Abstract
Rotator cuff (RC) tendon tears lead to negative structural and functional changes in the associated musculature. The structural features of muscle that predict function are termed “muscle architecture”. Although the architectural features of “normal” rotator cuff muscles are known, they are poorly understood in the context of cuff pathology. The purpose of this study was to investigate the effects of tear and repair on RC muscle architecture. To this end thirty cadaveric shoulders were grouped into one of four categories based on tear magnitude: Intact, Full-thickness tear (FTT), Massive tear (MT), or Intervention if sutures or hardware were present, and key parameters of muscle architecture were measured. We found that muscle mass and fiber length decreased proportionally with tear size, with significant differences between all groups. Conversely, sarcomere number was reduced in both FTT and MT with no significant difference between these two groups, in large part because sarcomere length was significantly reduced in MT but not FTT. The loss of muscle mass in FTT is due, in part, to subtraction of serial sarcomeres, which may help preserve sarcomere length. This indicates that function in FTT may be impaired, but there is some remaining mechanical loading to maintain “normal” sarcomere length-tension relationships. However, the changes resulting from MT suggest more severe limitations in force-generating capacity because sarcomere length-tension relationships are no longer normal. The architectural deficits observed in MT muscles may indicate deeper deficiencies in muscle adaptability to length change, which could negatively impact RC function despite successful anatomical repair.
Keywords: Rotator Cuff Tear, Muscle Architecture, Muscle Mechanics, Sarcomere
Introduction
Rotator cuff tears have a lifetime prevalence of 20%, with the likelihood of tear increasing with age1. While many studies rightfully focus on the repair of the RC tendon itself, the fate of the RC muscle following tear is also clinically important (Goutallier2, Patte3, Warner4). Specifically, it is necessary to understand, and ultimately through intervention change, the active and passive force-generating capacities of these muscles after prolonged RC injury.
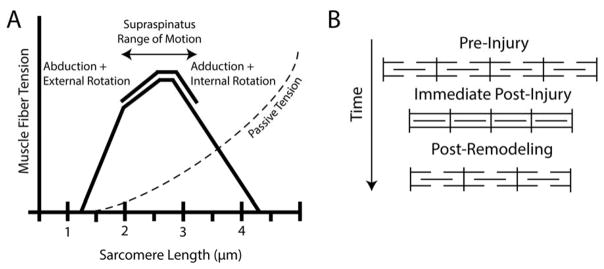
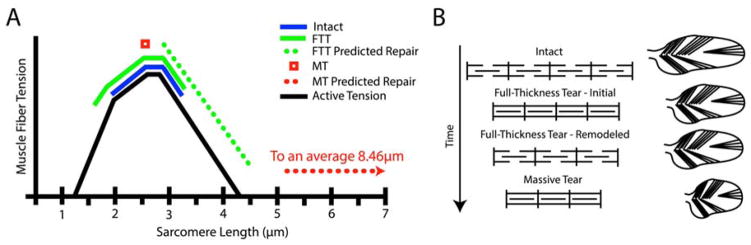
Muscle function is dictated by a set of structural features that cumulatively define the muscle’s distinct architecture. The critical parameters of muscle architecture include mass, muscle fiber length and pennation angle, and sarcomere length and number5. Fiber length determines muscle excursion and force-velocity relationships, where longer fibers permit a greater range of muscle lengths6 for a given force output and lower muscle velocities for a given joint velocity6. Increased pennation angle allows for increased fiber number and physiological cross-sectional area (PCSA), and therefore increased force producing capacity in a given muscle volume6. Finally, sarcomere length and number play a major role in the position of the muscle-joint system on the sarcomere length-tension curve (Figure 1A), which ultimately determines the force generating capacity of the muscle at a given length7. This is referred to as the sarcomere length-operating range of the muscle.
Figure 1.
(A) Classical hypothesis of sarcomere subtraction and maintenance of sarcomere length after muscle shortening injury. (B) Sarcomere length-tension curve with normal sarcomere length-operating range (solid line) and passive tension curve (dashed line) of the supraspinatus muscle indicated10.
When fiber lengths are chronically changed, the sarcomere length operating range is modulated by adding or subtracting sarcomeres in series8;9 (Figure 1B). In the case of torn RC’s, muscle fibers become shorter as the muscle retracts and become longer when the tear is repaired. Whether or not sarcomere number is reduced or increased in response to tear or repair is of great clinical interest for two reasons. First, this adaptation will have a direct impact on muscle passive tension, and therefore the forces acting on the repair site. Secondly, the preservation or disruption of the sarcomere length-operating range will ultimately impact the active force producing capacity of the muscle.
A previous study in human cadavers from Tomioka et al10 demonstrated that in torn rotator cuffs, fiber lengths were reduced while sarcomere lengths were not significantly different between intact and torn cuffs10. These data suggest that the muscle is adapting normally. However, the effect of tear size/severity on architectural measurements was not considered. Therefore, goal of this study was to characterize the architectural features of rotator cuff muscles in the presence of different tendon tear severities in order to understand the biological adaptations that dictate muscle function.
We hypothesized that, in the presence of small tendon tears, sarcomere number and length are maintained within the normal length-operating range, but in the presence of chronic and massively retracted tears, sarcomere length and number are no longer normally maintained. Furthermore, we calculated the theoretical post-repair sarcomere length for each tear category, and documented the actual architectural changes in a small series of specimens with repaired rotator cuffs and reverse total shoulder arthroplasties.
Methods
Thirty cadaveric shoulders with an average age of 83.9±8.7 years were used in this study. All cadavers were fixed (10% formaldehyde) in approximately the same position, with the shoulder at approximately neutral flexion and abduction angles and internally rotated approximately 30 degrees with the elbow flexed to approximately 10 degrees.
The RC muscles were exposed by removing the trapezius and deltoid muscles, and the acromion was removed at the level of the spinoglenoid notch to expose the distal portion of the supraspinatus muscle and tendon. Where applicable, anterior-posterior tear dimensions and retraction distance of the tendon stump from its footprint on the humerus were measured with digital calipers (Mitutoyo, accuracy 0.01mm) before dissecting the rotator cuff muscles from the scapula. Each sample was assigned to a group as being Intact (n=12), Full-Thickness (FTT, n=5), or Massive (MT, n=9). The distinction between FTT and Massive was made based on the size of the tear exceeding 5cm in any dimension. When evidence of repair was found in the form of sutures or hardware the integrity of the repair was noted based on the insertion of the tendon on the footprint of the humerus and the specimen was placed in a separate intervention group. The integrity of supraspinatus and infraspinatus repairs were graded independently (Table 1).
Table 1.
Specimen Demographic Data
| Tear State | Male | Female | Age (years) |
|---|---|---|---|
| Intact (N=12) | 8 | 4 | 81.0±8.4 |
| FTT (N=5) | 3 | 2 | 84.6±15.5 |
| MT (N=9) | 5 | 4 | 86.7±5.6 |
| Intervention | 1 | 3 | 85.5±4.4 |
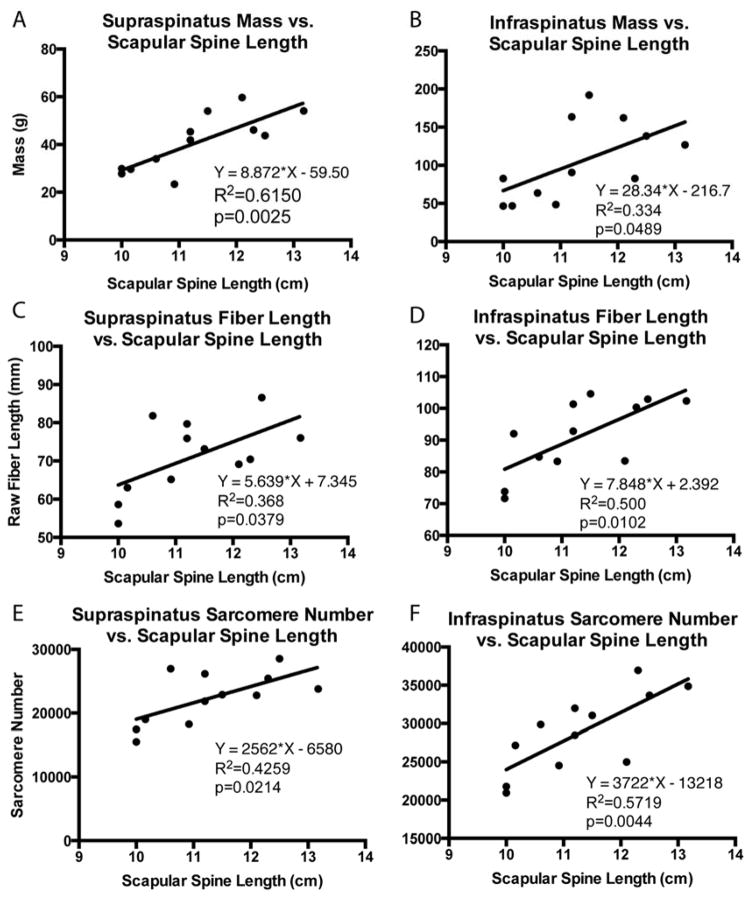
Architectural measurements of the supraspinatus and infraspinatus were made in accordance with previous studies5. Briefly, muscle mass, pennation angle, and length were recorded, and individual fascicles were dissected from distinct regions5 and raw fiber lengths were measured with calipers before being stored in PBS for subsequent sarcomere length measurements. Bony measurements were also recorded, including scapular and humeral head dimensions, to normalize for differences in skeletal dimensions among subjects.
Sarcomere length measurements were carried out using a laser diffraction as previously described11. Individual muscle fibers were dissected from fascicles and mounted on glass slides. Sarcomere length was recorded for six individual muscle fibers to obtain the average sarcomere length for that region. Sarcomere number was determined by dividing fiber length by sarcomere length. Physiological cross-sectional area (PCSA), the standard metric for describing the force-generating capacity of a muscle, was calculated using the following formula:
where density is assumed to be 1.06g/cm3 (12), Lf is fiber length adjusted for sarcomere length, and Ø is the average pennation angle for the muscle13. Finally, we calculated the supraspinatus sarcomere length that would result from repair of the tendon to the footprint on the humeral head in FTT and MT using the following equation:
where Ls_pre and Ls_post are sarcomere length pre- and post-repair, respectively.
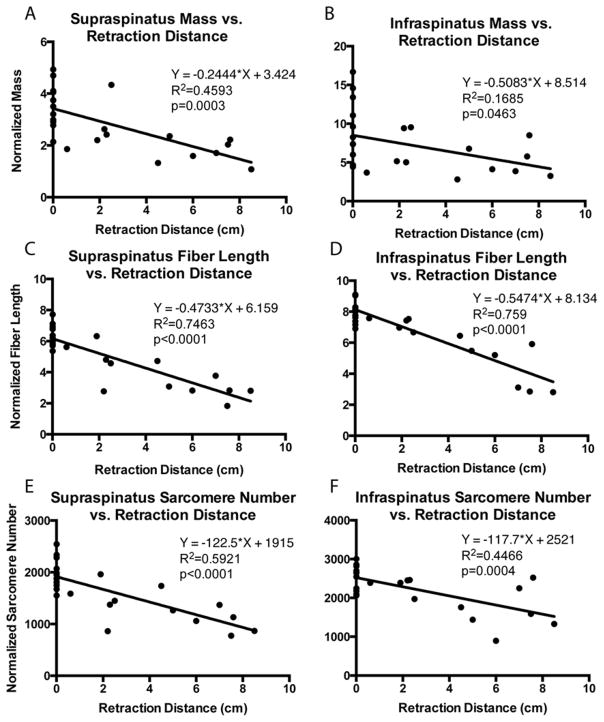
To adjust for differences in skeletal dimensions, comparisons of mass, fiber length, and sarcomere number were normalized by the length of the scapular spine from its most medial edge to the glenoid face (Figure 2). This method of normalization was used in analyzing the linear regression of architectural parameters to retraction distance. In four specimens, both shoulders were used. For the purpose of group comparisons, these shoulders were treated as separate samples due to the varying pathology and bony anatomy found on each side. Statistical comparisons between the intact, FTT and MT groups were carried out in GraphPad Prism 6 (GraphPad Software, Inc., La Jolla, CA, USA) software using linear regression to determine correlations and two-way repeated measures ANOVA by tear state and muscle region with post-hoc Tukey tests for significance (p<0.05). The intervention group is plotted for reference, but was excluded from statistical analysis due to the small sample size and heterogeneity of interventions within the group. Data are reported as mean±standard deviation, and p-values are indicated as follows: <0.1=#, <0.05=*, <0.01=**, <0.001=***, <0.0001=****.
Figure 2.
Linear regression of supraspinatus and infraspinatus muscle mass (A,B), muscle fiber length (C,D) and sarcomere number (E,F) with scapular spine length, demonstrating correlations between bony shoulder anatomy and muscle architecture parameters that accounts for inter-subject size variability. Equation of the regression line, the coefficient of determination, and the p-value are provided.
Results
No significant regional differences were found in muscle fiber or sarcomere lengths, nor were there any significant regional effects on these parameters in the presence of RC tear. Therefore, all architectural measurements were averaged and reported on a per-muscle basis.
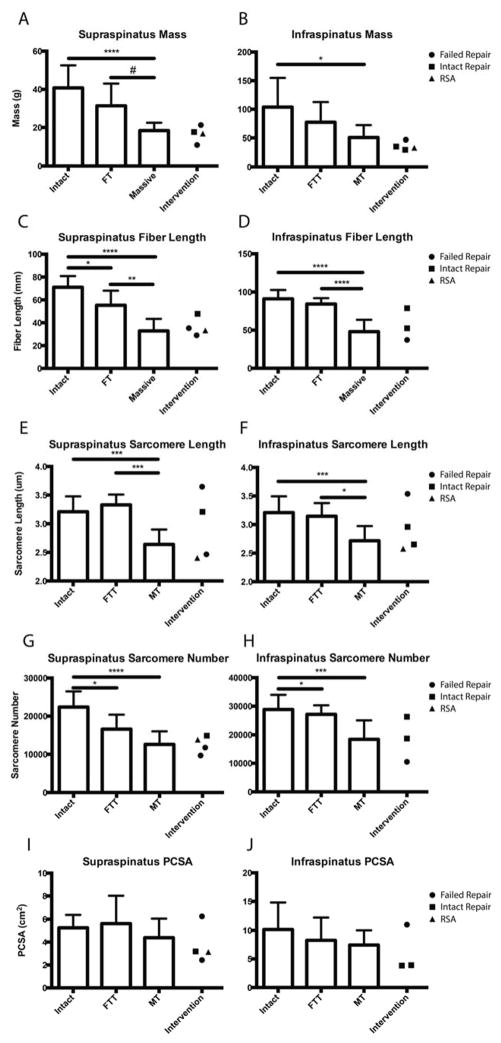
Muscle mass was significantly reduced in both FTT and MT compared to intact, with a trend toward further mass reduction in MT and repaired cuffs compared to FTT (Figure 3A,B). Linear regression showed that mass was negatively correlated with retraction distance in the supraspinatus with a similar, though weaker, trend in the infraspinatus (Figure 4A,B). The weaker relationships found in the infraspinatus are likely due to our definition of MT, as some infraspinatus tendons in the MT group may retain some mechanical connection to the bone. Interestingly, mass was reduced to the level of MT in the intervention group regardless of the state of the repair (Figure 3A,B). The pennation angle was not significantly altered in either FTT or MT, though there was a trend toward larger supraspinatus pennation angles in MT (18.1±8.0°, p=0.092) and the intervention group compared to intact RCs (10.4±4.5°).
Figure 3.
Average mass (A,B), raw muscle fiber length (C,D) sarcomere length (E,F), sarcomere number (G,H), and PCSA (I,J) of the supraspinatus and infraspinatus muscles grouped by tear state (Intact, FTT, MT), with samples demonstrating surgical intervention separated and indicated by symbol.
Figure 4.
Linear regression of normalized supraspinatus and infraspinatus muscle mass (A,B), fiber length (C,D), and sarcomere number (E,F) demonstrating negative correlations between muscle retraction distance and architecture parameters. Equation of the regression line, the coefficient of determination, and the p-value are provided.
In a similar trend to mass, raw muscle fiber lengths were significantly shorter in the supraspinatus of both FTT and MT (Figure 3C), with fiber shortening significantly correlated to muscle retraction distance in both supraspinatus and infraspinatus (Figure 4C,D). In the infraspinatus, MT’s presented with significantly shorter fibers. However, infraspinatus muscles with FTT did not demonstrate shorter fibers- likely due to localization of the tendon tear to the supraspinatus tendon (Figure 3D). Intervention did not increase muscle fiber length, as the samples from the intervention group were nearly identical to MT (Figure 3C,D).
Sarcomere length was significantly shorter in MT compared to intact and FTT in both the supraspinatus and infraspinatus (Figure 3E,F). Interestingly, the specimen with intact supraspinatus and infraspinatus repair contained normal sarcomere lengths in both muscles, while the remaining muscles demonstrated either extremely long or short sarcomeres. In these latter cases, the extreme to which the sarcomere length was altered was similar in both RC muscles. The predicted supraspinatus sarcomere length at repair for the FTT group was 4.56±0.56μm, while the predicted MT repair length was an extreme 8.46±2.75μm (Figure 5A).
Figure 5.
(A) Sarcomere length-tension curve demonstrating the approximate sarcomere operating range for each tear state (solid lines), using the operating ranges reported by Ward et. al10, extrapolated in the case of FTT to account for diminished sarcomere number. MT is represented by a single point (red square), as this muscle is mechanically disconnected from the joint. The dotted lines demonstrate the theoretical, immediate post-repair sarcomere length operating ranges of FTT (green) and MT (red). (B) The proposed progression of sarcomere-length modulation in FTT and MT, where FTT demonstrates classical sarcomere subtraction and sarcomere length modulation while MT fails to subtract serial sarcomeres, resulting in shortened sarcomeres.
In contrast to the sarcomere length data, no significant differences in supraspinatus sarcomere number were found between FTT and MT, with significantly more sarcomeres found only in the intact muscles compared to both tear states (Figure 3G). In the infraspinatus, only the MT group had significantly fewer sarcomeres than both intact and FTT (Figure 3H). This discrepancy between muscles is likely due to our definition of FTT, where the infraspinatus tendon is largely intact and, therefore, the muscle remains relatively unaltered. As with the previous architectural measurements, sarcomere number was negatively correlated with the extent of retraction in both muscles (Figure 4E,F).
No significant differences were observed in PCSA across all tear states (Figure 3I,J). Although masses were significantly different between groups, the shorter fiber lengths associated with tears cancelled out any potential changes in PCSA. It is important to note that this calculation does not take in to account changes in composition of the muscle, which detrimentally influences a key assumption of the PCSA calculation; that the total tissue volume is composed of functional contractile tissue.
Discussion
The purpose of this study was to determine the effect of tear severity on rotator cuff muscle architecture. Based on the high degree of muscle retraction and mechanical unloading observed in MT we hypothesized that unique architectural changes, particularly in terms of sarcomere length and number, would result from MT when compared to FTT and intact cuff muscle.
Muscle mass and fiber length were reduced with more severe RC tendon tears, similar to previous work10. We further demonstrated that these parameters are negatively correlated with tear size, where an increase in tear size leads to a decrease in mass and fiber length in a linear fashion. A secondary finding in this analysis was the correlation between scapular spine length and both mass and fiber length in the intact group (Figure 2), which combined with previous work14 will allow for corrections based on differences in both muscle volume and skeletal dimensions between subjects.
Sarcomere lengths were significantly shorter in MT compared to either intact or FTT, both of which were found to be similar to previous studies5; 10. Importantly, the length of sarcomeres in intact cuffs is greater than the reported optimal length for human sarcomeres15, suggesting that the sarcomeres of RC muscle are specifically maintained above optimal length. This is also consistent with previous findings5, but remains unique among human muscles. In the case of MT, the observed sarcomere length of 2.64μm is slightly below the optimal predicted length of 2.8μm15, which suggests that muscle retraction in MT has shortened sarcomeres to such an extent that MT muscle resides on the plateau of the length-tension curve, where passive tensions are minimal (Figure 5A). This is important because any muscle shortening would result in movement of the muscle down the ascending limb of the length tension curve, where active and passive tensions are low, which in turn will inhibit function. This finding contradicts previous reports, which showed maintenance of sarcomere length in the presence of tendon tears and muscle fiber length shortening10, and supports our hypothesis that maintenance of sarcomere length and number is in fact dependent on tear severity.
Of critical clinical importance is the effect of RC repair on sarcomere length and the subsequent location of the muscle on the length-tension curve. The predicted post-repair resting sarcomere length in the FTT group was just outside the range of active force generation, suggesting that on average complete anatomical repair to the tendon footprint is possible and unlikely to cause significant muscle damage due to sarcomere overstrain. However, the predicted repair length in the massive tears shows that anatomical repair to the footprint would overstretch sarcomeres, which would yield poor active force production, high passive tensions, and would likely result in damage to the contractile apparatus (Figure 5A). The importance of mechanical load in sarcomere length modulation is highlighted in the case of the repaired RC’s, where only the anatomically intact repair demonstrated normal sarcomere lengths. In failed repairs, without the normal loading condition, sarcomere lengths appeared to be unregulated, and were found at both long and short extremes. Together, these data suggest that in the case of successful repair, normal sarcomere length may be restored. However, more evidence is required to confirm this hypothesis and determine the initial and final conditions under which sarcomere length is modulated in this manner.
Sarcomere number was significantly reduced in FTT compared to intact, which is the inevitable result of maintaining sarcomere length in a shorter muscle fiber6. Surprisingly, though there was a significant difference between fiber lengths in FTT and MT, the sarcomere numbers were not significantly different between the two. Mathematically, this is explained by the shorter sarcomeres in MT, as shorter sarcomeres allow for shorter fibers without loss of serial sarcomeres, but biologically it indicates that the muscle is no longer adapting appropriately to its changing mechanical environment (Figure 5B).
While PCSA is indicative of a normal muscle’s force generating capacity and is expected to decrease in atrophic muscles6, the PCSA data presented here must be viewed cautiously. At face value, it appears that PCSA is preserved across tear states. However, this interpretation is highly misleading based on known compositional changes, such as fat accumulation and fibrosis16; 17, that are unaccounted for in normal PCSA calculations.
The limitations of this study are similar to previous studies, in that minor alterations to muscle structure (<3%) are known to occur during and after fixation, namely that there may be slight contraction of the muscle fibers and overall shrinkage of the tissue18. While these effects are eliminated with in situ fixation of intact muscle, the presence of the unattached tendon in RC tear could lead to minor fixation-induced shortening. Though these are legitimate concerns, it has been shown that neither rigor mortis nor formalin fixation alter sarcomere length in a meaningful way19; 20. And, given the large discrepancies in fiber and sarcomere length observed, it is highly unlikely that the differences between tear states found here are due to fixation artifacts.
We speculate that the discrepancies in sarcomere length between intact and FTT muscle and MT muscle is the result of a deficiency in remodeling ability that only affects the more severely damaged MT muscle. Previous studies have shown that serial sarcomeres are added or subtracted in order to maintain a specific length8; 9, which is thought to be determined by the muscle’s physiological function and the relative length of the contractile proteins found in that muscle5. As such, the “set-point” sarcomere length may differ across muscles, though the optimal sarcomere length in terms of force-generating capacity is believed to be the same across all muscles within a species7. In the RC muscles, this type of remodeling appears to occur in FTT, where serial sarcomere subtraction maintains sarcomere length as fiber length is reduced by muscle retraction. But the cause for the disruption of this process in MT, where sarcomere lengths remain chronically short, is not clear (Figure 7B). One possibility is that the regulatory cues present in intact and FTT muscles are altered in MT due to the high degree of unloading and altered biological environment21; 22 that they experience. Without the proper mechanical and biochemical cues, normal remodeling does not occur and sarcomeres shorten proportionally to the shortening of the fibers without subsequent sarcomere subtraction and sarcomere length maintenance. This hypothesis is further supported by the data from repaired shoulders. Specifically, sarcomere length was only maintained in the single specimen with and intact repair site. Since healthy RC muscle is maintained above optimal sarcomere length in the anatomically neutral position, the shortened sarcomeres in MT may be construed as an inability to maintain non-optimal length, which results in sarcomeres returning to at or near slack sarcomere length.
In conclusion, we have demonstrated that MT leads to unique changes in muscle architecture. Both FTT and MT show decreased mass and fiber length proportional to the degree of muscle retraction. However, where FTT demonstrates serial sarcomere subtraction and maintained sarcomere length consistent with previous work, MT sarcomere lengths are shortened with a relative preservation of serial sarcomeres compared to FTT. This indicates a possible defect in the remodeling ability of the MT muscle, which may partially explain the irreversibility of many of the pathologic changes that occur in RC muscle with massive tendon tears. It is also possible that reconnecting the muscle mechanically would improve its ability to adapt, though both of these hypotheses require further investigation.
Acknowledgments
The authors gratefully acknowledge the UC San Diego Anatomical Gifts Program. Without the tissues donated by patients and families, this experiment would not have been possible.
Funding for the project was provided by NIH R01HD073180 (Ward) and the UCSD Frontiers in Innovation Scholars Program.
Footnotes
Author Contribution Statements:
MC Gibbons – Experimental design, sample harvesting/preparation, data collection, data analysis, manuscript synthesis
EJ Sato – Experimental design, sample harvesting/preparation, data collection
D Bachasson – Sample harvesting/preparation, data collection
T Cheng – Sample harvesting/preparation, data collection
H Azimi – Sample harvesting/preparation, data collection
S Schenk – Experimental design, manuscript preparation
AJ Engler – Experimental design, manuscript preparation
A Singh – Experimental design, data analysis, manuscript preparation
SR Ward – Experimental design, data analysis, manuscript preparation
References
- 1.Yamamoto A, Takagishi K, Osawa T, et al. Prevalence and risk factors of a rotator cuff tear in the general population. Journal of Shoulder and Elbow Surgery. 2010;19:116–120. doi: 10.1016/j.jse.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 2.Goutallier D, Postel J-M, Bernageau J, et al. Fatty muscle degeneration in cuff ruptures: pre-and postoperative evaluation by CT scan. Clinical orthopaedics and related research. 1994;304:78–83. [PubMed] [Google Scholar]
- 3.PATTE D. Classification of rotator cuff lesions. Clinical orthopaedics and related research. 1990;254:81–86. [PubMed] [Google Scholar]
- 4.Warner JJ, Higgins L, Parsons I, et al. Diagnosis and treatment of anterosuperior rotator cuff tears. Journal of Shoulder and Elbow Surgery. 2001;10:37–46. doi: 10.1067/mse.2001.112022. [DOI] [PubMed] [Google Scholar]
- 5.Ward SR, Hentzen ER, Smallwood LH, et al. Rotator cuff muscle architecture: implications for glenohumeral stability. Clinical orthopaedics and related research. 2006;448:157–163. doi: 10.1097/01.blo.0000194680.94882.d3. [DOI] [PubMed] [Google Scholar]
- 6.Lieber RL. Skeletal muscle structure, function, and plasticity. Lippincott Williams & Wilkins; 2002. [Google Scholar]
- 7.Gordon A, Huxley AF, Julian F. The variation in isometric tension with sarcomere length in vertebrate muscle fibres. The Journal of physiology. 1966;184:170–192. doi: 10.1113/jphysiol.1966.sp007909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burkholder T. Sarcomere number adaptation after retinaculum transection in adult mice. Journal of Experimental Biology. 1998;201:309–316. [PubMed] [Google Scholar]
- 9.Friden J, Ponten E, Lieber RL. Effect of muscle tension during tendon transfer on sarcomerogenesis in a rabbit model. The Journal of hand surgery. 2000;25:138–143. doi: 10.1053/jhsu.2000.jhsu025a0138. [DOI] [PubMed] [Google Scholar]
- 10.Tomioka T, Minagawa H, Kijima H, et al. Sarcomere length of torn rotator cuff muscle. Journal of Shoulder and Elbow Surgery. 2009;18:955–959. doi: 10.1016/j.jse.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 11.Lieber RL, Yeh Y, Baskin RJ. Sarcomere length determination using laser diffraction. Effect of beam and fiber diameter. Biophysical journal. 1984;45:1007. doi: 10.1016/S0006-3495(84)84246-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ward SR, Lieber RL. Density and hydration of fresh and fixed human skeletal muscle. Journal of biomechanics. 2005;38:2317–2320. doi: 10.1016/j.jbiomech.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 13.Powell PL, Roy RR, Kanim P, et al. Predictability of skeletal muscle tension from architectural determinations in guinea pig hindlimbs. Journal of Applied Physiology. 1984;57:1715–1721. doi: 10.1152/jappl.1984.57.6.1715. [DOI] [PubMed] [Google Scholar]
- 14.Holzbaur KR, Murray WM, Gold GE, et al. Upper limb muscle volumes in adult subjects. Journal of biomechanics. 2007;40:742–749. doi: 10.1016/j.jbiomech.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 15.Lieber RL, Loren GJ, Friden J. In vivo measurement of human wrist extensor muscle sarcomere length changes. Journal of Neurophysiology. 1994;71:874–881. doi: 10.1152/jn.1994.71.3.874. [DOI] [PubMed] [Google Scholar]
- 16.Steinbacher P, Tauber M, Kogler S, et al. Effects of rotator cuff ruptures on the cellular and intracellular composition of the human supraspinatus muscle. Tissue and Cell. 2010;42:37–41. doi: 10.1016/j.tice.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 17.Lundgreen K, Lian ØB, Engebretsen L, et al. Lower muscle regenerative potential in full-thickness supraspinatus tears compared to partial-thickness tears. Acta orthopaedica. 2013;84:565–570. doi: 10.3109/17453674.2013.858289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cutts A. Shrinkage of muscle fibres during the fixation of cadaveric tissue. Journal of anatomy. 1988;160:75. [PMC free article] [PubMed] [Google Scholar]
- 19.Locker RH. Striation patterns of ox muscle in rigor mortis. The Journal of biophysical and biochemical cytology. 1959;6:419–422. doi: 10.1083/jcb.6.3.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bendall J. Postmortem changes in muscle. The structure and function of muscle. 1973;2:244–309. [Google Scholar]
- 21.De Giorgi S, Saracino M, Castagna A. Degenerative disease in rotator cuff tears: what are the biochemical and histological changes? Joints. 2013;2:26–28. [PMC free article] [PubMed] [Google Scholar]
- 22.Choo A, McCarthy M, Pichika R, et al. Muscle Gene Expression Patterns in Human Rotator Cuff Pathology. The Journal of Bone & Joint Surgery. 2014;96:1558–1565. doi: 10.2106/JBJS.M.01585. [DOI] [PMC free article] [PubMed] [Google Scholar]