Abstract
Background
Reforms to the legal status of medical and non-medical cannabis are underway in many jurisdictions, including Canada, as are renewed efforts to scale-up HIV treatment-as-prevention (TasP) initiatives. It has been suggested that high-intensity cannabis use may be associated with sub-optimal HIV treatment outcomes. Thus, using data from a setting with a community-wide treatment-as-prevention (TasP) initiative coinciding with increasing access to medical cannabis, we sought to investigate the possible impact of high-intensity cannabis use on HIV clinical outcomes.
Methods
Data was derived from the ACCESS study, a prospective cohort of HIV-positive people who use illicit drugs (PWUD) in Vancouver, Canada. Cohort data was confidentially linked to comprehensive clinical profiles, including records of all antiretroviral therapy (ART) dispensations and longitudinal plasma HIV-1 RNA viral load (VL) monitoring. We used generalized estimating equations (GEEs) to estimate the longitudinal bivariable and multivariable relationships between at least daily cannabis use and two key clinical outcomes: overall engagement in ART care, and achieving a non-detectable VL among ART-exposed participants.
Results
Between December 2005 and June 2015, 874 HIV-positive PWUD (304 [35%] non-male) were included in this study. In total, 788 (90%) were engaged in HIV care at least once over the study period, of whom 670 (85%) achieved non-detectable VL at least once. In multivariable analyses, ≥ daily cannabis use did not predict lower odds of ART care (Adjusted Odds Ratio [AOR]: 1.02, 95% confidence interval [CI]: 0.77–1.36) or VL non-detectability among ART-exposed (AOR: 0.96, 95% CI: 0.75–1.21).
Conclusion
Our results showed no statistically significant impact of daily cannabis use on the likelihood of ART care or VL non-detectability among ART-exposed HIV-positive PWUD. These findings are reassuring in light of the impending legalization of cannabis in Canada and ongoing efforts to expand TasP initiatives.
Keywords: Cannabis, Cannabis Legalization, Human Immunodeficiency Virus, Treatment as Prevention, Antiretroviral Therapy, People who Use Illicit Drugs
INTRODUCTION
The health and policy implications of cannabis use are an emerging priority in clinical and public health research, as laws surrounding both medical and non-medical use continue to be reformed. Specifically, regulatory regimes to allow legal access to medicinal cannabis have been enacted across Canada (“Marihuana for Medical Purposes Regulations, SOR/2013-119,” 2012) and in 23 US states (National Conference of State Legislatures, 2016). More recently, laws prohibiting non-medicinal cannabis use have been repealed and systems of legal production, distribution, and sale have been enacted in some US states, including Washington, Oregon, Colorado, and Alaska (National Conference of State Legislatures, 2016). In Canada, the newly-elected federal government has committed to “legalizing and restricting access” to non-medical cannabis (Office of the Prime Minister, 2015). Although it is estimated that over half of the populations in the United States (Ingraham, 2015) and Canada (de Grandpré, 2015) support cannabis legalization, these changes raise a host of potential public health issues. One consideration is the potential impact of cannabis use on other large-scale public health interventions, such as HIV treatment-as-prevention (TasP).
A new cornerstone of the global strategy to control the HIV pandemic (UNAIDS, 2014), TasP seeks to scale-up access to antiretroviral therapy (ART) among all individuals living with HIV infection, including members of typically harder-to-reach groups, such as people who use illicit drugs (PWUD) (World Health Organization, 2012), in order to simultaneously curb HIV/AIDS-associated morbidity, mortality and viral transmission. Although this approach has been proven effective through population-level analyses (Montaner et al., 2010; Montaner et al., 2014) and a multicenter randomized controlled trial (Cohen et al., 2011), its effect on PWUD has not been completely evaluated. In particular, HIV-positive PWUD face numerous behavioural, social and structural barriers to optimal engagement in health care (Lucas et al., 2001; Wood et al., 2003), often as a result of stigma, discrimination, marginalization, lack of licit employment opportunities, and criminalization (Milloy et al., 2012; Richardson et al., 2015; Small et al., 2009; Westergaard et al., 2011; Wood et al., 2003).
Previous studies indicate that approximately 15–40% of people living with HIV/AIDS use cannabis to treat symptoms of the disease and side effects of its treatment (Belle-Isle, 2006; Braitstein et al., 2001; Fogarty et al., 2007; Furler et al., 2004; Prentiss et al., 2004; Woolridge et al., 2005). However, more recent research suggests that high-intensity cannabis use may be linked to poorer HIV treatment outcomes, including exhibiting a detectable viral load (Bonn-Miller et al., 2014). Emerging research also suggests that some PWUD may use cannabis to substitute for higher-risk drug use patterns, including opioid use (Kral et al., 2015; Lau et al., 2015; Lucas et al., 2013; Lucas et al., 2015). In this sense, cannabis use may prove beneficial for TasP efforts among PWUD, given the known association between illicit opioid use with reduced likelihood of adherence to ART (Azar et al., 2015; Jeevanjee et al., 2014; Rosen et al., 2013; Shannon et al., 2005), and subsequent viral treatment failure (Nolan et al., 2011).
Vancouver, British Columbia, Canada was the setting of an explosive HIV outbreak among PWUD in the mid-1990s (Tyndall et al., 2003). This led to the establishment of a variety of innovative harm reduction programs, including needle and syringe distribution (Werb et al., 2013), supervised injection (Wood et al., 2004), and opioid substitution programs (Nosyk et al., 2015). In 2006, the Vancouver Police Department announced a de facto policy of cannabis use decriminalization in an effort to shift focus towards illicit drug manufacturing and distribution rather than personal use (The Vancouver Province, 2006). Coinciding with the HIV/AIDS outbreak, Canada’s first medical cannabis dispensary opened its doors in Vancouver in 1997, setting a local culture of tolerance for medical cannabis dispensaries (City of Vancouver, 2015). Most recently, the proliferation of over 100 retail dispensaries since 2013 has prompted Vancouver’s municipal government to develop a regulatory framework for these businesses (City of Vancouver, 2016). Over the last decade, Vancouver has also been the focus of a province-wide TasP effort (Montaner et al., 2010; Montaner et al., 2014). Thus, in light of high levels of access to medical cannabis in Vancouver, we conducted the present study to characterize the impact of high intensity cannabis use on engagement in HIV/AIDS care and achieving viral suppression among HIV-positive PWUD.
METHODS
Study Sample
Data for this study was derived from the AIDS Care Cohort to evaluate Exposure to Survival Services (ACCESS), an ongoing observational prospective cohort of HIV-positive PWUD. In brief, as previously described (Tyndall et al., 2003; Wood et al., 2009), participants are recruited through extensive street outreach from the Downtown Eastside (DTES) neighbourhood, an area with high levels of illicit drug use, poverty, and homelessness. Eligibility criteria include being aged 18 years or older, providing written informed consent, HIV-seropositivity through a positive blood test, and using an illicit drug (other than cannabis) in the 30 days prior to enrolment. Participants receive a $30 (CAD) honorarium for each study visit. The University of British Columbia/Providence Healthcare Research Ethics Board has provided ethical approval for this study.
At baseline and semi-annually, all participants complete an interviewer-administered questionnaire eliciting information on a range of lifetime and recent (i.e., previous six months) socio-demographic, environmental, behavioural, and health-related exposures and characteristics. At each interview visit, participants are examined by a study nurse and undergo blood testing for plasma HIV-1 RNA viral load (VL). Participants provide their personal health number (PHN), a personal identifier issued to all residents of the province of British Columbia for medical billing and tracking. Using each participant’s PHN, study information is confidentially linked to the British Columbia Centre for Excellence in HIV/AIDS (BC-CfE) Drug Treatment Program (DTP). The DTP is a confidential data repository for HIV-related treatment and monitoring data, and holds all VL observations and ART dispensation records. Medical care, laboratory monitoring, and ART for all HIV-positive residents of BC are available through the province’s universal medical system at no cost to the patient, and without co-payments of deductibles.
Measures
This study included participants recruited from December 2005 to June 2015. We restricted to individuals with ≥1 VL observation within 360 days of their baseline interview. The first outcome of interest was engagement in ART care in the previous six months, defined as having been dispensed ART for ≥1 (vs. 0 days). Data for this measure was obtained through pharmacy refill records held by the DTP. The second outcome of interest was viral suppression (i.e., non-detectable VL) in the previous six months, defined as VL <50 copies/mL. We restricted analyses of VL to participants with ≥1 day recent ART dispensation. Consistent with previous work (Milloy et al., 2015b), if more than one VL observation was collected within a six-month follow-up, the mean of all VL observations was used. If no observations were available within a six-month follow-up, we assumed a detectable VL unless the participant was on ART and pharmacy records indicated they had filled a prescription for >95% of all days, as confirmed with pharmacy refill data available through the DTP. All VL measurements were determined with the Roche Amplicor Monitoring assay (Roche Molecular Systems, Pleasanton, CA, U.S.A.).
At each follow-up, participants were asked to indicate which (if any) non-injection drugs they had used in the previous six months, and the average frequency with which they used each drug (where applicable). The primary independent variable of interest was high-intensity cannabis use, defined as self-reported ≥ daily cannabis in the previous six months (vs. < daily cannabis use). We included other factors hypothesized to confound the relationship between cannabis use and the outcome variables. These variables included age at time of interview; gender (male vs. non-male); ancestry (Caucasian vs. non-Caucasian); unstable housing (defined as living in a single room occupancy hotel, shelter or other transitional housing, or living on the street (Zivanovic et al., 2015); yes vs. no); employment (yes vs. no); alternative income generation (defined as engaging in any of the following income-generating activities: sex work, informal recycling, squeegeeing [i.e., window-washing], panhandling, selling drugs, theft, robbery or stealing (Richardson et al., 2015), yes vs. no); incarceration (yes vs. no); addiction treatment (yes vs. no); injection drug use (yes vs. no); binge drug use (yes vs. no); and binge alcohol use (yes vs. no). To account for disease progression, we also included CD4+ cell count (per 100 cells/mL plasma). We determined CD4+ cell count in the same manner as VL, but if data was not available for any six-month follow-up period, we used the most recent observation. Similar to both outcome measures, all non-fixed variables are time-updated and refer to exposures or behaviours in the six-month period prior to the interview.
Analysis
First, we used Pearson’s χ-square (for categorical variables) and Wilcoxon rank-sum (for continuous variables) to compare baseline characteristics of the sample, stratified by cannabis use (≥ daily vs. < daily). Then, we investigated the longitudinal bivariable and multivariable relationship between daily cannabis use and each outcome using generalized estimating equations (GEEs). This approach uses repeated measures to identify factors potentially associated with a time-updated binary outcome (Lee et al., 2007). A GEE model was chosen for its ability to estimate the within- and between-subject correlation of each independent variable with the outcome of interest, as participants may report daily cannabis use and/or achieve a treatment outcome of interest during some biannual follow-up periods but not others. This method uses an exchangeable correlation structure to provide standard errors for each parameter, adjusted for multiple observations per person. We used this approach to build two bivariable models to examine the relationship between daily cannabis use and each outcome of interest. To build each multivariable model, we included all variables that were significant at p<0.10 in bivariable analysis into each of the full models, and used a stepwise approach to fit a series of reduced models. For each outcome model, we compared the coefficient value for the main independent variable (daily cannabis use) and dropped the secondary variable associated with the smallest relative change. We continued this iterative process until the minimum change exceeded 5%. We also examined potential interaction between cannabis use (≥ daily vs. < daily) and other explanatory variables for both outcomes, dichotomizing continuous explanatory variables at the median. Data was analyzed with R (version 2.15.1, R Foundation for Statistical Computing, Vienna, Austria), and all p-values are two-sided.
RESULTS
Between December 2005 and June 2015, 875 HIV-seropositive PWUD were recruited into the study. Of these, 874 (99%) participants had a VL measurement during the study and were included in the analysis. In total, the sample included 570 (65.2%) males, and collectively contributed 7644 baseline and follow-up interviews. Participants had a median age of 42.8 (Interquartile Range [IQR]: 36.5–48.3) years at baseline, and contributed a median of 9 (IQR: 4–13) study interviews.
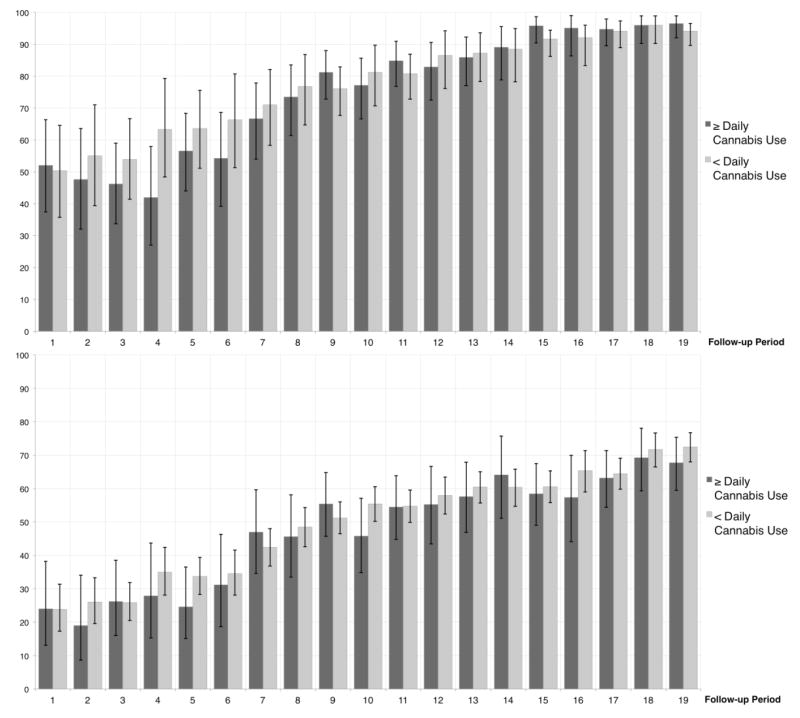
The baseline characteristics of the participants, stratified by daily cannabis use, are shown in Table 1. At baseline, 215 (24.6%) participants reported daily cannabis use in the previous six months, and the prevalence of recent cannabis use remained relatively high over the study period for both ≥ daily use (median: 20.6%, IQR: 19.6–21.5%) and any use (46.5%, IQR: 45.3–47.6%). At baseline, a total of 540 (61.8%) participants were engaged on ART care and 282 (32.2%) had an undetectable viral load. Over the study period, 788 (90.2%) participants were engaged in ART at least once, and, of these, 670 (85.0%) achieved viral suppression for at least one 180-day period. There was a clear increasing trend in the proportion of participants engaging in ART care as well as the proportion of ART-engaged participants achieving viral suppression over the over the study period (Figure 1).
TABLE 1.
BASELINE CHARACTERISTICS OF 874 HIV-POSITIVE PEOPLE WHO USE ILLICIT DRUGS, STRATIFIED BY DAILY CANNABIS USE IN THE PREVIOUS SIX MONTHS
| Characteristic | ≥Daily Cannabis Use | Odds Ratio (95% CI*) | p - value | |
|---|---|---|---|---|
| No 659 (75.4 %) |
Yes 215 (24.6%) |
|||
| Age€ | ||||
| Median (IQR) | 43.3 (36.7–49.0) | 41.4 (35.5–46.5) | 0.97 (0.96 – 0.99) | 0.005 |
| Gender | ||||
| Male | 405 (61.5) | 165 (76.7) | 1.00 | |
| Female | 254 (38.5) | 50 (23.3) | 0.48 (0.34 – 0.69) | < 0.001 |
| Ethnicity | < | |||
| Non-White | 302 (45.8) | 88 (40.9) | 1.00 | |
| White | 357 (54.2) | 127 (59.1) | 1.22 (0.89 – 1.67) | 0.210 |
| Unstable housing‡ | ||||
| No | 197 (29.9) | 76 (35.3) | 1.00 | |
| Yes | 462 (70.1) | 139 (64.7) | 0.78 (0.56 – 1.08) | 0.134 |
| Employment‡ | ||||
| No | 539 (81.8) | 179 (83.3) | 1.00 | |
| Yes | 120 (18.2) | 36 (16.7) | 0.90 (0.60 – 1.36) | 0.626 |
| Alternative income generation‡ | ||||
| No | 413 (62.7) | 131 (60.9) | 1.00 | |
| Yes | 246 (37.3) | 84 (39.1) | 1.08 (0.78 – 1.48) | 0.647 |
| Incarcerated‡ | ||||
| No | 568 (86.2) | 187 (87.0) | 1.00 | |
| Yes | 91 (13.8) | 28 (13.0) | 0.93 (0.59 – 1.47) | 0.771 |
| Addiction Treatment‡ | ||||
| No | 330 (50.1) | 122 (56.7) | 1.00 | |
| Yes | 329 (49.9) | 93 (43.3) | 0.76 (0.56 – 1.04) | 0.089 |
| Injection drug use‡ | ||||
| No | 124 (18.8) | 48 (22.3) | 1.00 | |
| Yes | 535 (81.2) | 167 (77.7) | 0.81 (0.55 – 1.17) | 0.261 |
| Binge drug use‡ | ||||
| No | 371 (56.3) | 126 (58.6) | 1.00 | |
| Yes | 288 (43.7) | 89 (41.4) | 0.91 (0.67 – 1.24) | 0.553 |
| Binge alcohol use‡ | ||||
| No | 510 (77.4) | 137 (63.7) | 1.00 | |
| Yes | 149 (22.6) | 78 (36.3) | 1.95 (1.40 – 2.71) | < 0.001 |
| ART dispensation‡ | ||||
| 0 days | 249 (37.8) | 85 (39.5) | 1.00 | |
| ≥1 days | 410 (62.2) | 130 (60.5) | 0.93 (0.68 – 1.27) | 0.647 |
| HIV-1 RNA viral load‡ | ||||
| ≥50 copies/mL | 446 (67.7) | 146 (67.9) | 1.00 | |
| < 50 copies/mL | 213 (32.3) | 69 (32.0) | 0.99 (0.71 – 1.38) | 0.950 |
| CD4 count (cells/mL)€‡ | ||||
| Median (IQR) | 3.5 (2.2–4.5) | 4.0 (2.2–5.2) | 1.09 (1.02 – 1.17) | 0.017 |
Note: Some columns may not add up to 100%, as participants may choose not to answer sensitive questions
95% CI = 95% Confidence Interval
IQR: Interquartile Range
Denotes events in the previous six months
Wilcoxon rank sum test used for continuous variables
Figure 1.
Top: Proportion (95% CI) of participants engaged in antiretroviral therapy care at each follow-up period, stratified by ≥daily cannabis use. Bottom: Proportion (95% CI) of antiretroviral therapy-exposed participants reaching viral suppression at each follow-up period, stratified by ≥daily cannabis use.
Table 2 provides the bivariable and multivariable estimates of the relationship between periods of daily cannabis use and periods of ART engagement and VL suppression. As shown, in an analysis adjusted for potential confounders, daily cannabis use was not significantly associated with ART engagement (Adjusted Odds Ratio [AOR]: 1.02, 95% Confidence Interval [95% CI]: 0.77 – 1.36) or VL suppression among ART-exposed (AOR: 0.96, 95% CI: 0.75 – 1.21).
TABLE 2.
BIVARIABLE AND MULTIVARIABLE GENERALIZED ESTIMATING EQUATION MODELS OF FACTORS ASSOCIATED WITH RECENT ENGAGEMENT ON ANTIRETROVIRAL THERAPY (N = 874, LEFT), AND RECENT UNDETECTABLE PLASMA VIRAL LOAD (N = 788, RIGHT) BETWEEN DECEMBER 2005 AND JUNE 2015 AMONG HIV-POSITIVE PEOPLE WHO USE ILLICIT DRUGS IN VANCOUVER, CANADA
| Characteristic | Odds Ratio (95% CI§) | |||
|---|---|---|---|---|
| Model Series 1 | Model Series 2 | |||
| ART Engagement | Non-detectable VL | |||
| Unadjusted | Adjusted | Unadjusted | Adjusted | |
| Cannabis Use£ | ||||
| < Daily | 1.00 | 1.00 | 1.00 | 1.00 |
| ≥ Daily | 0.94 (0.78 – 1.12) | 1.02 (0.77 – 1.36) | 1.05 (0.89 – 1.24) | 0.96 (0.75 – 1.21) |
| Age | ||||
| Per year older | 1.17 (1.14 – 1.19)** | 1.08 (1.06 – 1.10)** | 1.09 (1.07 – 1.11)** | 1.04 (1.03 – 1.06)** |
| Gender | ||||
| Male | 1.00 | 1.00 | 1.00 | 1.00 |
| Female | 0.65 (0.51 – 0.83)* | 0.73 (0.54 – 1.00) | 0.75 (0.62 – 0.92)* | 0.78 (0.62 – 0.99)* |
| Ethnicity | ||||
| Non-Caucasian | 1.00 | - | 1.00 | - |
| Caucasian | 1.11 (0.87 – 1.41) | - | 1.17 (0.97 – 1.41) | - |
| Unstable housing£ | ||||
| No | 1.00 | 1.00 | 1.00 | - |
| Yes | 0.81 (0.70 – 0.94)* | 0.94 (0.73 – 1.20) | 0.82 (0.72 – 0.93)* | - |
| Employment£ | ||||
| No | 1.00 | - | 1.00 | - |
| Yes | 1.02 (0.87 – 1.20) | - | 1.06 (0.93 – 1.22) | - |
| Alternative income generation£ | ||||
| No | 1.00 | 1.00 | 1.00 | - |
| Yes | 0.58 (0.51 – 0.66)** | 0.82 (0.64 – 1.04) | 0.68 (0.60 – 0.78)** | - |
| Incarcerated£ | ||||
| No | 1.00 | 1.00 | 1.00 | - |
| Yes | 0.48 (0.39 – 0.60)** | 0.66 (0.50 – 0.88)* | 0.62 (0.50 – 0.76)** | - |
| Addiction treatment£ | ||||
| No | 1.00 | 1.00 | 1.00 | 1.00 |
| Yes | 1.98 (1.67 – 2.35)** | 2.28 (1.79 – 2.90)** | 1.38 (1.21 – 1.58)** | 1.66 (1.36 – 2.03)** |
| Injection drug use£ | ||||
| No | 1.00 | 1.00 | 1.00 | - |
| Yes | 0.61 (0.52 – 0.73)** | 0.71 (0.55 – 0.92)* | 0.64 (0.55 – 0.74)** | - |
| Binge drug use£ | ||||
| No | 1.00 | - | 1.00 | 1.00 |
| Yes | 0.81 (0.73 – 0.90)** | - | 0.79 (0.71 – 0.87)** | 0.79 (0.69 – 0.91)* |
| Binge alcohol use£ | ||||
| No | 1.00 | 1.00 | 1.00 | - |
| Yes | 0.88 (0.77 – 0.99)* | 1.01 (0.81 – 1.27) | 0.93 (0.82 – 1.04) | - |
| CD4 cell count£ | ||||
| Per 100 cells/mL plasma increase | 1.19 (1.11 – 1.26)** | - | 1.48 (1.40 – 1.57)** | 1.34 (1.27 – 1.42)** |
95% CI = 95% Confidence Interval
In the previous six months
p<0.05
p<0.001
To confirm that our findings were not a result of combining cannabis users who engaged in < daily use and cannabis non-users in the reference group, we built a three-level variable and separately examined the associations for ≥ daily use (vs. no use) and ≥ daily use (vs. no use). In these analyses, we did not detect a significant association between ≥ daily cannabis use and ART engagement (AOR: 1.02, 95% CI: 0.76 – 1.37) or VL suppression (AOR: 0.92, 95% CI: 0.72 – 1.18). Similarly, there was not a significant association between <daily use and ART engagement (AOR: 1.13, 95% CI: 0.90 – 1.42) or VL suppression (AOR: 0.88, 95% CI: 0.74 – 1.04). Upon examining potential interactions, a significant interaction was observed between cannabis use and binge alcohol use for ART engagement, where ≥ daily use was significantly negatively associated with ART engagement during periods of binge alcohol use (OR: 0.59, 95% CI: 0.43 – 0.80), but remained unassociated during periods of no binge alcohol use (OR: 1.16, 95% CI: 0.94 – 1.14). We did not identify an interaction between daily cannabis use and binge alcohol use for VL.
DISCUSSION
In our study, the first to our knowledge to examine the relationships between high-intensity cannabis use in the context of a community-wide TasP initiative, approximately half of participants reported any cannabis use at each six-month follow-up period. This proportion is approximately twice as high as previously recorded in general samples of people living with HIV/AIDS in other North American (de Jong et al., 2005; Prentiss et al., 2004; Rosen et al., 2013) and European (Garin et al., 2015) jurisdictions, where prevalence of any cannabis more closely approximates our presently recorded median 20% prevalence of daily use. However, our estimates closely resemble those recorded in a French cohort of patients co-infected with HIV and HCV (Marcellin et al., 2016). Despite the high prevalence of cannabis use in our setting, we did not find evidence to suggest that the likelihood of either engaging in ART or achieving VL non-detectability (among those on ART) was compromised during periods of high-intensity cannabis use. However, it should be noted that the odds of ART engagement among those who used cannabis ≥ daily differed significantly during periods of binge alcohol use, whereby ART engagement was less likely for daily cannabis users during periods of binge alcohol use.
In terms of HIV/AIDS-related outcomes, including VL, there appears to be little consensus with regard to whether cannabis use poses a significant threat. We identified two observational studies of patients in clinical settings that examined VL according to any cannabis use, which reached opposing conclusions about whether cannabis use is associated with increased (Ghosn et al., 2014) or decreased (Thames et al., 2016) VL. As the current study was particularly concerned with individuals who engage in at least daily cannabis use, we identified one randomized controlled trial (RCT) and two cross-sectional studies in which high frequency of cannabis use was shared across a study group. The first cross-sectional study examined VL among individuals who met DSM-IV criteria for cannabis dependence versus non-dependent cannabis users and non-users, and found that dependent users (who reported slightly more than daily use, on average) were significantly more likely to have a detectable VL, compared with the non-dependent users (who reported < daily use, on average), or the non-users (Bonn-Miller et al., 2014). Contrastingly, and more in line with the presently recorded findings, in the second cross-sectional study, which was conducted among a representative sample of patients on HIV treatment in the state of Florida, daily cannabis use was not significantly associated with viral suppression (Okafor et al., 2016). In the RCT, no significant differences were found in short-term change in mean VL between participants randomized to smoke cannabis (3.95% tetrahydrocannabinol daily), ingest dronobinol (2.5 mg daily), or ingest a placebo daily (Abrams et al., 2003). Inconsistencies across studies may be a result of variations in the relationship between cannabis use and ART adherence, both related to the above-described differences in cannabis use frequency/dependence categorizations as well as differences in the overall sample populations and confounding variables taken into account. Previously, using data from the ACCESS cohort we demonstrated that high-intensity cannabis use was not associated with a greater or lesser likelihood of optimal ART adherence (Slawson et al., 2014). While similar findings have been recorded in studies of more general HIV-positive samples (i.e., inclusive of both drug-using and non drug-using individuals) (Rosen et al., 2013), others have concluded adherence rates are significantly lower among cannabis users (Tucker et al., 2003; Wilson et al., 2004). In addition to cannabis influencing VL through this behavioural adherence pathway, there is emerging evidence for a potential additional biological pathway. In brief, Molina and colleagues, have shown that cannabinoid administration attenuates the progression of simian immunodeficiency virus infection (Molina et al., 2011), and we have previously found, within a subset of the current study population with incident HIV infections, that ART-naive individuals who used cannabis daily in the year after seroconversion had 0.51 log10 copies/mL lower plasma VL, on average, during this period (Milloy et al., 2015a).
An important characteristic that is unique to our study is a shared history of extensive poly-drug use (including opioids, stimulants, alcohol and tobacco), which is broadly known to correspond to poorer HIV treatment outcomes (Celentano & Lucas, 2007; Lucas et al., 2002). In light of emerging research in similar settings demonstrating the use of cannabis to curb the frequency of other illicit drug use, including opioid use (Kral et al., 2015; Lau et al., 2015; Lucas et al., 2013; Lucas et al., 2015), it is possible that some individuals engage in daily cannabis use as a means of reducing their use of other illicit drugs. If true, this shift in drug use pattern may be accompanied by a higher likelihood of engaging in ART care and adhering optimally to ART simply due to improved management of higher risk illicit drug use (Palepu et al., 2003; Rosen et al., 2013). However, this hypothesis remains to be tested. As well, some daily cannabis users in this group may be using the drug as a way to manage symptoms related to HIV/AIDS and/or ART (e.g., nausea (Carr & Cooper, 2000)). Cannabis use has long been common among people living with HIV/AIDS (Braitstein et al., 2001), and patients have reported benefitting from its use, particularly with regard to certain HIV/AIDS-related comorbidities and symptoms including reduced anxiety and depression, improved appetite, and reduced pain (Prentiss et al., 2004; Woolridge et al., 2005). In addition, patients on ART may experience negative side effects, including nausea and vomiting, for which cannabis may be beneficial (Woolridge et al., 2005). In a cross-sectional study from Northern California, de Jong and colleagues found that cannabis use was positively associated with optimal ART adherence among HIV-positive patients who experienced nausea, and negatively associated with optimal ART adherence among HIV-positive patients who did not report nausea (de Jong et al., 2005). Although this study focused on adherence rather than VL, their finding illustrates the behavioural and clinical heterogeneity of HIV-positive individuals who use cannabis, and the effect this likely has on associations with virologic response. Finally, we noted that ART engagement was less likely during periods of daily cannabis use and binge alcohol use, demonstrating that problematic alcohol use should not be overlooked in this population that contends with several other issues related to illicit drugs. Our finding may be explained in part by potential harms of simultaneous or concurrent use of cannabis with alcohol. When used together, impairment tends to increase more than either one alone (Bramness et al., 2010; Dubois et al., 2015), and simultaneous use has also been linked with poor social problems (e.g., interpersonal conflict) and health outcomes (e.g., depression) that may prevent engagement in the health care system (Midanik et al., 2007). However, likelihood of VL suppression among daily cannabis users was unaffected by binge alcohol use. This may reflect sample restrictions for the VL analysis, which excluded those who did not engage in HIV care – possibly consisting of binge drinkers with the most severe alcohol use disorders.
In the current study, frequent cannabis use was not statistically significantly associated with either sub-optimal engagement in ART care or detectable VL among HIV-positive PWUD, even in a setting where access to medical cannabis has scaled-up rapidly. These findings are notable given the impending legalization of non-medical cannabis in Canada. The findings also demonstrate that frequent cannabis use should not be considered a deterrent to ART prescribing among HIV-positive patients who currently use, or have a history of using, illicit drugs. These findings speak to the continued promise of ongoing HIV treatment-as-prevention strategies among PWUD as being potentially compatible with a framework for legalized cannabis within the British Columbia context. Future research will be necessary to understand the actual pathway(s) responsible for the associations here described and whether our findings hold in other HIV-positive populations. Considering the presently recorded high prevalence of moderate and high-intensity cannabis use among PWUD living with HIV/AIDS, our research brings up an additional consideration pertaining to safer modes of cannabis consumption. While the long-term pulmonary effects of cannabis smoking are not clear, short-term effects include symptoms of chronic bronchitis (Joshi et al., 2014), and previous research among PWUD (Shin et al., 2013) and the general population (Moore et al., 2005) shows that the majority of people who use cannabis also smoke tobacco. Although our findings are largely encouraging in terms of access and response to HIV care for cannabis users, there is a continued need to investigate potential strategies and policies aimed at minimizing the short- and long-term harms associated with inhalation of the toxic components of cannabis and cigarette smoke in this population. It should be noted that alternatives modes of consumption (e.g., ingestion) are increasingly becoming more commonplace in Vancouver as its cannabis industry continues to proliferate.
This study is subject to some limitations. First, findings should be interpreted in light of the fact that ACCESS is not a random sample. As a result, our findings may not generalize to PWUD in other settings. Second, while we used objective measures wherever possible, including the two outcomes of interest, we relied on self-report to obtain the main independent variable of interest. Misclassification may have occurred as a result, specifically in cases of underreporting related to recall or response biases. However, we do not anticipate that these reporting rates would be differentially distributed according to engagement in ART care or VL suppression. While generalized estimating equations are advantageous for longitudinally analyzing repeated measures over time, this method cannot account for temporality of event within each six-month period. As a result, causality cannot be inferred between the exposure and outcome. Recognizing that hepatitis C virus (HCV) co-infection is common among PWUD living with HIV (Kim et al., 2013), we were unable to address links between cannabis use and HCV treatment access in this sample, as newer direct acting antiviral-based treatment has yet to be scaled up in this setting. Finally, as is the case with all observational research, the current estimates are subject to bias from unmeasured confounding. Specifically, mental health conditions, including cannabis dependence, could not be assessed in the present study.
To conclude, PWUD constitute a key population for treatment scale-up as part of TasP efforts in many settings. In the current setting, which offers the opportunity to study the impact of cannabis use on HIV/AIDS-related outcomes under a community-wide HIV/AIDS TasP policy, as well as a rapidly expanding quasi-legal cannabis market, with the exception of co-use of frequent cannabis and binge alcohol, we did not find evidence to support the hypothesis that high intensity cannabis use compromises key individual-level goals of TasP. Our findings demonstrate that PWUD who engage in daily cannabis use are not at a significant disadvantage and should therefore not be excluded from ART scale-up efforts.
Acknowledgments
We would like to thank the participants of this study for their contribution to the research. We would also like to thank current and past researchers and staff, particularly Tricia Collingham, Carmen Rock, Kristie Starr, Lorena Mota, Deborah Graham, Peter Vann, Jennifer Matthews, and Steve Kain, for their administrative assistance. This study was supported by the US National Institutes of Health (R01-DA021525). Dr. Milloy is supported in part by the United States National Institutes of Health (R01-DA021525) and a Scholar Award from the Michael Smith Foundation for Health Research. This research was presented at the International Society for the Study of Drug Policy Regional Meeting of the Americas on Cannabis Policy, thanks in part to support from BOTEC Analysis and the NYU Marron Institute of Urban Management. None of the funding sources were involved in the study design, data collection, analysis, or interpretation.
Footnotes
Conflict of Interest Statement
Dr. Milloy’s institution has received unstructured support for his research from NG Biomed Ltd., a firm seeking to become a licensed producer of medical cannabis. Dr. Montaner has received grants from, served as an ad hoc adviser to, or spoken at events sponsored by Abbott, Argos Thera- peutics, Bioject Inc., Boehringer Ingelheim, BMS, Gilead Sciences, GlaxoSmithKline, Hoffmann-La Roche, Janssen-Ortho, Merck Frosst, Panacos, Pfizer Ltd., Schering, Serono Inc., TheraTechnologies, Tibotec (J&J), and Trimeris. All other authors declare that they have no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abrams DI, Hilton JF, Leiser RJ, Shade SB, Elbeik TA, Aweeka FT, et al. Short-term effects of cannabinoids in patients with HIV-1 infection: a randomized, placebo-controlled clinical trial. Annals of Internal Medicine. 2003;139:258–266. doi: 10.7326/0003-4819-139-4-200308190-00008. [DOI] [PubMed] [Google Scholar]
- Azar P, Wood E, Nguyen P, Luma M, Montaner J, Kerr T, et al. Drug use patterns associated with risk of non-adherence to antiretroviral therapy among HIV-positive illicit drug users in a Canadian setting: a longitudinal analysis. BMC Infectious Diseases. 2015;15:193. doi: 10.1186/s12879-015-0913-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belle-Isle L. Cannabis as Therapy for People Living with HIV/AIDS. Canadian AIDS Society; Ottawa: 2006. Retrieved from http://sagecollection.ca/en/resources/final-report-cannabis-therapy-people-living-hivaids-our-right-our-choice. [Google Scholar]
- Bonn-Miller M, Oser M, Bucossi M, Trafton J. Cannabis use and HIV antiretroviral therapy adherence and HIV-related symptoms. Journal of Behavioral Medicine. 2014;37:1–10. doi: 10.1007/s10865-012-9458-5. [DOI] [PubMed] [Google Scholar]
- Braitstein P, Kendall T, Chan K, Wood E, Montaner JSG, O’Shaughnessy MV, et al. Mary-Jane and her patients: Sociodemographic and clinical characteristics of HIV-positive individuals using medical marijuana and antiretroviral agents. AIDS. 2001;15:532–533. doi: 10.1097/00002030-200103090-00016. [DOI] [PubMed] [Google Scholar]
- Bramness JG, Khiabani HZ, Morland J. Impairment due to cannabis and ethanol: Clinical signs and additive effects. Addiction. 2010;105:1080–1087. doi: 10.1111/j.1360-0443.2010.02911.x. [DOI] [PubMed] [Google Scholar]
- Carr A, Cooper DA. Adverse effects of antiretroviral therapy. The Lancet. 2000;356:1423–1430. doi: 10.1016/S0140-6736(00)02854-3. http://dx.doi.org/10.1016/S0140-6736(00)02854-3. [DOI] [PubMed] [Google Scholar]
- Celentano DD, Lucas G. Optimizing treatment outcomes in HIV-infected patients with substance abuse issues. Clinical Infectious Diseases. 2007;45:S318–S323. doi: 10.1086/522557. [DOI] [PubMed] [Google Scholar]
- City of Vancouver. Regulation of Retail Dealers - Medical Marijuana-Related Uses. Vancouver: 2015. Retrieved from http://council.vancouver.ca/20150428/documents/rr1.pdf. [Google Scholar]
- City of Vancouver. Regulations for medical marijuana-related businesses. Retrieved May 13, 2016 from http://vancouver.ca/doing-business/medical-marjiuana-related-business-regulations.aspx.
- Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. Prevention of HIV-1 infection with early antiretroviral therapy. New England Journal of Medicine. 2011;365:493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Grandpré H. Deux Canadiens sur trois prêts à légaliser la marijuana [Two-thirds of Canadians support legalizing marijuana] [La Presse] Retrieved June 17, 2016 from http://www.lapresse.ca/actualites/201511/03/01-4917106-deux-canadiens-sur-trois-prets-a-legaliser-la-marijuana.php.
- de Jong BC, Prentiss D, McFarland W, Machekano R, Israelski DM. Marijuana use and its association with adherence to antiretroviral therapy among HIV-infected persons with moderate to severe nausea. Journal of Acquired Immune Deficiency Syndromes. 2005:38. doi: 10.1097/00126334-200501010-00008. [DOI] [PubMed] [Google Scholar]
- Dubois S, Mullen N, Weaver B, Bedard M. The combined effects of alcohol and cannabis on driving: Impact on crash risk. Forensic Science International. 2015;248:94–100. doi: 10.1016/j.forsciint.2014.12.018. [DOI] [PubMed] [Google Scholar]
- Fogarty A, Rawstorne P, Prestage G, Crawford J, Grierson J, Kippax S. Marijuana as therapy for people living with HIV/AIDS: Social and health aspects. AIDS Care. 2007;19:295–301. doi: 10.1080/09540120600841930. [DOI] [PubMed] [Google Scholar]
- Furler MD, Einarson TR, Millson M, Walmsley S, Bendayan R. Medicinal and recreational marijuana use by patients infected with HIV. AIDS Patient Care and STDs. 2004;18:215–228. doi: 10.1089/108729104323038892. [DOI] [PubMed] [Google Scholar]
- Garin N, Velasco C, De Pourcq JT, Lopez B, del Gutierrez MM, Haro JM, et al. Recreational drug use among individuals living with HIV in Europe: review of the prevalence, comparison with the general population and HIV guidelines recommendations. Frontiers in Microbiology. 2015;6:690. doi: 10.3389/fmicb.2015.00690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosn J, Leruez-Ville M, Blanche J, Delobelle A, Beaudoux C, Mascard L, et al. HIV-1 DNA levels in peripheral blood mononuclear cells and cannabis use are associated with intermittent HIV shedding in semen of men who have sex with men on successful antiretroviral regimens. Clinical Infectious Diseases. 2014;58:1763–1770. doi: 10.1093/cid/ciu187. [DOI] [PubMed] [Google Scholar]
- Ingraham C. A majority favors marijuana legalization for first time, according to nation’s most authoritative survey [Washington Post] Retrieved February 21, 2016 from https://www.washingtonpost.com/news/wonk/wp/2015/03/04/majority-of-americans-favor-marijuana-legalization-for-first-time-according-to-the-nations-most-authoritative-survey/
- Jeevanjee S, Penko J, Guzman D, Miaskowski C, Bangsberg DR, Kushel MB. Opioid analgesic misuse is associated with incomplete antiretroviral adherence in a cohort of HIV-infected indigent adults in San Francisco. AIDS and Behavior. 2014;18:1352–1358. doi: 10.1007/s10461-013-0619-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi M, Joshi A, Bartter T. Marijuana and lung diseases. Current Opinion in Pulmonary Medicine. 2014;20:173–179. doi: 10.1097/mcp.0000000000000026. [DOI] [PubMed] [Google Scholar]
- Kim AY, Onofrey S, Church DR. An epidemiologic update on hepatitis C infection in persons living with or at risk of HIV infection. Journal of Infectious Diseases. 2013;207(Suppl 1):S1–6. doi: 10.1093/infdis/jis927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kral AH, Wenger L, Novak SP, Chu D, Corsi KF, Coffa D, et al. Is cannabis use associated with less opioid use among people who inject drugs? Drug and Alcohol Dependence. 2015 doi: 10.1016/j.drugalcdep.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau N, Sales P, Averill S, Murphy F, Sato SO, Murphy S. A safer alternative: Cannabis substitution as harm reduction. Drug and Alcohol Review. 2015;34:654–659. doi: 10.1111/dar.12275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Herzog TA, Meade CD, Webb MS, Brandon TH. The use of GEE for analyzing longitudinal binomial data: a primer using data from a tobacco intervention. Addictive Behaviors. 2007;32:187–193. doi: 10.1016/j.addbeh.2006.03.030. [DOI] [PubMed] [Google Scholar]
- Lucas GM, Cheever LW, Chaisson RE, Moore RD. Detrimental effects of continued illicit drug use on the treatment of HIV-1 infection. Journal of Acquired Immune Deficiency Syndromes. 2001:27. doi: 10.1097/00126334-200107010-00006. [DOI] [PubMed] [Google Scholar]
- Lucas GM, Gebo KA, Chaisson RE, Moore RD. Longitudinal assessment of the effects of drug and alcohol abuse on HIV-1 treatment outcomes in an urban clinic. AIDS. 2002:16. doi: 10.1097/00002030-200203290-00012. [DOI] [PubMed] [Google Scholar]
- Lucas P, Reiman A, Earleywine M, McGowan SK, Oleson M, Coward MP, et al. Cannabis as a substitute for alcohol and other drugs: A dispensary-based survey of substitution effect in Canadian medical cannabis patients. Addiction Research and Theory. 2013;21:435–442. doi: 10.3109/16066359.2012.733465. [DOI] [Google Scholar]
- Lucas P, Walsh Z, Crosby K, Callaway R, Belle-Isle L, Kay R, et al. Substituting cannabis for prescription drugs, alcohol and other substances among medical cannabis patients: The impact of contextual factors. Drug and Alcohol Review. 2015 doi: 10.1111/dar.12323. [DOI] [PubMed] [Google Scholar]
- Marcellin F, Lions C, Rosenthal E, Roux P, Sogni P, Wittkop L, et al. No significant effect of cannabis use on the count and percentage of circulating CD4 T-cells in HIV-HCV co-infected patients (ANRS CO13-HEPAVIH French cohort) Drug and Alcohol Review. 2016 doi: 10.1111/dar.12398. [DOI] [PubMed] [Google Scholar]
- Marihuana for Medical Purposes Regulations, SOR/2013-119. Canada Gazette, Part II. 2012;146(50) Retrieved from http://gazette.gc.ca/rp-pr/p1/2012/2012-12-15/html/reg4-eng.html. [Google Scholar]
- Midanik LT, Tam TW, Weisner C. Concurrent and simultaneous drug and alcohol use: results of the 2000 National Alcohol Survey. Drug and Alcohol Dependence. 2007;90:72–80. doi: 10.1016/j.drugalcdep.2007.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milloy MJ, Kerr T, Bangsberg DR, Buxton J, Parashar S, Guillemi S, et al. Homelessness as a structural barrier to effective antiretroviral therapy among HIV-seropositive illicit drug users in a Canadian setting. AIDS Patient Care and STDs. 2012;26:60–67. doi: 10.1089/apc.2011.0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milloy MJ, Marshall B, Kerr T, Richardson L, Hogg B, Guillemi S, et al. High-intensity cannabis use associated with lower plasma human immunodeficiency virus-1 RNA viral load among recently infected people who use injection drugs. Drug and Alcohol Review. 2015a;34:135–140. doi: 10.1111/dar.12223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milloy MJ, Wood E, Kerr T, Hogg B, Guillemi S, Harrigan PR, et al. Increased prevalence of controlled viremia and decreased rates of HIV drug resistance among HIV-positive people who use illicit drugs during a community-wide Treatment-as-Prevention initiative. Clinical Infectious Diseases. 2015b doi: 10.1093/cid/civ929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina PE, Winsauer P, Zhang P, Walker E, Birke L, Amedee A, et al. Cannabinoid administration attenuates the progression of simian immunodeficiency virus. AIDS Res Hum Retroviruses. 2011;27:585–592. doi: 10.1089/AID.2010.0218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montaner J, Lima VD, Barrios R, Yip B, Wood E, Kerr T, et al. Association of highly active antiretroviral therapy coverage, population viral load, and yearly new HIV diagnoses in British Columbia, Canada: A population-based study. The Lancet. 2010;376:532–539. doi: 10.1016/S0140-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montaner JSG, Lima VD, Harrigan PR, Lourenço L, Yip B, Nosyk B, et al. Expansion of HAART coverage is associated with sustained decreases in HIV/AIDS morbidity, mortality and HIV transmission: The “HIV Treatment as Prevention” experience in a Canadian setting. PLoS One. 2014;9:e87872. doi: 10.1371/journal.pone.0087872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore BA, Augustson EM, Moser RP, Budney AJ. Respiratory effects of marijuana and tobacco use in a U.S. sample. Journal of General Internal Medicine. 2005;20:33–37. doi: 10.1111/j.1525-1497.2004.40081.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Conference of State Legislatures. State Medical Marijuana Laws. Retrieved February 21, 2016 from http://www.ncsl.org/research/health/state-medical-marijuana-laws.aspx.
- Nolan S, Milloy MJ, Zhang R, Kerr T, Hogg RS, Montaner JS, et al. Adherence and plasma HIV RNA response to antiretroviral therapy among HIV-seropositive injection drug users in a Canadian setting. AIDS Care. 2011;23:980–987. doi: 10.1080/09540121.2010.543882. [DOI] [PubMed] [Google Scholar]
- Nosyk B, Min JE, Colley G, Lima VD, Yip B, Milloy MJ, et al. The causal effect of opioid substitution treatment on HAART medication refill adherence. AIDS. 2015;29:965–973. doi: 10.1097/qad.0000000000000642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minister of Justice and Attorney General of Canada Mandate Letter. Government of Canada; 2015. Retrieved from http://pm.gc.ca/eng/minister-justice-and-attorney-general-canada-mandate-letter. [Google Scholar]
- Okafor CN, Zhou Z, Burrell LE, 2nd, Kelso NE, Whitehead NE, Harman JS, et al. Marijuana use and viral suppression in persons receiving medical care for HIV-infection. American Journal of Drug and Alcohol Abuse. 2016:1–8. doi: 10.1080/00952990.2016.1191505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palepu A, Tyndall M, Yip B, O’Shaughnessy MV, Hogg RS, Montaner JSG. Impaired virologic response to highly active antiretroviral therapy associated with ongoing injection drug use. Journal of Acquired Immune Deficiency Syndromes. 2003;32:522–526. doi: 10.1097/00126334-200304150-00009. [DOI] [PubMed] [Google Scholar]
- Prentiss D, Power R, Balmas G, Tzuang G, Israelski DM. Patterns of marijuana use among patients with HIV/AIDS followed in a public health care setting. Journal of Acquired Immune Deficiency Syndromes. 2004;35:38–45. doi: 10.1097/00126334-200401010-00005. [DOI] [PubMed] [Google Scholar]
- Richardson LA, Kerr TH, Dobrer S, Puskas CM, Guillemi SA, Montaner JS, et al. Socioeconomic marginalization and plasma HIV-1 RNA nondetectability among individuals who use illicit drugs in a Canadian setting. AIDS. 2015;29:2487–2495. doi: 10.1097/qad.0000000000000853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen MI, Black AC, Arnsten JH, Goggin K, Remien RH, Simoni JM, et al. Association between use of specific drugs and antiretroviral adherence: Findings from MACH 14. AIDS and Behavior. 2013;17:142–147. doi: 10.1007/s10461-011-0124-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon K, Kerr T, Lai C, Ishida T, Wood E, Montaner JS, et al. Nonadherence to antiretroviral therapy among a community with endemic rates of injection drug use. Journal of the International Association of Physicians in AIDS Care. 2005;4:66–72. doi: 10.1177/1545109705284353. [DOI] [PubMed] [Google Scholar]
- Shin SS, Moreno PG, Rao S, Garfein RS, Novotny TE, Strathdee SA. Cigarette smoking and quit attempts among injection drug users in Tijuana, Mexico. Nicotine and Tobacco Research. 2013;15:2060–2068. doi: 10.1093/ntr/ntt099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slawson G, Milloy MJ, Balneaves L, Simo A, Guillemi S, Hogg R, et al. High-intensity cannabis use and adherence to antiretroviral therapy among people who use illicit drugs in a Canadian setting. AIDS and Behavior. 2014:1–8. doi: 10.1007/s10461-014-0847-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small W, Wood E, Betteridge G, Montaner J, Kerr T. The impact of incarceration upon adherence to HIV treatment among HIV-positive injection drug users: a qualitative study. AIDS Care. 2009;21:708–714. doi: 10.1080/09540120802511869. [DOI] [PubMed] [Google Scholar]
- Thames AD, Mahmood Z, Burggren AC, Karimian A, Kuhn TP. Combined effects of HIV and marijuana use on neurocognitive functioning and immune status. AIDS Care. 2016;28:628–632. doi: 10.1080/09540121.2015.1124983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Vancouver Province. Police release a new policy for drug crimes [Canada.com] Retrieved April 11, 2016 from http://www.canada.com/theprovince/news/story.html?id=b5640c14-69d6-4ee9-8986-4160faeff270.
- Tucker JS, Burnam MA, Sherbourne CD, Kung FY, Gifford AL. Substance use and mental health correlates of nonadherence to antiretroviral medications in a sample of patients with human immunodeficiency virus infection. The American Journal of Medicine. 2003;114:573–580. doi: 10.1016/s0002-9343(03)00093-7. http://dx.doi.org/10.1016/S0002-9343(03)00093-7. [DOI] [PubMed] [Google Scholar]
- Tyndall MW, Currie S, Spittal P, Li K, Wood E, O’Shaughnessy MV, et al. Intensive injection cocaine use as the primary risk factor in the Vancouver HIV-1 epidemic. AIDS. 2003;17:887–893. doi: 10.1097/01.aids.0000050859.71999.ae. [DOI] [PubMed] [Google Scholar]
- UNAIDS. 90-90-90: An ambitious treatment target to help end the AIDS epidemic. Geneva: 2014. Retrieved from http://www.unaids.org/en/resources/documents/2014/90-90-90. [Google Scholar]
- Werb D, Kerr T, Buxton J, Shoveller J, Richardson C, Montaner J, et al. Patterns of injection drug use cessation during an expansion of syringe exchange services in a Canadian setting. Drug and Alcohol Dependence. 2013;132:535–540. doi: 10.1016/j.drugalcdep.2013.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westergaard RP, Kirk GD, Richesson DR, Galai N, Mehta SH. Incarceration predicts virologic failure for HIV-infected injection drug users receiving antiretroviral therapy. Clinical Infectious Diseases. 2011;53:725–731. doi: 10.1093/cid/cir491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson KJ, Doxanakis A, Fairley CK. Predictors for non-adherence to antiretroviral therapy. Sexual Health. 2004;1:251–257. doi: 10.1071/sh04020. [DOI] [PubMed] [Google Scholar]
- Wood E, Kerr T, Marshall BDL, Li K, Zhang R, Hogg RS, et al. Longitudinal community plasma HIV-1 RNA concentrations and incidence of HIV-1 among injecting drug users: prospective cohort study. British Medical Journal. 2009:338. doi: 10.1136/bmj.b1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood E, Kerr T, Montaner JS, Strathdee SA, Wodak A, Hankins CA, et al. Rationale for evaluating North America’s first medically supervised safer-injecting facility. The Lancet Infectious Diseases. 2004;4:301–306. doi: 10.1016/s1473-3099(04)01006-0. [DOI] [PubMed] [Google Scholar]
- Wood E, Montaner JSG, Yip B, Tyndall MW, Schechter MT, O’Shaughnessy MV, et al. Adherence and plasma HIV RNA responses to highly active antiretroviral therapy among HIV-1 infected injection drug users. Canadian Medical Association Journal. 2003;169:656–661. [PMC free article] [PubMed] [Google Scholar]
- Woolridge E, Barton S, Samuel J, Osorio J, Dougherty A, Holdcroft A. Cannabis use in HIV for pain and other medical symptoms. Journal of Pain and Symptom Management. 2005;29:358–367. doi: 10.1016/j.jpainsymman.2004.07.011. http://dx.doi.org/10.1016/j.jpainsymman.2004.07.011. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Antiretroviral Treatment as Prevention (TasP) of HIV and TB. World Health Organization; Geneva: 2012. Retrieved from http://apps.who.int/iris/bitstream/10665/70904/1/WHO_HIV_2012.12_eng.pdf. [Google Scholar]
- Zivanovic R, Milloy MJ, Hayashi K, Dong H, Sutherland C, Kerr T, et al. Impact of unstable housing on all-cause mortality among persons who inject drugs. BMC Public Health. 2015;15 doi: 10.1186/s12889-015-1479-x. [DOI] [PMC free article] [PubMed] [Google Scholar]