Abstract
Sleep disturbances have been reliably reported in patients with schizophrenia, thus suggesting that abnormal sleep may represent a core feature of this disorder. Traditional electroencephalographic studies investigating sleep architecture have found reduced deep Non-Rapid Eye Movement (NREM) sleep, or slow wave sleep (SWS), and increased REM density. However, these findings have been inconsistently observed, and have not survived meta-analysis. By contrast, several recent EEG studies exploring brain activity during sleep have established marked deficits in sleep spindles in schizophrenia, including first-episode and early–onset patients, compared to both healthy and psychiatric comparison subjects. Spindles are waxing and waning, 12–16 Hz NREM sleep oscillations that are generated within the thalamus by the thalamic reticular nucleus (TRN), and are then synchronized and sustained in the cortex. While the functional role of sleep spindles still needs to be fully established, increasing evidence has shown that sleep spindles are implicated in learning and memory, including sleep dependent memory consolidation, and spindle parameters have been associated to general cognitive ability and IQ. In this article we will review the EEG studies demonstrating sleep spindle deficits in patients with schizophrenia, and show that spindle deficits can predict their reduced cognitive performance. We will then present data indicating that spindle impairments point to a TRN-MD thalamus-prefrontal cortex circuit deficit, and discuss about the possible molecular mechanisms underlying thalamo-cortical sleep spindle abnormalities in schizophrenia.
Introduction
Sleep disturbances have been consistently observed in psychotic disorders, and especially in schizophrenia, since their first clinical description(Bleuler, 1950), and recent evidence indicates that sleep dysfunction contribute to psychotic symptoms, including delusions and hallucinations(Reeve et al., 2015). Sleep is also a fruitful approach to investigate the neurobiology of schizophrenia, given that abnormal brain connectivity and plasticity are core pathological features of the disorder(Stephan et al., 2009), which can be observed in the sleep electroencephalogram (EEG)(Sprecher et al., 2015). EEG activity during sleep in largely unaffected by confounds frequently occurring during wakefulness in patients with schizophrenia, including fluctuation in the level of arousal, attention, and motivation. Furthermore, the two main Non-Rapid Eye Movement (NREM) sleep oscillations, slow waves and spindles, reflect the activity of distinct, complementary circuits within the thalamo-cortical system. Specifically, slow waves are characterized by large amplitude, positive-negative 1 Hz EEG oscillations, which are initiated by cortical neurons and propagated by cortico-cortical and cortico-thalamo-cortical connections(Steriade, 2006), whereas sleep spindles are waxing/waning, 12–16 Hz oscillations, which are generated by the interplay of the thalamic reticular nucleus (TRN) with other thalamic nuclei, and then sustained and relayed to the cortex by thalamo-thalamic and thalamo-cortical loops(Huguenard and McCormick, 2007).
While the role of slow wave in learning and memory is well established (for a review, see (Huber and Born, 2014), there is increasing evidence suggesting that sleep spindles are implicated in memory consolidation and plasticity(Molle and Born, 2011), and can be considered a proxy measure for the individual's learning potential(Fogel and Smith, 2011). For example, both animals and humans studies have shown an increase in spindle density and/or activity during non-REM sleep after learning (Eschenko et al., 2006; Gais et al., 2002; Morin et al., 2008; Schabus et al., 2004). Enhanced spindle activity was observed after acquisition of both declarative memory tasks and procedural motor skills, and in some instances this enhancement correlated with the overnight improvement in task performance (Clemens et al., 2005, 2006; Nishida and Walker, 2007; Tamaki et al., 2008). Furthermore, this spindle activity increase was localized in cortical areas most strongly involved in the prior learning of the task, including the prefrontal cortex after encoding of difficult word pairs(Clemens et al., 2005; Schmidt et al., 2006), the parietal cortex after a visuospatial task(Clemens et al., 2006) and the contra-lateral motor cortex following finger motor-skill learning(Nishida and Walker, 2007).
In what follows we will review evidence of sleep disturbances in schizophrenia, with particular emphasis on a number of recent studies showing marked sleep spindle deficits in schizophrenia, including first-break and early onset patients, compared to both healthy and psychiatric control subjects. These spindle deficits predict an impaired cognitive function in patients with schizophrenia, and are associated with worse psychotic symptoms. The neurobiology underlying sleep spindle impairments will also be discussed, and especially the presence of a TRN-thalamus-prefrontal cortex circuitry deficit in schizophrenia. We will then speculate on the molecular mechanisms implicated, which include GABA and NMDA receptor dysfunction, and how clarifying these mechanisms may help predict conversion to psychosis in high-risk individuals as well offer novel molecular targets for patients with schizophrenia.
Sleep architecture abnormalities in schizophrenia
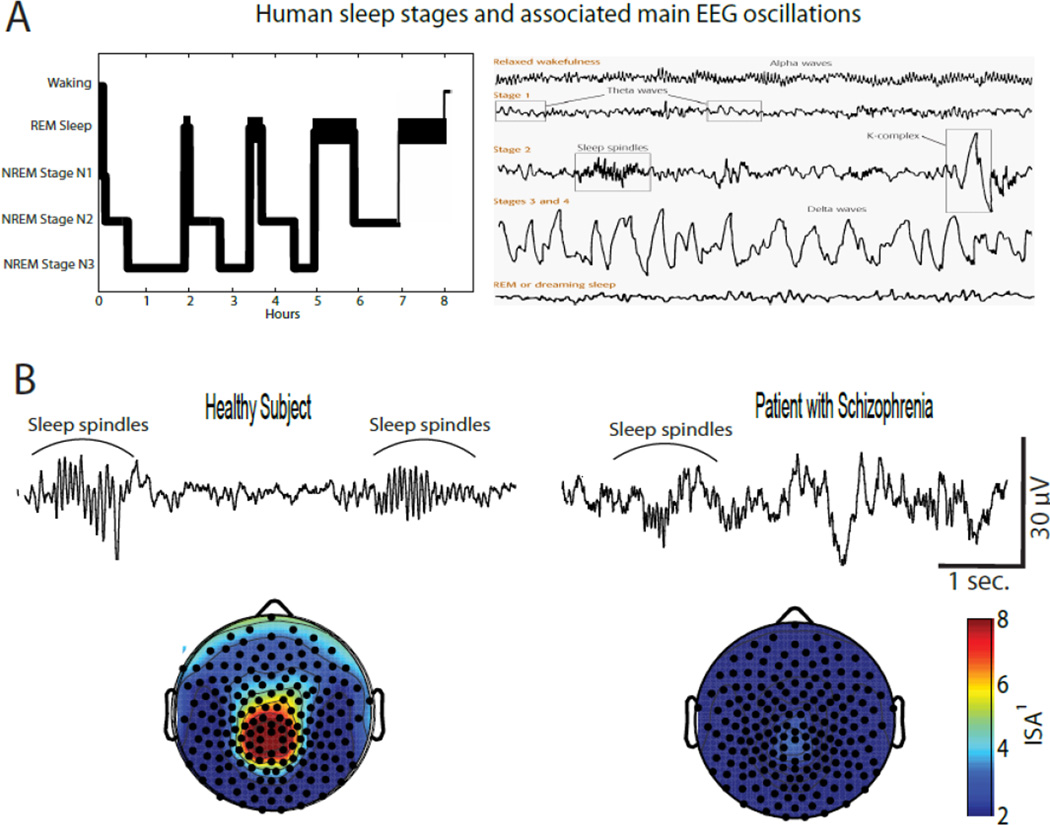
In healthy humans, sleep consists of several cycles characterized by episodes of NREM, or dreamless sleep, followed by episodes of REM sleep, when dreaming activity usually occurs (Figure 1A). NREM sleep can be further divided in stage N1, N2, and N3, which are characterized by progressively deeper sleep and are accompanied by representative EEG oscillations, including sleep spindles (N2) and slow waves (N3), to the extent that NREM N3 is also described as slow wave sleep (SWS, Figure 1A). Sleep disturbances, such as profound insomnia and fragmented, unrestful sleep are often experienced by patients with schizophrenia, especially during acute periods of psychosis, and objective sleep EEG abnormalities have been reported by several studies. Traditional EEG studies have focused on the sleep EEG architecture, and a decrease in REM latency (Benson et al., 1991; Hudson et al., 1993; Tandon et al., 1992), total NREM sleep (Benson et al., 1991; Hoffmann et al., 2000) as well as a reduction in the amount of NREM SWS (Benson et al., 1991; Benson et al., 1996; Hiatt et al., 1985; Keshavan et al., 1990) are the most commonly reported findings. However, meta-analyses have failed to establish a consistent, significant difference in any of these sleep architecture parameters between patients with schizophrenia and healthy or psychiatric control subjects (Benca et al., 1992; Chouinard et al., 2004).
Figure 1.
Spindle deficits have been reported in schizophrenia, including early onset and first-break patients
Over the past several years, an increasing number of studies have gone beyond sleep architecture to investigate the spontaneous brain activity during sleep, including representative EEG oscillations like sleep spindles, which dominate NREM N2 sleep, and slow waves, which characterize NREM N3, also called slow wave sleep (SWS, Figure 1A). Three sleep EEG studies found reduced slow wave power in schizophrenia patients to be associated with marked SWS decrease (Goder et al., 2006; Hiatt et al., 1985; Keshavan et al., 1998), whereas four studies were unable to establish any difference in slow wave activity between schizophrenia patients compared to normal subjects (Ferrarelli et al., 2007; Ferrarelli et al., 2010; Manoach et al., 2014; Tekell et al., 2005). Furthermore, in one of these studies patients with schizophrenia had no reduction in several slow wave parameters, including incidence, amplitude, up-slope and downslope when compared to both healthy and psychiatric controls (Ferrarelli et al., 2010).
Regarding sleep spindles, a handful of early sleep studies, which were performed with a few EEG channels and on relatively small sample sizes (N≤11), found higher spindle counts during the first NREM sleep episode in five patients with schizophrenia(Hiatt et al., 1985) or no difference in whole night spindle activity between nine(Van Cauter et al., 1991) and eleven(Poulin et al., 2003) schizophrenia patients and healthy controls. By contrast, several recent studies on larger group of schizophrenia patients (N≥18) have consistently reported marked spindle deficits in schizophrenia relative to comparison groups (Figure 1B).
Specifically, by performing sleep high density (hd)-EEG recording with a 256 channel montage we established that schizophrenia patients (N=18) had a reduction in several spindle parameters, including duration, amplitude, and density compared to healthy (N=17) and depressed (N=15) subjects during their first NREM sleep episode (Ferrarelli et al., 2007). In a follow-up, whole night hd-EEG sleep study we confirmed these spindle deficits in a larger group of patients with schizophrenia (N=49) compared to healthy subjects (N=44) as well as antipsychotic medicated psychiatric controls (N=20), thus suggesting the spindle impairments were present throughout the night and were unlikely related to medication status(Ferrarelli et al., 2010). A recent report established reduced spindle activity in first episode, antipsychotic-naive schizophrenia patients, but found no spindle deficit in non-schizophrenia psychotic patients compared to healthy controls (Manoach et al., 2014). A decrease in spindle density and amplitude was also seen in asymptomatic first-degree relatives of schizophrenia patients, which further indicate that spindle impairments are unrelated to antipsychotic medication and may be specific for schizophrenia, though replication in larger samples of patients is required. Of note, reduced sleep spindle density has also been found in nine adolescent meeting criteria for early onset schizophrenia compared to healthy controls (Tesler et al., 2015).
Spindle reduction is associated to worse cognitive function and psychotic symptoms in schizophrenia
In healthy individuals, sleep spindles have been implicated in the consolidation of both procedural (Fogel and Smith, 2006; Nishida and Walker, 2007; Tamaki et al., 2008) and declarative (Clemens et al., 2006; Schabus et al., 2008) memory occurring during sleep. Higher spindle activity tend to occur in specific brain circuits involved in pre-sleep learning, and this localized spindle increase predict a post-sleep improvement in performance(Clemens et al., 2006; Tamaki et al., 2013). In several studies employing the motor sequence task (MST), which assesses sleep-dependent motor procedural memory, significant performance improvements occurred after sleep, but not after a comparable amount of wakefulness(Fischer et al., 2005; Walker et al., 2003), and these improvements correlated with sleep spindle density(Barakat et al., 2011; Nishida and Walker, 2007). By contrast, in patients with schizophrenia post-sleep MST performance did not significantly improve(Manoach et al., 2004; Manoach et al., 2010; Wamsley et al., 2012), and in one of these studies this lack of improvement correlated with a reduction in spindle density(Wamsley et al., 2012). Furthermore, schizophrenia patients showed decreased sleep-dependent consolidation in a declarative memory task together with reduced sleep spindles, and these two deficits were significantly correlated(Goder et al., 2015). There is also some indication the sleep spindles may reflect learning potential and general cognitive ability (IQ)(Fogel and Smith, 2011), although the exact relationship between spindle parameters, IQ, and memory consolidation still need to be elucidated. For example, we failed to establish a correlation between sleep spindles and performance in the Raven’s Progressive Matrices Test, which is usually utilized to measure intelligence, in chronic patients with schizophrenia (Ferrarelli et al., 2010), whereas another study reported that sleep spindle deficits were associated with lower IQ in first-break psychotic patients, which included both schizophrenia and non-schizophrenia psychotic disorders(Manoach et al., 2014).
As far as the relationship between spindle activity and clinical symptoms, several sleep studies have also shown an inverse correlation between sleep spindles deficits and the severity of psychosis in schizophrenia, including both chronic (Ferrarelli et al., 2010; Wamsley et al., 2012) and early onset (Tesler et al., 2015) patients. In one study, which failed to establish a correlation between positive symptoms and spindle activity, the authors reported that the patient population was characterized by mild and homogeneous symptomatology(Goder et al., 2015), whereas in antipsychotic-naïve, first-episode patients with schizophrenia spindle amplitude was inversely related with the level of positive symptoms(Manoach et al., 2014).
Reduced spindle activity point to a TRN-thalamus-prefrontal cortical circuit defect in schizophrenia
Converging evidence from electrophysiological and neuroimaging studies suggest that reduced spindle activity reflects dysfunctions within the thalamo-cortical system. Here we will review data pointing to a TRN-MD thalamus-PFC circuit deficit in schizophrenia.
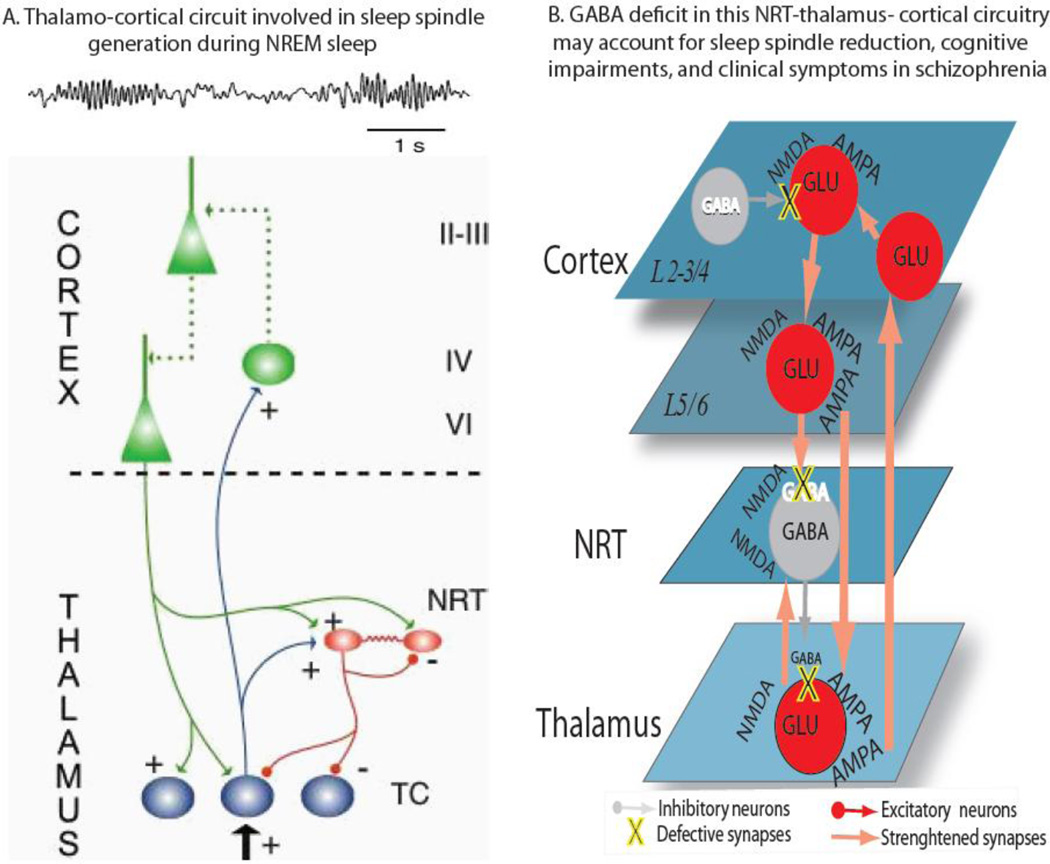
The TRN is strategically placed between the thalamus and the cortex, since it receives excitatory afferents from both cortical and thalamic neurons and sends gamma aminobutyric acid (GABA)-ergic inhibitory projections to all thalamic nuclei (Figure 2A). Because of its location and unique anatomical connectivity, it has been proposed that the TRN represent an attentional searchlight(Crick, 1984), and that may be implicated in regulating a variety of brain functions, including the generation of sleep spindles (Pinault, 2004). The TRN is also likely plays important role in initiating the spindle oscillation, as suggested by seminal work from Steriade et al. showing that sleep spindles occurred in reticular neurons even after it was completely disconnected from other thalamic nuclei. These initial findings have been corroborated by subsequent computational and experimental studies (Bazhenov et al., 2000; Bazhenov et al., 1999; Destexhe et al., 1994; Golomb et al., 1994; Wang and Rinzel, 1993). More recently, by employing a combination of optogenetics and multi-electrode recording in behaving mice Halassa et a (Halassa et al., 2011) demonstrated that brief selective drive of TRN switched the thalamocortical firing mode from tonic to bursting and generated state-dependent neocortical spindles. It should be however noted that thalamo-thalamic as well as corticothalamic loops are critically involved in the synchronization and the maintenance of spindle oscillations (Figure 2A) (Fuentealba and Steriade, 2005). In vitro recordings have shown that sleep spindles are generated by the reciprocal interaction between the GABAergic cells of the TRN and excitatory thalamo-cortical sensory relay neurons(von Krosigk et al., 1993). Furthermore, Bartho et al. has shown that ongoing network activity between TRN and TC neurons controls the length of sleep spindles (Bartho et al., 2014). It is also important to point out the most of the electrophysiological animal data on sleep spindles comes from studies primarily investigating the relationship between TRN and sensory thalamic nuclei. Importantly, higher order limbic and midline nuclei of the thalamus seem to generate sleep spindles differently from sensory nuclei, as demonstrated by an elegant study in mice by Sheroziya and Timofeev (Sheroziya and Timofeev, 2014), whereas there are no intracellular recordings in MD neuron during spindle activity. These studies are needed in order to clarify the role of MD thalamus in sleep spindle generation as well as its implication in spindle deficits in schizophrenia. In one of the few studies looking at the both sensory and limbic reticular neurons Halassa et al. employed a combination of connectivity-based optogenetic tagging and TRN ensemble recording to demonstrate that visually-tagged reticular neurons activity was directly correlated, whereas limbic-tagged neurons were negatively correlated to cortical spindle power during NREM sleep(Halassa et al., 2014). The same research group also demonstrated that a reduction in TRN visual, but not limbic neurons was observed during a visual detection attentional task, and optogenetically-induced reduction in these neurons predicted an improvement in performance in mice, thus confirming a role for TRN neurons in regulating attentional states (Halassa et al., 2014). Related findings were observed by recording visual TRN neurons in awake monkeys, wherein McAlonan et al. demonstrated that the activity of these neurons is modulated by shifts in visual attention, and this activity changes affect visual processing in the lateral geniculate nucleus of the thalamus via feedback reticular inhibitory projections(McAlonan et al., 2006). Aherns et al. has recently shown that deficiency of the ErbB4 gene in somatostatin-expressing TRN neurons markedly altered behaviors dependent on sensory selection in mice, and particularly their ability to switch attention between conflicting sensory cues. Furthermore, by using a combination of electrophysiology and intracellular chloride photometry Wimmer et al. demonstrated that visual TRN dynamically controlled visual thalamic gain through PFC-regulated feedforward inhibition (Wimmer et al., 2015). Finally, using single-unit activity in the TRN neurons of anesthetized rats, Krause et al. showed gated response to paired-tone auditory stimuli, which was disrupted by amphetamine and reversed by haloperidol (Krause et al., 2003).
Figure 2.
Altogether, these findings indicate that TRN neurons play a critical role in both bottom-up and top-down processes, including spindle generation, auditory sensory gating(Hajos et al., 2008) and attention modulation(McAlonan et al., 2006), which are known to be defective in patients with schizophrenia(Ferrarelli, 2015; Javitt and Freedman, 2015). Furthermore sleep spindles have been linked to cognitive functions, including memory consolidation(Lustenberger et al., 2015; Molle and Born, 2011) and learning potential(Fogel and Smith, 2011) in healthy subjects, and several studies have reported that sleep spindles deficits were associated with reduced cognitive performance in finger-tapping motor sequence(Manoach et al., 2010; Wamsley et al., 2012), procedural learning(Seeck-Hirschner et al., 2010), as well as working memory(Buchmann et al., 2014) tasks in patients with schizophrenia. Nonetheless, future experimental work is needed to establish more directly the implication of TRN in these higher order cognitive deficits. The implication of the thalamus in the pathophysiology of schizophrenia was initially based on the observation that the clinical symptoms of this disorder could not be localized in a single cortical area(Andreasen et al., 1986), or just restricted to the cerebral cortex(Andreasen et al., 1998). It also relied on studies demonstrating the implication of thalamo-cortical circuits in brain functions, including sensory-motor relay, attention and memory, commonly found to be defective in schizophrenia patients(Carrera and Bogousslavsky, 2006). Since then, growing evidence from both post-mortem and neuroimaging studies point to thalamic abnormalities in schizophrenia. Several post-mortem studies have established significant thalamic changes, especially in the medio-dorsal (MD) nucleus, which included reduction in total number of neurons and overall volume, in patients with schizophrenia compared to healthy controls (Byne et al., 2002; Danos et al., 2005; Young et al., 2000). However, some negative findings have also been reported (Cullen et al., 2003; Kreczmanski et al., 2007), which could be related to the relative small sample size of these studies. A reduction in MD volume has been more consistently assessed by anatomical MRI studies in schizophrenia, whereas smaller whole thalami (WT) has been found in some studies, but not in several others, thus suggesting the implication of only specific thalamic nuclei in the neurobiology of this disorder. Notably, in a recent study we performed MRI-based volume analysis of WT, lateral geniculate nucleus (LGN), and MD nuclei bilaterally and established that only MD volumes were significantly reduced in patients with schizophrenia compared to healthy controls(Buchmann et al., 2014). Furthermore, these smaller MD volumes were correlated with decreased sleep spindle density in a frontal region, thus suggesting a role for the MD in spindle generation(Buchmann et al., 2014). While these findings were observed in chronic patients with schizophrenia, reduction in MD volumes have been also reported in first-episode patients (Gilbert et al., 2001; Salgado-Pineda et al., 2003).
The implication of PFC in a TRN-MD-cortical circuit thought to be defective in schizophrenia come from two lines of evidence. First, anatomical (Goldman-Rakic and Porrino, 1985; Jones, 2002) and functional (Jones, 2009; Mitchell, 2015) studies in both primates and healthy humans have shown that the MD thalamus is heavily interconnected with the Prefrontal cortex (PFC). Second, several recent studies utilizing resting-state fMRI to investigate functional connectivity within the thalamo-cortical system have consistently reported abnormalities in MD-PFC connections. An initial study employing a seed-based analysis approach found reduced PFC-MD connectivity in eleven patients with schizophrenia compared to 12 health controls (Welsh et al., 2010). This finding was confirmed in a much larger sample (N=62) of schizophrenia patients together with an increase in thalamic connectivity with sensorimotor cortical areas in these patients relative to normal subjects (N=77)(Woodward et al., 2012). Another fMRI study established this thalamus-PFC hypo-connectivity together with the thalamus-sensorimotor cortices hyper-connectivity in an even large group of schizophrenia patients (N=90), and also showed that these parameters were significantly correlated by employing an anatomical and data-driven clustering analysis (Anticevic et al., 2014). Furthermore, in a resting-state Region of Interest (ROI) fMRI investigation on 105 healthy subjects and 148 psychotic patients Woodward et al. demonstrated reduced PFC-thalamus and increased sensorimotor-thalamic connectivity in both chronic and early-state psychosis, and the PFC-thalamus reduction correlated with impaired cognitive functioning, including verbal learning and memory(Woodward and Heckers, 2015). The presence of MD-PFC deficits in schizophrenia was also reported by a sleep hd-EEG study, where decreased frontal sleep spindles were associated to smaller MD thalamic volumes, and the cortical currents underlying these frontal spindles were localized in PFC(Buchmann et al., 2014). While promising these findings needs to be replicated in studies with larger groups of patients. It would also be important to perform electrophysiological and optogenetic studies in animals to explore the relationship between TRN and MD thalamic in relation to spindle generation and modulation within the thalamo-cortical network.
What are the molecular mechanisms underlying spindle deficits in schizophrenia?
At this stage, we can only speculate on the molecular substrates responsible for reduced sleep spindle activity in patients with schizophrenia.
A possible molecular mechanism involve a reduced binding or expression of N-methyl-D-aspartate (NMDA) glutamate receptors within the thalamo-cortical system. Postmortem studies have found a reduction of NMDA glutamate receptors in both MD thalamus and PFC in patients with schizophrenia(Pakkenberg et al., 2009), whereas pharmacological manipulations with NMDA antagonists, including ketamine and phencyclidine (PCP), produce schizophrenia-like psychosis in healthy individuals(Bergeron and Coyle, 2012). Furthermore, it has been shown that second-generation antipsychotic medications, including Clozapine, could revert a PCP-mediated blockade of NMDA receptors in both the MD thalamus (Santana et al., 2011) and PFC (Jardemark et al., 2010), while subanaestethic, acute ketamine treatment in mice resulted in impairments in a circuitry involving the TRN, MD thalamus, and PFC (Dawson et al., 2013). It has also been shown that rats receiving chronic treatment with a low dose of PCP developed hypo-frontality and PFC GABA interneuron deficits combined with reduced metabolic and GABAergic marker activity in the TRN (Cochran et al., 2003), and that the reticular neurons changes occurred first(Cochran et al., 2002), thus suggesting that prefrontal deficits may be secondary to thalamic dysfunction(Pratt and Morris, 2015). Another pharmacological study employing single unit and local field potential thalamic recordings in combination with electrocorticogram found that PCP reduced the discharge rate of TRN as well as the power and phase coherence between TRN and PFC in anesthetized rats, whereas clozapine countered the effects of PCP in the cortex, but not in the thalamus(Troyano-Rodriguez et al., 2014).
A defect in GABA-ergic neurotransmission could also underlie sleep spindles abnormalities in schizophrenia. The TRN consists of GABA-ergic neurons, and electrophysiological experiments have demonstrated their role in spindle generation. Specifically, GABAA receptor-mediated depolarization in reticular neurons activates T-type Ca+ channels, which are responsible for the burst-spiking associated to the spindle oscillation, whereas both GABAA and GABAB receptors are involved in synchronizing the spindle oscillatory activity within the thalamus (Sun et al., 2012). TRN GABA activity also mediates sensory auditory gating in rats, which is disrupted by amphetamine and reversed by haloperidol (Krause et al., 2003), all findings in keeping with electrophysiological observations of sensory processing dysfunction in schizophrenia (Javitt and Freedman, 2015). Another line of evidence comes from post-mortem studies demonstrating a reduction in glutamate decarboxylase 67, an enzyme involved in GABA synthesis and in GABA membrane transporter density in PFC interneurons in schizophrenia patients(Lewis et al., 2005), whereas treatment studies have shown that some of the beneficial effects of Clozapine as well as Electroconvulsive Therapy (ECT) and Transcranial Magnetic Stimulation (TMS) in schizophrenia patients are related to an increase in GABA-mediated inhibitory neurotransmission on excitatory cortical neurons(Daskalakis et al., 2008; Kaster et al., 2015; Taylor and Tso, 2015).
Future directions
So far, a reduction in spindle activity was found in both chronic and early course schizophrenia patients as well as in first degree relatives of schizophrenia probands, whereas no spindle abnormalities have been reported in antipsychotic medicated non-schizophrenia patients or in antipsychotic naïve, non-schizophrenia psychotic patients. Additional studies involving other psychiatric populations as well as individuals at high risk for mental illness will help to assess the specificity of spindle deficits in schizophrenia, including the possibility to predict those who will develop this disorder, thus establishing the role of sleep spindles as a candidate biomarker or endophenotype for schizophrenia. As an initial step in this direction, a recent neuroimaging study showed that in healthy individuals spindle density was inversely correlated with magical ideation, an index of liability to psychosis, as well as with MRS Glutamate levels in the thalamus (Lustenberger et al., 2015).
In previous work we suggested that a defect in TRN neurons function would result in reduced activation of NMDA post-synaptic receptors in other thalamic nuclei, thus increasing the firing rate of thalamic relay neurons to the cortex. Hyper-activated cortical neurons would then determine psychotic symptoms, while cortico-thalamic excitatory feedbacks would sustain this effect(Ferrarelli and Tononi, 2011). Based on accumulating evidence, which has been reviewed here, we now suggest that more diffuse deficits within this TRN-MD thalamus-cortical circuitry involving both NMDA and GABA receptors are implicated in the sleep spindles as well as other neuro-physiological and cognitive abnormalities commonly reported in schizophrenia patients (Figure 2B). Additional studies are needed to test this hypothesis as well as to better characterize the molecular underpinnings of sleep spindle abnormalities in schizophrenia.
It will also be important to assess whether targeting with pharmacological and nonpharmacological interventions some of these pathophysiological mechanisms may improve sleep spindle impairments together with the associated clinical and cognitive symptoms in patients with schizophrenia. Some initial pharmacological work on small sample sizes have shown that eszopiclone, which shows greater synaptic efficacy on GABA neurons in the TRN compared to other hypnotics(Jia et al., 2009), significantly increased spindles but not sleep dependent memory in one study(Wamsley et al., 2013), whereas in another study eszopiclone improved working memory, but not symptoms(Tek et al., 2014) in patients with schizophrenia. Furthermore, in a study applying transcranial direct current stimulation (tDCS) during N2 sleep, where most of sleep spindles occur, it was found that tDCS was able to enhance sleep related declarative memory consolidation in patients with schizophrenia, although spindle parameters were not measured(Goder et al., 2013).
Conclusion
In this review we presented accumulating evidence of reduced sleep spindles in schizophrenia, including first-break and early-onset patients, compared to both healthy and psychiatric comparison subjects. This spindle reduction is associated to worse clinical symptoms, can predict an impaired cognitive performance, and points to dysfunction within the thalamo-cortical system. Future work aimed at further characterizing the neuronal circuits and molecular mechanisms implicated in spindle deficits could significantly contribute to clarify the neurobiology of schizophrenia; it could also provide novel insight into the treatment, early detection, and eventually even the prevention of this devastating mental illness.
Acknowledgments
We would like to thank all patients who agreed to participate to the studies reviewed in the manuscript.
Role of the funding source
This work was supported by the Lane's Schizophrenia Research Fund (Dr. Ferrarelli & Tononi); and by a National Institutes of Health/National Institute of Mental Health Conte Center grant, 1P20MH-077967-01A1 (Dr. Tononi).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
Dr. Tononi has served as a consultant for Tikvah Therapeutics and Respironics; he has received speaker’s honoraria from Sanofi-Aventis and Respironics; he has received research support from Sanofi-Aventis as well as from Respironics (Philips). Ferrarelli reports no financial relationships with commercial interests.
Contributors:
Fabio Ferrarelli: study design and funding, data collection, contributed to data analysis, contributed to write the manuscript
Giulio Tononi: study design and funding, contributed to write the manuscript
References
- Andreasen N, Nasrallah HA, Dunn V, Olson SC, Grove WM, Ehrhardt JC, Coffman JA, Crossett JH. Structural abnormalities in the frontal system in schizophrenia. A magnetic resonance imaging study. Arch Gen Psychiatry. 1986;43(2):136–144. doi: 10.1001/archpsyc.1986.01800020042006. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, Paradiso S, O'Leary DS. "Cognitive dysmetria" as an integrative theory of schizophrenia: a dysfunction in cortical-subcortical-cerebellar circuitry? Schizophr Bull. 1998;24(2):203–218. doi: 10.1093/oxfordjournals.schbul.a033321. [DOI] [PubMed] [Google Scholar]
- Anticevic A, Cole MW, Repovs G, Murray JD, Brumbaugh MS, Winkler AM, Savic A, Krystal JH, Pearlson GD, Glahn DC. Characterizing thalamo-cortical disturbances in schizophrenia and bipolar illness. Cereb Cortex. 2014;24(12):3116–3130. doi: 10.1093/cercor/bht165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barakat M, Doyon J, Debas K, Vandewalle G, Morin A, Poirier G, Martin N, Lafortune M, Karni A, Ungerleider LG, Benali H, Carrier J. Fast and slow spindle involvement in the consolidation of a new motor sequence. Behav Brain Res. 2011;217(1):117–121. doi: 10.1016/j.bbr.2010.10.019. [DOI] [PubMed] [Google Scholar]
- Bartho P, Slezia A, Matyas F, Faradzs-Zade L, Ulbert I, Harris KD, Acsady L. Ongoing network state controls the length of sleep spindles via inhibitory activity. Neuron. 2014;82(6):1367–1379. doi: 10.1016/j.neuron.2014.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazhenov M, Timofeev I, Steriade M, Sejnowski T. Spiking-bursting activity in the thalamic reticular nucleus initiates sequences of spindle oscillations in thalamic networks. J Neurophysiol. 2000;84(2):1076–1087. doi: 10.1152/jn.2000.84.2.1076. [DOI] [PubMed] [Google Scholar]
- Bazhenov M, Timofeev I, Steriade M, Sejnowski TJ. Self-sustained rhythmic activity in the thalamic reticular nucleus mediated by depolarizing GABAA receptor potentials. Nat Neurosci. 1999;2(2):168–174. doi: 10.1038/5729. [DOI] [PubMed] [Google Scholar]
- Benca RM, Obermeyer WH, Thisted RA, Gillin JC. Sleep and psychiatric disorders. A meta-analysis. Arch Gen Psychiatry. 1992;49(8):651–668. doi: 10.1001/archpsyc.1992.01820080059010. discussion 669–670. [DOI] [PubMed] [Google Scholar]
- Benson KL, Faull KF, Zarcone VP., Jr Evidence for the role of serotonin in the regulation of slow wave sleep in schizophrenia. Sleep. 1991;14(2):133–139. doi: 10.1093/sleep/14.2.133. [DOI] [PubMed] [Google Scholar]
- Benson KL, Sullivan EV, Lim KO, Lauriello J, Zarcone VP, Jr, Pfefferbaum A. Slow wave sleep and computed tomographic measures of brain morphology in schizophrenia. Psychiatry Res. 1996;60(2–3):125–134. doi: 10.1016/0165-1781(96)02705-9. [DOI] [PubMed] [Google Scholar]
- Bergeron R, Coyle JT. NAAG, NMDA receptor and psychosis. Curr Med Chem. 2012;19(9):1360–1364. doi: 10.2174/092986712799462685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleuler E. Dementia praecox or the group of schizophrenias. New York: International Universities Press; 1950. [Google Scholar]
- Buchmann A, Dentico D, Peterson MJ, Riedner BA, Sarasso S, Massimini M, Tononi G, Ferrarelli F. Reduced mediodorsal thalamic volume and prefrontal cortical spindle activity in schizophrenia. Neuroimage. 2014;102(Pt 2):540–547. doi: 10.1016/j.neuroimage.2014.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byne W, Buchsbaum MS, Mattiace LA, Hazlett EA, Kemether E, Elhakem SL, Purohit DP, Haroutunian V, Jones L. Postmortem assessment of thalamic nuclear volumes in subjects with schizophrenia. Am J Psychiatry. 2002;159(1):59–65. doi: 10.1176/appi.ajp.159.1.59. [DOI] [PubMed] [Google Scholar]
- Carrera E, Bogousslavsky J. The thalamus and behavior: effects of anatomically distinct strokes. Neurology. 2006;66(12):1817–1823. doi: 10.1212/01.wnl.0000219679.95223.4c. [DOI] [PubMed] [Google Scholar]
- Chouinard S, Poulin J, Stip E, Godbout R. Sleep in untreated patients with schizophrenia: a meta-analysis. Schizophr Bull. 2004;30(4):957–967. doi: 10.1093/oxfordjournals.schbul.a007145. [DOI] [PubMed] [Google Scholar]
- Clemens Z, Fabo D, Halasz P. Overnight verbal memory retention correlates with the number of sleep spindles. Neuroscience. 2005;132(2):529–535. doi: 10.1016/j.neuroscience.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Clemens Z, Fabo D, Halasz P. Twenty-four hours retention of visuospatial memory correlates with the number of parietal sleep spindles. Neurosci Lett. 2006;403(1–2):52–56. doi: 10.1016/j.neulet.2006.04.035. [DOI] [PubMed] [Google Scholar]
- Cochran SM, Fujimura M, Morris BJ, Pratt JA. Acute and delayed effects of phencyclidine upon mRNA levels of markers of glutamatergic and GABAergic neurotransmitter function in the rat brain. Synapse. 2002;46(3):206–214. doi: 10.1002/syn.10126. [DOI] [PubMed] [Google Scholar]
- Cochran SM, Kennedy M, McKerchar CE, Steward LJ, Pratt JA, Morris BJ. Induction of metabolic hypofunction and neurochemical deficits after chronic intermittent exposure to phencyclidine: differential modulation by antipsychotic drugs. Neuropsychopharmacology. 2003;28(2):265–275. doi: 10.1038/sj.npp.1300031. [DOI] [PubMed] [Google Scholar]
- Crick F. Function of the thalamic reticular complex: the searchlight hypothesis. Proc Natl Acad Sci U S A. 1984;81(14):4586–4590. doi: 10.1073/pnas.81.14.4586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen TJ, Walker MA, Parkinson N, Craven R, Crow TJ, Esiri MM, Harrison PJ. A postmortem study of the mediodorsal nucleus of the thalamus in schizophrenia. Schizophr Res. 2003;60(2–3):157–166. doi: 10.1016/s0920-9964(02)00297-9. [DOI] [PubMed] [Google Scholar]
- Danos P, Schmidt A, Baumann B, Bernstein HG, Northoff G, Stauch R, Krell D, Bogerts B. Volume and neuron number of the mediodorsal thalamic nucleus in schizophrenia: a replication study. Psychiatry Res. 2005;140(3):281–289. doi: 10.1016/j.pscychresns.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Daskalakis ZJ, Christensen BK, Fitzgerald PB, Moller B, Fountain SI, Chen R. Increased cortical inhibition in persons with schizophrenia treated with clozapine. J Psychopharmacol. 2008;22(2):203–209. doi: 10.1177/0269881107084002. [DOI] [PubMed] [Google Scholar]
- Dawson N, Morris BJ, Pratt JA. Subanaesthetic ketamine treatment alters prefrontal cortex connectivity with thalamus and ascending subcortical systems. Schizophr Bull. 2013;39(2):366–377. doi: 10.1093/schbul/sbr144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Destexhe A, Contreras D, Sejnowski TJ, Steriade M. A model of spindle rhythmicity in the isolated thalamic reticular nucleus. J Neurophysiol. 1994;72(2):803–818. doi: 10.1152/jn.1994.72.2.803. [DOI] [PubMed] [Google Scholar]
- Eschenko O, Molle M, Born J, Sara SJ. Elevated sleep spindle density after learning or after retrieval in rats. J Neurosci. 2006;26(50):12914–12920. doi: 10.1523/JNEUROSCI.3175-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrarelli F. Sleep in patients with schizophrenia. Curr Sleep Med Rep. 2015;1(2):150–156. doi: 10.1007/s40675-015-0010-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrarelli F, Huber R, Peterson MJ, Massimini M, Murphy M, Riedner BA, Watson A, Bria P, Tononi G. Reduced sleep spindle activity in schizophrenia patients. Am J Psychiatry. 2007;164(3):483–492. doi: 10.1176/ajp.2007.164.3.483. [DOI] [PubMed] [Google Scholar]
- Ferrarelli F, Peterson MJ, Sarasso S, Riedner BA, Murphy MJ, Benca RM, Bria P, Kalin NH, Tononi G. Thalamic dysfunction in schizophrenia suggested by whole-night deficits in slow and fast spindles. Am J Psychiatry. 2010;167(11):1339–1348. doi: 10.1176/appi.ajp.2010.09121731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrarelli F, Tononi G. The thalamic reticular nucleus and schizophrenia. Schizophr Bull. 2011;37(2):306–315. doi: 10.1093/schbul/sbq142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer S, Nitschke MF, Melchert UH, Erdmann C, Born J. Motor memory consolidation in sleep shapes more effective neuronal representations. J Neurosci. 2005;25(49):11248–11255. doi: 10.1523/JNEUROSCI.1743-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogel SM, Smith CT. Learning-dependent changes in sleep spindles and Stage 2 sleep. J Sleep Res. 2006;15(3):250–255. doi: 10.1111/j.1365-2869.2006.00522.x. [DOI] [PubMed] [Google Scholar]
- Fogel SM, Smith CT. The function of the sleep spindle: a physiological index of intelligence and a mechanism for sleep-dependent memory consolidation. Neurosci Biobehav Rev. 2011;35(5):1154–1165. doi: 10.1016/j.neubiorev.2010.12.003. [DOI] [PubMed] [Google Scholar]
- Fuentealba P, Steriade M. The reticular nucleus revisited: intrinsic and network properties of a thalamic pacemaker. Prog Neurobiol. 2005;75(2):125–141. doi: 10.1016/j.pneurobio.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Gais S, Molle M, Helms K, Born J. Learning-dependent increases in sleep spindle density. J Neurosci. 2002;22(15):6830–6834. doi: 10.1523/JNEUROSCI.22-15-06830.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert AR, Rosenberg DR, Harenski K, Spencer S, Sweeney JA, Keshavan MS. Thalamic volumes in patients with first-episode schizophrenia. Am J Psychiatry. 2001;158(4):618–624. doi: 10.1176/appi.ajp.158.4.618. [DOI] [PubMed] [Google Scholar]
- Goder R, Aldenhoff JB, Boigs M, Braun S, Koch J, Fritzer G. Delta power in sleep in relation to neuropsychological performance in healthy subjects and schizophrenia patients. J Neuropsychiatry Clin Neurosci. 2006;18(4):529–535. doi: 10.1176/jnp.2006.18.4.529. [DOI] [PubMed] [Google Scholar]
- Goder R, Baier PC, Beith B, Baecker C, Seeck-Hirschner M, Junghanns K, Marshall L. Effects of transcranial direct current stimulation during sleep on memory performance in patients with schizophrenia. Schizophr Res. 2013;144(1–3):153–154. doi: 10.1016/j.schres.2012.12.014. [DOI] [PubMed] [Google Scholar]
- Goder R, Graf A, Ballhausen F, Weinhold S, Baier PC, Junghanns K, Prehn-Kristensen A. Impairment of sleep-related memory consolidation in schizophrenia: relevance of sleep spindles? Sleep Med. 2015;16(5):564–569. doi: 10.1016/j.sleep.2014.12.022. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Porrino LJ. The primate mediodorsal (MD) nucleus and its projection to the frontal lobe. J Comp Neurol. 1985;242(4):535–560. doi: 10.1002/cne.902420406. [DOI] [PubMed] [Google Scholar]
- Golomb D, Wang XJ, Rinzel J. Synchronization properties of spindle oscillations in a thalamic reticular nucleus model. J Neurophysiol. 1994;72(3):1109–1126. doi: 10.1152/jn.1994.72.3.1109. [DOI] [PubMed] [Google Scholar]
- Hajos M, Hoffmann WE, Kocsis B. Activation of cannabinoid-1 receptors disrupts sensory gating and neuronal oscillation: relevance to schizophrenia. Biol Psychiatry. 2008;63(11):1075–1083. doi: 10.1016/j.biopsych.2007.12.005. [DOI] [PubMed] [Google Scholar]
- Halassa MM, Chen Z, Wimmer RD, Brunetti PM, Zhao S, Zikopoulos B, Wang F, Brown EN, Wilson MA. State-dependent architecture of thalamic reticular subnetworks. Cell. 2014;158(4):808–821. doi: 10.1016/j.cell.2014.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halassa MM, Siegle JH, Ritt JT, Ting JT, Feng G, Moore CI. Selective optical drive of thalamic reticular nucleus generates thalamic bursts and cortical spindles. Nat Neurosci. 2011;14(9):1118–1120. doi: 10.1038/nn.2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiatt JF, Floyd TC, Katz PH, Feinberg I. Further evidence of abnormal non-rapid-eye-movement sleep in schizophrenia. Arch Gen Psychiatry. 1985;42(8):797–802. doi: 10.1001/archpsyc.1985.01790310059007. [DOI] [PubMed] [Google Scholar]
- Hoffmann R, Hendrickse W, Rush AJ, Armitage R. Slow-wave activity during non-REM sleep in men with schizophrenia and major depressive disorders. Psychiatry Res. 2000;95(3):215–225. doi: 10.1016/s0165-1781(00)00181-5. [DOI] [PubMed] [Google Scholar]
- Huber R, Born J. Sleep, synaptic connectivity, and hippocampal memory during early development. Trends Cogn Sci. 2014;18(3):141–152. doi: 10.1016/j.tics.2013.12.005. [DOI] [PubMed] [Google Scholar]
- Hudson JI, Lipinski JF, Keck PE, Jr, Aizley HG, Vuckovic A, Zierk KC, Pope HG., Jr Polysomnographic characteristics of schizophrenia in comparison with mania and depression. Biol Psychiatry. 1993;34(3):191–193. doi: 10.1016/0006-3223(93)90391-p. [DOI] [PubMed] [Google Scholar]
- Huguenard JR, McCormick DA. Thalamic synchrony and dynamic regulation of global forebrain oscillations. Trends Neurosci. 2007;30(7):350–356. doi: 10.1016/j.tins.2007.05.007. [DOI] [PubMed] [Google Scholar]
- Jardemark K, Marcus MM, Shahid M, Svensson TH. Effects of asenapine on prefrontal N-methyl-D-aspartate receptor-mediated transmission: involvement of dopamine D1 receptors. Synapse. 2010;64(11):870–874. doi: 10.1002/syn.20803. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Freedman R. Sensory processing dysfunction in the personal experience and neuronal machinery of schizophrenia. Am J Psychiatry. 2015;172(1):17–31. doi: 10.1176/appi.ajp.2014.13121691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia F, Goldstein PA, Harrison NL. The modulation of synaptic GABA(A) receptors in the thalamus by eszopiclone and zolpidem. J Pharmacol Exp Ther. 2009;328(3):1000–1006. doi: 10.1124/jpet.108.146084. [DOI] [PubMed] [Google Scholar]
- Jones EG. Thalamic organization and function after Cajal. Prog Brain Res. 2002;136:333–357. doi: 10.1016/s0079-6123(02)36029-1. [DOI] [PubMed] [Google Scholar]
- Jones EG. Synchrony in the interconnected circuitry of the thalamus and cerebral cortex. Ann N Y Acad Sci. 2009;1157:10–23. doi: 10.1111/j.1749-6632.2009.04534.x. [DOI] [PubMed] [Google Scholar]
- Kaster TS, de Jesus D, Radhu N, Farzan F, Blumberger DM, Rajji TK, Fitzgerald PB, Daskalakis ZJ. Clozapine potentiation of GABA mediated cortical inhibition in treatment resistant schizophrenia. Schizophr Res. 2015;165(2–3):157–162. doi: 10.1016/j.schres.2015.04.015. [DOI] [PubMed] [Google Scholar]
- Keshavan MS, Reynolds CF, 3rd, Miewald MJ, Montrose DM, Sweeney JA, Vasko RC, Jr, Kupfer DJ. Delta sleep deficits in schizophrenia: evidence from automated analyses of sleep data. Arch Gen Psychiatry. 1998;55(5):443–448. doi: 10.1001/archpsyc.55.5.443. [DOI] [PubMed] [Google Scholar]
- Keshavan MS, Reynolds CF, Kupfer DJ. Electroencephalographic sleep in schizophrenia: a critical review. Compr Psychiatry. 1990;31(1):34–47. doi: 10.1016/0010-440x(90)90052-t. [DOI] [PubMed] [Google Scholar]
- Krause M, Hoffmann WE, Hajos M. Auditory sensory gating in hippocampus and reticular thalamic neurons in anesthetized rats. Biol Psychiatry. 2003;53(3):244–253. doi: 10.1016/s0006-3223(02)01463-4. [DOI] [PubMed] [Google Scholar]
- Kreczmanski P, Heinsen H, Mantua V, Woltersdorf F, Masson T, Ulfig N, Schmidt-Kastner R, Korr H, Steinbusch HW, Hof PR, Schmitz C. Volume, neuron density and total neuron number in five subcortical regions in schizophrenia. Brain. 2007;130(Pt 3):678–692. doi: 10.1093/brain/awl386. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6(4):312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- Lustenberger C, Wehrle F, Tushaus L, Achermann P, Huber R. The Multidimensional Aspects of Sleep Spindles and Their Relationship to Word-Pair Memory Consolidation. Sleep. 2015;38(7):1093–1103. doi: 10.5665/sleep.4820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoach DS, Cain MS, Vangel MG, Khurana A, Goff DC, Stickgold R. A failure of sleep-dependent procedural learning in chronic, medicated schizophrenia. Biol Psychiatry. 2004;56(12):951–956. doi: 10.1016/j.biopsych.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Manoach DS, Demanuele C, Wamsley EJ, Vangel M, Montrose DM, Miewald J, Kupfer D, Buysse D, Stickgold R, Keshavan MS. Sleep spindle deficits in antipsychotic-naive early course schizophrenia and in non-psychotic first-degree relatives. Front Hum Neurosci. 2014;8:762. doi: 10.3389/fnhum.2014.00762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoach DS, Thakkar KN, Stroynowski E, Ely A, McKinley SK, Wamsley E, Djonlagic I, Vangel MG, Goff DC, Stickgold R. Reduced overnight consolidation of procedural learning in chronic medicated schizophrenia is related to specific sleep stages. J Psychiatr Res. 2010;44(2):112–120. doi: 10.1016/j.jpsychires.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAlonan K, Cavanaugh J, Wurtz RH. Attentional modulation of thalamic reticular neurons. J Neurosci. 2006;26(16):4444–4450. doi: 10.1523/JNEUROSCI.5602-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell AS. The mediodorsal thalamus as a higher order thalamic relay nucleus important for learning and decision-making. Neurosci Biobehav Rev. 2015;54:76–88. doi: 10.1016/j.neubiorev.2015.03.001. [DOI] [PubMed] [Google Scholar]
- Molle M, Born J. Slow oscillations orchestrating fast oscillations and memory consolidation. Prog Brain Res. 2011;193:93–110. doi: 10.1016/B978-0-444-53839-0.00007-7. [DOI] [PubMed] [Google Scholar]
- Morin A, Doyon J, Dostie V, Barakat M, Hadj Tahar A, Korman M, Benali H, Karni A, Ungerleider LG, Carrier J. Motor sequence learning increases sleep spindles and fast frequencies in post-training sleep. Sleep. 2008;31(8):1149–1156. [PMC free article] [PubMed] [Google Scholar]
- Nishida M, Walker MP. Daytime naps, motor memory consolidation and regionally specific sleep spindles. PLoS One. 2007;2(4):e341. doi: 10.1371/journal.pone.0000341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pakkenberg B, Scheel-Kruger J, Kristiansen LV. Schizophrenia; from structure to function with special focus on the mediodorsal thalamic prefrontal loop. Acta Psychiatr Scand. 2009;120(5):345–354. doi: 10.1111/j.1600-0447.2009.01447.x. [DOI] [PubMed] [Google Scholar]
- Pinault D. The thalamic reticular nucleus: structure, function and concept. Brain Res Brain Res Rev. 2004;46(1):1–31. doi: 10.1016/j.brainresrev.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Poulin J, Daoust AM, Forest G, Stip E, Godbout R. Sleep architecture and its clinical correlates in first episode and neuroleptic-naive patients with schizophrenia. Schizophr Res. 2003;62(1–2):147–153. doi: 10.1016/s0920-9964(02)00346-8. [DOI] [PubMed] [Google Scholar]
- Pratt JA, Morris BJ. The thalamic reticular nucleus: a functional hub for thalamocortical network dysfunction in schizophrenia and a target for drug discovery. J Psychopharmacol. 2015;29(2):127–137. doi: 10.1177/0269881114565805. [DOI] [PubMed] [Google Scholar]
- Reeve S, Sheaves B, Freeman D. The role of sleep dysfunction in the occurrence of delusions and hallucinations: A systematic review. Clin Psychol Rev. 2015;42:96–115. doi: 10.1016/j.cpr.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salgado-Pineda P, Baeza I, Perez-Gomez M, Vendrell P, Junque C, Bargallo N, Bernardo M. Sustained attention impairment correlates to gray matter decreases in first episode neuroleptic-naive schizophrenic patients. Neuroimage. 2003;19(2 Pt 1):365–375. doi: 10.1016/s1053-8119(03)00094-6. [DOI] [PubMed] [Google Scholar]
- Santana N, Troyano-Rodriguez E, Mengod G, Celada P, Artigas F. Activation of thalamocortical networks by the N-methyl-D-aspartate receptor antagonist phencyclidine: reversal by clozapine. Biol Psychiatry. 2011;69(10):918–927. doi: 10.1016/j.biopsych.2010.10.030. [DOI] [PubMed] [Google Scholar]
- Schabus M, Gruber G, Parapatics S, Sauter C, Klosch G, Anderer P, Klimesch W, Saletu B, Zeitlhofer J. Sleep spindles and their significance for declarative memory consolidation. Sleep. 2004;27(8):1479–1485. doi: 10.1093/sleep/27.7.1479. [DOI] [PubMed] [Google Scholar]
- Schabus M, Hoedlmoser K, Pecherstorfer T, Anderer P, Gruber G, Parapatics S, Sauter C, Kloesch G, Klimesch W, Saletu B, Zeitlhofer J. Interindividual sleep spindle differences and their relation to learning-related enhancements. Brain Res. 2008;1191:127–135. doi: 10.1016/j.brainres.2007.10.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt C, Peigneux P, Muto V, Schenkel M, Knoblauch V, Munch M, de Quervain DJ, Wirz-Justice A, Cajochen C. Encoding difficulty promotes postlearning changes in sleep spindle activity during napping. J Neurosci. 2006;26(35):8976–8982. doi: 10.1523/JNEUROSCI.2464-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeck-Hirschner M, Baier PC, Sever S, Buschbacher A, Aldenhoff JB, Goder R. Effects of daytime naps on procedural and declarative memory in patients with schizophrenia. J Psychiatr Res. 2010;44(1):42–47. doi: 10.1016/j.jpsychires.2009.05.008. [DOI] [PubMed] [Google Scholar]
- Sheroziya M, Timofeev I. Global intracellular slow-wave dynamics of the thalamocortical system. J Neurosci. 2014;34(26):8875–8893. doi: 10.1523/JNEUROSCI.4460-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprecher KE, Ferrarelli F, Benca RM. Sleep and plasticity in schizophrenia. Curr Top Behav Neurosci. 2015;25:433–458. doi: 10.1007/7854_2014_366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan KE, Friston KJ, Frith CD. Dysconnection in schizophrenia: from abnormal synaptic plasticity to failures of self-monitoring. Schizophr Bull. 2009;35(3):509–527. doi: 10.1093/schbul/sbn176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M. Grouping of brain rhythms in corticothalamic systems. Neuroscience. 2006;137(4):1087–1106. doi: 10.1016/j.neuroscience.2005.10.029. [DOI] [PubMed] [Google Scholar]
- Sun YG, Wu CS, Renger JJ, Uebele VN, Lu HC, Beierlein M. GABAergic synaptic transmission triggers action potentials in thalamic reticular nucleus neurons. J Neurosci. 2012;32(23):7782–7790. doi: 10.1523/JNEUROSCI.0839-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamaki M, Huang TR, Yotsumoto Y, Hamalainen M, Lin FH, Nanez JE, Sr, Watanabe T, Sasaki Y. Enhanced spontaneous oscillations in the supplementary motor area are associated with sleep-dependent offline learning of finger-tapping motor-sequence task. J Neurosci. 2013;33(34):13894–13902. doi: 10.1523/JNEUROSCI.1198-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamaki M, Matsuoka T, Nittono H, Hori T. Fast sleep spindle (13–15 hz) activity correlates with sleep-dependent improvement in visuomotor performance. Sleep. 2008;31(2):204–211. doi: 10.1093/sleep/31.2.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tandon R, Shipley JE, Taylor S, Greden JF, Eiser A, DeQuardo J, Goodson J. Electroencephalographic sleep abnormalities in schizophrenia. Relationship to positive/negative symptoms and prior neuroleptic treatment. Arch Gen Psychiatry. 1992;49(3):185–194. doi: 10.1001/archpsyc.1992.01820030017003. [DOI] [PubMed] [Google Scholar]
- Taylor SF, Tso IF. GABA abnormalities in schizophrenia: A methodological review of in vivo studies. Schizophr Res. 2015;167(1–3):84–90. doi: 10.1016/j.schres.2014.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tek C, Palmese LB, Krystal AD, Srihari VH, DeGeorge PC, Reutenauer EL, Guloksuz S. The impact of eszopiclone on sleep and cognition in patients with schizophrenia and insomnia: a doubleblind, randomized, placebo-controlled trial. Schizophr Res. 2014;160(1–3):180–185. doi: 10.1016/j.schres.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tekell JL, Hoffmann R, Hendrickse W, Greene RW, Rush AJ, Armitage R. High frequency EEG activity during sleep: characteristics in schizophrenia and depression. Clin EEG Neurosci. 2005;36(1):25–35. doi: 10.1177/155005940503600107. [DOI] [PubMed] [Google Scholar]
- Tesler N, Gerstenberg M, Franscini M, Jenni OG, Walitza S, Huber R. Reduced sleep spindle density in early onset schizophrenia: a preliminary finding. Schizophr Res. 2015;166(1–3):355–357. doi: 10.1016/j.schres.2015.04.042. [DOI] [PubMed] [Google Scholar]
- Troyano-Rodriguez E, Llado-Pelfort L, Santana N, Teruel-Marti V, Celada P, Artigas F. Phencyclidine inhibits the activity of thalamic reticular gamma-aminobutyric acidergic neurons in rat brain. Biol Psychiatry. 2014;76(12):937–945. doi: 10.1016/j.biopsych.2014.05.019. [DOI] [PubMed] [Google Scholar]
- Van Cauter E, Linkowski P, Kerkhofs M, Hubain P, L'Hermite-Baleriaux M, Leclercq R, Brasseur M, Copinschi G, Mendlewicz J. Circadian and sleep-related endocrine rhythms in schizophrenia. Arch Gen Psychiatry. 1991;48(4):348–356. doi: 10.1001/archpsyc.1991.01810280064009. [DOI] [PubMed] [Google Scholar]
- von Krosigk M, Bal T, McCormick DA. Cellular mechanisms of a synchronized oscillation in the thalamus. Science. 1993;261(5119):361–364. doi: 10.1126/science.8392750. [DOI] [PubMed] [Google Scholar]
- Walker MP, Brakefield T, Seidman J, Morgan A, Hobson JA, Stickgold R. Sleep and the time course of motor skill learning. Learn Mem. 2003;10(4):275–284. doi: 10.1101/lm.58503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wamsley EJ, Shinn AK, Tucker MA, Ono KE, McKinley SK, Ely AV, Goff DC, Stickgold R, Manoach DS. The effects of eszopiclone on sleep spindles and memory consolidation in schizophrenia: a randomized placebo-controlled trial. Sleep. 2013;36(9):1369–1376. doi: 10.5665/sleep.2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wamsley EJ, Tucker MA, Shinn AK, Ono KE, McKinley SK, Ely AV, Goff DC, Stickgold R, Manoach DS. Reduced sleep spindles and spindle coherence in schizophrenia: mechanisms of impaired memory consolidation? Biol Psychiatry. 2012;71(2):154–161. doi: 10.1016/j.biopsych.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XJ, Rinzel J. Spindle rhythmicity in the reticularis thalami nucleus: synchronization among mutually inhibitory neurons. Neuroscience. 1993;53(4):899–904. doi: 10.1016/0306-4522(93)90474-t. [DOI] [PubMed] [Google Scholar]
- Welsh RC, Chen AC, Taylor SF. Low-frequency BOLD fluctuations demonstrate altered thalamocortical connectivity in schizophrenia. Schizophr Bull. 2010;36(4):713–722. doi: 10.1093/schbul/sbn145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimmer RD, Schmitt LI, Davidson TJ, Nakajima M, Deisseroth K, Halassa MM. Thalamic control of sensory selection in divided attention. Nature. 2015;526(7575):705–709. doi: 10.1038/nature15398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward ND, Heckers S. Mapping Thalamocortical Functional Connectivity in Chronic and Early Stages of Psychotic Disorders. Biol Psychiatry. 2015 doi: 10.1016/j.biopsych.2015.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward ND, Karbasforoushan H, Heckers S. Thalamocortical dysconnectivity in schizophrenia. Am J Psychiatry. 2012;169(10):1092–1099. doi: 10.1176/appi.ajp.2012.12010056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young KA, Manaye KF, Liang C, Hicks PB, German DC. Reduced number of mediodorsal and anterior thalamic neurons in schizophrenia. Biol Psychiatry. 2000;47(11):944–953. doi: 10.1016/s0006-3223(00)00826-x. [DOI] [PubMed] [Google Scholar]