans infection. J Immunol. 2013;191:238–248. doi: 10.4049/jimmunol.1203435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guilliams M, Lambrecht B, Hammad H. Division of labor between lung dendritic cells and macrophages in the defense against pulmonary infections. Mucosal Immunol. 2013;6:464–473. doi: 10.1038/mi.2013.14. [DOI] [PubMed] [Google Scholar]
- 29.Canton J, Neculai D, Grinstein S. Scavenger receptors in homeostasis and immunity. Nat Rev Immunol. 2013;13:621–634. doi: 10.1038/nri3515. [DOI] [PubMed] [Google Scholar]
- 30.Huffnagle GB, Chen GH, Curtis JL, McDonald RA, Strieter RM, Toews GB. Down-regulation of the afferent phase of T cell-mediated pulmonary inflammation and immunity by a high melanin-producing strain of Cryptococcus neoformans. J Immunol. 1995;155:3507–3516. [PubMed] [Google Scholar]
- 31.Lipscomb MF, Huffnagle GB, Lovchik JA, Lyons CR, Pollard AM, Yates JL. The role of T lymphocytes in pulmonary microbial defense mechanisms. Arch Pathol Lab Med. 1993;117:1225–1232. [PubMed] [Google Scholar]
- 32.Xu J, Eastman AJ, Flaczyk A, Neal LM, Zhao G, Carolan J, Malachowski AN, Stolberg VR, Yosri M, Chensue SW. Disruption of Early Tumor Necrosis Factor Alpha Signaling Prevents Classical Activation of Dendritic Cells in Lung-Associated Lymph Nodes and Development of Protective Immunity against Cryptococcal Infection. MBio. 2016;7:e00510–00516. doi: 10.1128/mBio.00510-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Plantinga M, Guilliams M, Vanheerswynghels M, Deswarte K, Branco-Madeira F, Toussaint W, Vanhoutte L, Neyt K, Killeen N, Malissen B. Conventional and monocyte-derived CD11b+ dendritic cells initiate and maintain T helper 2 cell-mediated immunity to house dust mite allergen. Immunity. 2013;38:322–335. doi: 10.1016/j.immuni.2012.10.016. [DOI] [PubMed] [Google Scholar]
- 34.Wager CL, Wormley F. Classical versus alternative macrophage activation: the Ying and the Yang in host defense against pulmonary fungal infections. Mucosal Immunol. 2014;7:1023–1035. doi: 10.1038/mi.2014.65. [DOI] [PubMed] [Google Scholar]
- 35.Chen GH, McDonald RA, Wells JC, Huffnagle GB, Lukacs NW, Toews GB. The gamma interferon receptor is required for the protective pulmonary inflammatory response to Cryptococcus neoformans. Infect Immun. 2005;73:1788–1796. doi: 10.1128/IAI.73.3.1788-1796.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Decken K, Köhler G, Palmer-Lehmann K, Wunderlin A, Mattner F, Magram J, Gately MK, Alber G. Interleukin-12 is essential for a protective Th1 response in mice infected with Cryptococcus neoformans. Infect Immun. 1998;66:4994–5000. doi: 10.1128/iai.66.10.4994-5000.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Davis MJ, Tsang TM, Qiu Y, Dayrit JK, Freij JB, Huffnagle GB, Olszewski MA. Macrophage M1/M2 polarization dynamically adapts to changes in cytokine microenvironments in Cryptococcus neoformans infection. MBio. 2013;4:e00264–00213. doi: 10.1128/mBio.00264-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Italiani P, Boraschi D. From monocytes to M1/M2 macrophages: phenotypical vs. functional differentiation. Front Immunol. 2015:47. doi: 10.3389/fimmu.2014.00514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jablonski KA, Amici SA, Webb LM, de Dios Ruiz-Rosado J, Popovich PG, Partida-Sanchez S, Guerau-de-Arellano M. Novel markers to delineate murine M1 and M2 macrophages. PLoS One. 2015;10:e0145342. doi: 10.1371/journal.pone.0145342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Plüddemann A, Mukhopadhyay S, Sankala M, Savino S, Pizza M, Rappuoli R, Tryggvason K, Gordon S. SR-A, MARCO and TLRs differentially recognise selected surface proteins from Neisseria meningitidis: an example of fine specificity in microbial ligand recognition by innate immune receptors. Journal of innate immunity. 2008;1:153–163. doi: 10.1159/000155227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Traynor TR, Kuziel WA, Toews GB, Huffnagle GB. CCR2 expression determines T1 versus T2 polarization during pulmonary Cryptococcus neoformans infection. J Immunol. 2000;164:2021–2027. doi: 10.4049/jimmunol.164.4.2021. [DOI] [PubMed] [Google Scholar]
- 42.Traynor TR, Herring AC, Dorf ME, Kuziel WA, Toews GB, Huffnagle GB. Differential roles of CC chemokine ligand 2/monocyte chemotactic protein-1 and CCR2 in the development of T1 immunity. J Immunol. 2002;168:4659–4666. doi: 10.4049/jimmunol.168.9.4659. [DOI] [PubMed] [Google Scholar]
- 43.Serbina NV, Jia T, Hohl TM, Pamer EG. Monocyte-mediated defense against microbial pathogens. Annu Rev Immunol. 2008;26:421–452. doi: 10.1146/annurev.immunol.26.021607.090326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hollifield M, Ghanem EB, de Villiers WJ, Garvy BA. Scavenger receptor A dampens induction of inflammation in response to the fungal pathogen Pneumocystis carinii. Infect immun. 2007;75:3999–4005. doi: 10.1128/IAI.00393-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huffnagle GB, Toews GB, Burdick MD, Boyd MB, McAllister KS, McDonald RA, Kunkel SL, Strieter RM. Afferent phase production of TNF-alpha is required for the development of protective T cell immunity to Cryptococcus neoformans. J Immunol. 1996;157:4529–4536. [PubMed] [Google Scholar]
- 46.Hoag KA, Lipscomb MF, Izzo AA, Street NE. IL-12 and IFN-gamma are required for initiating the protective Th1 response to pulmonary cryptococcosis in resistant C.B-17 mice. Am J Respir Cell Mol Biol. 1997;17:733–739. doi: 10.1165/ajrcmb.17.6.2879. [DOI] [PubMed] [Google Scholar]