Abstract
Lessons Learned.
Oral targeted agents are desirable for treatment of Kaposi sarcoma (KS); however, in patients with HIV, drug–drug interactions must be considered. In this study to treat KS, sorafenib was poorly tolerated at doses less than those approved by the U.S. Food and Drug Administration for hepatocellular carcinoma and other cancers, and showed only modest activity.
Sorafenib's metabolism occurs via the CYP3A4 pathway, which is inhibited by ritonavir, a commonly used antiretroviral agent used by most patients in this study. Strong CYP3A4 inhibition by ritonavir may contribute to the observed sorafenib toxicity.
Alternate antiretroviral agents without predicted interactions are preferred for co‐administration in patients with HIV and cancers for which sorafenib is indicated.
Background.
We conducted a phase Ib study of sorafenib, a vascular epithelial growth factor receptor (VEGFR), c‐kit, and platelet derived growth factor receptor (PDGFR)‐targeted treatment in Kaposi sarcoma (KS). We evaluated drug–drug interactions between sorafenib and ritonavir, an HIV medication with strong CYP3A4 inhibitory activity.
Methods.
Two cohorts were enrolled: HIV‐related KS on ritonavir (Cohort R) and HIV‐related or classical KS not receiving ritonavir (Cohort NR). Sorafenib dose level 1 in cohort R (R1) was 200 mg daily and 200 mg every 12 hours in cohort NR (NR1). Steady‐state pharmacokinetics were evaluated at cycle 1, day 8. KS responses and correlative factors were assessed.
Results.
Ten patients (nine HIV+) were enrolled: R1 (eight), NR1 (two). Median CD4+ count (HIV+) was 500 cells/µL. Dose‐limiting toxicities (DLTs) were grade 3 elevated lipase (R1), grade 4 thrombocytopenia (R1), and grade 3 hand‐foot syndrome (NR1). Two of seven evaluable patients had a partial response (PR; 29%; 95% CI 4%–71%). Steady‐state area under the curve of the dosing interval (AUCTAU) of sorafenib was not significantly affected by ritonavir; however, a trend for decreased AUCTAU of the CYP3A4 metabolite sorafenib‐N‐oxide (3.8‐fold decrease; p = .08) suggests other metabolites may be increased.
Conclusion.
Sorafenib was poorly tolerated, and anti‐KS activity was modest. Strong CYP3A4 inhibitors may contribute to sorafenib toxicity, and ritonavir has previously been shown to be a CYP3A4 inhibitor. Alternate antiretroviral agents without predicted interactions should be used when possible for concurrent administration with sorafenib. The Oncologist 2017;22:505–e49
Abstract
经验总结
• 口服靶向药物是卡波西肉瘤(KS)的理想治疗药物, 但用于HIV患者时, 必须考虑到药物相互作用。在本项有关KS治疗的研究中, 索拉非尼在低于美国食品药品监督管理局批准用于治疗肝细胞癌和其他癌症的剂量下耐受性较差, 并且仅表现出较低的活性。
• 索拉非尼通过CYP3A4途径代谢, 而本研究中大部分患者经常使用的抗逆转录病毒药物利托那韦可抑制该途径。研究中观察到的索拉非尼毒性可能是由于利托那韦对CYP3A4的强效抑制作用所致。
• 合并感染HIV且适合接受索拉非尼治疗的癌症患者应首选合并使用预期无相互作用的抗逆转录病毒药物, 用以替代利托那韦。
摘要
背景.索拉非尼是一种以血管内皮生长因子受体(VEGFR)、c‐kit受体和血小板源性生长因子受体(PDGFR)为靶点治疗卡波西肉瘤(KS)的药物。我们使用索拉非尼进行了一项Ib期研究, 评价其与利托那韦(具有强效CYP3A4抑制活性的HIV药物)之间的药物相互作用。
方法.本研究入组了两个队列:接受利托那韦治疗的HIV相关KS患者(队列R)和未接受利托那韦治疗的HIV相关KS或经典型KS患者(队列NR)。队列R的索拉非尼剂量水平1(R1)为200 mg每日一次, 队列NR的索拉非尼剂量水平1(NR1)为200 mg每12小时一次。在第1周期第8天评价了稳态药代动力学。评估了KS缓解情况和相关因素。
结果.入组了10例患者(9例为HIV+):R1中8例, NR1中2例。中位CD4+计数(HIV+)为500 细胞数/μL。剂量限制性毒性(DLT)为3级脂肪酶升高(R1)、4级血小板减少症(R1)和3级手足综合征(NR1)。7例可评价患者中有2例达到了部分缓解(PR;29%;95% CI:4%‐71%)。利托那韦未显著影响索拉非尼的稳态给药间隔曲线下面积(AUCTAU), 但是, CYP3A4代谢物索拉非尼‐N‐氧化物的AUCTAU 有降低趋势(降低3.8倍; p = 0.08), 表明其他代谢物的生成可能有所增加。
结论.索拉非尼耐受性较差, 抗KS活性不高。CYP3A4强抑制剂可能引起索拉非尼毒性, 而既往研究表明利托那韦属于CYP3A4抑制剂。如果可能涉及与索拉非尼合用, 应选择预期无相互作用的抗逆转录病毒药物, 用以替代利托那韦。The Oncologist 2017;22:505‐e49
Discussion
Preclinical data supported evaluation of sorafenib in KS. Our primary objective was to evaluate the safety of sorafenib in KS patients and ritonavir–sorafenib pharmacokinetic (PK) interactions [1]. Sorafenib was poorly tolerated, with two patients experiencing DLTs at the first dose level (one in each cohort). The overall response rate (ORR) in seven evaluable patients was 29% (95% CI 4%–71%). Although the maximum tolerated dose (MTD) was not determined, accrual was terminated after review of Cohort R1 safety and efficacy data.
Importantly, patients had well‐controlled HIV and preserved CD4 counts. Such patients generally tolerate standard chemotherapy dosing when co‐administered with appropriate antiretroviral therapy (ART). Poor tolerability was most likely due to drug–drug interactions. Maximum plasma concentration (CMAX) and AUC0‐12h of sorafenib following a 200 mg dose at steady state observed in this trial were within reported ranges [2], [3], [4]. The effects of drug–drug interactions and genetic variants on hepatic metabolism are important [5], [6], [7], and co‐administration with ritonavir, a strong CYP3A4 inhibitor, is a possible contributor to the poor tolerability in Cohort R1 [7], [8]. A phase I study of sunitinib, another CYP3A4‐metabolized drug, in patients with HIV and cancer demonstrated that HIV patients not taking ritonavir tolerated standard dosing, whereas patients receiving ritonavir had higher toxicities at lower doses. Ritonavir was associated with decreases in the sunitinib active metabolite but not the parent drug [7]. In our study, we demonstrated a similar trend toward a 3.8‐fold decrease in the CYP3A4 main active metabolite sorafenib‐N‐oxide [9] in patients receiving ritonavir, while parent sorafenib exposures were only modestly affected. Shunting of metabolism towards other pathways yielding more toxic metabolites may alter tolerability (Fig. 1) and explain the toxicity observed. A limitation of this study is the small sample size, and conclusions on the use of sorafenib with ritonavir cannot be based on PK data alone. Nonetheless, our findings suggest that sorafenib has modest activity and does not have a favorable activity/toxicity profile in patients with KS, and that use of concurrent ritonavir‐based ART and sorafenib should be avoided.
Figure 1.
Hepatic metabolism of sorafenib. Elimination of sorafenib occurs mainly in the liver through CYP3A4 oxidative metabolism. M2 is produced by oxidation of sorafenib via CYP3A4 and is the major circulating active metabolite. M7 is produced through the glucoronidation of the parent compound by UGT1A9. Ritonavir is a strong inhibitor of the CYP3A4 pathway, and inhibition of CYP3A4 may lead to the increased production of other metabolites through alternate pathways. Figure modified from PharmGKB pathway with permission from PharmGKB and Stanford University (https://www.pharmgkb.org/pathway/PA165959537).
Abbreviations: M, metabolite; M2, Sorafenib N‐oxide; R, ritonavir.
Although these results do not support its further study or use in KS, our PK and safety findings inform treatment of patients with HIV and cancers for which sorafenib is indicated, particularly those with hepatocellular carcinoma, a tumor with increasing incidence [10]. Caution in using sorafenib in patients with HIV and cancers for which it is approved is advised. Although this study did not conclusively show that ritonavir affected sorafenib metabolism, the results are suggestive, and concurrent ritonavir or other strong CYP3A4 inhibitors should be avoided. ART without predicted strong CYP3A4 interactions should be preferred for concurrent treatment of HIV in patients with cancers best treated by sorafenib.
Trial Information
- Disease
Kaposi's sarcoma
- Stage of disease/treatment
Any
- Prior Therapy
No designated number of regimens
- Type of study – 1
Phase I
- Type of study – 2
3 + 3 phase I design
- Primary endpoint
Toxicity
- Primary endpoint
Pharmacokinetics
- Primary endpoint
Pharmacodynamic
- Primary endpoint
Safety
- Secondary endpoint
Efficacy
- Additional details of endpoints or study design
- Eligibility and treatment. Adults with histologically confirmed KS and at least five evaluable cutaneous lesions or non‐cutaneous measurable disease were eligible, regardless of HIV status. HIV‐positive subjects must have been on ART ≥3 months with progressive disease (PD) or ≥4 months without disease regression and willing to adhere to concurrent ART during this study of sorafenib. Patients with symptomatic visceral KS, except oral cavity disease, were excluded. There was no CD4+ T‐cell count criterion. The initial dosing was less frequent in cohort R because of concerns of the effect of CYP34 inhibition on drug metabolism. Sorafenib was given continuously over 21‐day cycles. Dose escalation by 100% was planned in each cohort up to the recommended dose of 400 mg every 12 hours.
- Safety monitoring and dose‐limiting toxicity evaluation. Safety was evaluated by history and physical and laboratory investigations at baseline every 7 days during cycle 1 (C1), then on day 1 of subsequent cycles. CD4+ T‐cell counts and HIV RNA levels in HIV‐positive patients were obtained at the end of C1 and every 3 months. Adverse events (AEs) were evaluated using NCI Common Terminology Criteria for Adverse Events version 3.0; after January 1, 2011, version 4.0 was utilized. DLTs were determined over 12 weeks on sorafenib, with a minimum 6‐week evaluation required for a participant not taken off treatment for a DLT. Any grade 4 AE was considered a DLT except for lymphopenia, CD4+ T‐cell lymphocytopenia, neutropenia, anemia, or transient creatine phosphokinase (CPK) elevation; these required the following additional criteria to be considered a DLT: absolute neutrophil count <500 cells/mm3 for ≥5 days and/or accompanied by ≥grade 2 fever; CD4+ T‐cell decrease >80% and ≥50 cell/mm3 from entry on two successive determinations despite controlled HIV; anemia unresponsive to erythropoietin within 1 week and no other identified causes; and CPK elevation occurring in the absence of causal exercise or trauma. Grade 3 AEs at least possibly due to sorafenib were considered DLTs with the exception of elevation of hepatic transaminases <500 IU, total bilirubin <4.8 mg/dL (direct <0.3 mg/dL and indirect <4.5 mg/dL) in patients on a protease inhibitor, asymptomatic hyperuricemia or hypophosphatemia, amylase elevation due to non‐pancreatic origin, grade 3 rash that decreases to grade 1 by week 6 and does not recur on drug rechallenge, or hypertension managed with modification of medications. Preexisting manifestations of HIV, KS, or HIV therapy were not considered DLTs. A dose was considered not tolerable if two or more of six evaluable patients developed a DLT. The MTD was defined as the highest dose level where ≤fewer than one of six patients experienced a DLT.
- Pharmacokinetics (PK) methods. Steady‐state PKs were evaluated C1, day 8. Blood was collected pre‐dose and at 1, 2, 4, 8, and 12 hours (before the next dose if on a every 12 hour dosing) and 16 and 24 hours (before the next dose) after sorafenib. Plasma concentrationsof sorafenib and its major active CYP3A4 metabolite sorafenib N‐oxide were measured by liquid chromatography-tandem mass spectrometry as previously described. Noncompartmental PK assessment was performed using Phoenix WinNonlin v6.4 (Certara Pharsight Corp, Cary, NC). Maximum plasma concentration (CMAX) was recorded as observed values, and the area under the plasma‐concentration time curve was calculated using the Linear Trapezoidal rule. AUCTAU was calculated to compare the two groups and normalize the additive effect of twice daily versus once daily dosing.
- Pharmacodynamics. Sorafenib's effect on select serum factors, Kaposi sarcoma‐associated herpes virus (KSHV) viral load (VL) and CD4+ T‐cell count were evaluated. Correlative assays were performed on biospecimens collected at baseline and the end of C1. Serum IL‐1b, IL‐6, IL‐8, IL‐10, IL‐12p40, 1L‐12p70, IFN‐Υ, TNF‐alpha, MCP1, MIP1A, bFGF, IP‐10, GM‐CSF, FIT‐1, PIGF, VEGF‐A, VEGF‐C, and VEGF‐D were evaluated using a custom V‐Plex Assay (Meso‐Scale Discovery, Gaithersburg, MD) and Sector Imager (Meso‐Scale Discovery). Peripheral blood mononuclear cell (PMBC)‐associated KSHV VL was measured using previously described methods.
- Statistical considerations. The primary objectives were to assess safety and pharmacokinetics of sorafenib in both cohorts. The study allowed for closure after primary objectives were met in Cohort R and did not require completion of Cohort NR. A secondary objective was to preliminarily assess antitumor effect. ORR, defined as the proportion of patients whose best response was PR or better with exact 95% confidence intervals, was calculated for all patients and for evaluable patients. PK parameters were compared between cohorts to assess the effects of ritonavir on sorafenib. Nonparametric Mann–Whitney test was applied to compare differences in sorafenib, sorafenib‐N‐oxide, the ratio sorafenib‐N‐oxide:sorafenib, and AUC TAU between patients administered 200 mg sorafenib daily with 200 mg ritonavir or sorafenib twice daily alone. Association between grade 3–4 AEs and AUCTAU was evaluated by unpaired t test. For comparisons between cohorts, p < .05 was considered significant while .05 < p < .1 would indicate a trend. Sorafenib's effect on serum factors, KSHV VL, and CD4+ T‐cell count was evaluated by comparing differences from baseline to the end of C1 using Wilcoxon signed rank test. Analytes with serum levels below the lower limit of detection in a majority of samples were excluded. Analyses were repeated excluding one patient subsequently diagnosed with KSHV‐associated multicentric Castleman disease (KSHV‐MCD), which is associated with abnormalities in human IL‐6 and IL‐10. Analyses were considered exploratory without formal adjustment for multiple comparisons. A p‐value <.01 was considered to reflect a significant change while .01 < p < .05 would indicate a trend.
- Investigator's analysis
Active but too toxic as administered in this study
Drug Information
- Drug 1
- Generic/Working name
Sorafenib
- Trade name
Nexavar
- Company name
Bayer
- Drug type
Small molecule
- Dose
milligrams (mg) per flat dose
- Route
oral (po)
- Schedule of administration
Cohort R ‐ dose level 1 was 200 mg orally once daily. Cohort NR‐ dose level 1 was 200 mg twice daily. In all cohorts, sorafenib was given orally continuously over 21‐day cycles.
Patient Characteristics
- Number of patients, male
10
- Number of patients, female
0
- Stage
- In HIV infected patients
-
KS prognostic factors1: n (%)—T1: 6 (67%); I1: 1 (11%), S1: 1 (11%)
Revised TS stage2 (AIDS KS prognostic criteria): Good, 8 (89%); Poor, 1 (11%)
1Risk factors based on AIDS Clinic Trials Group (ACTG) staging criteria. T1: edema or ulceration, extensive oral mucosa KS, or visceral KS; I1: CD4+ T‐cells <150 cells/mL; S1: history of opportunistic infections or thrush, and/or “B” symptoms present, and/or Karnofsky Score <70%, and/or other HIV‐related disease.
2Revised AIDS KS prognostic criteria, excludes CD4+ as a risk factor.
- Age
Median (range): 49 years (35–72 years)
- Number of prior systemic therapies
Median (range): 2 (0–4)
- Performance Status: ECOG
-
0 — 4 (40%)
1 — 6 (60%)
2 — 0
3 — 0
unknown —
- Other
Patients accrued between January 2006 and February 2012. Patient characteristics are as follows:
- All patients
-
Race: Black 2 (20%); White 8 (80%)
Detectable circulating KSHV: 7 (70%)
Tumor associated edema: 6 (60%)
Greater than 50 KS lesions: 10 (100%)
Prior therapy for KS: 8 (80%)
HIV seropositive: 9 (90%)
Median time since last KS treatment (months): 22 (range 2–108)
- In HIV infected patients
-
CD4+ (cells/microL) median (range): 500 (35–747)
CD4+ <200 cells/microL: 1 (11%)
HIV VL <50 copies/mL: 7 (78%)
Median time on antiretroviral therapy (ART) (months*): 22 (range 3.5–108)
*Defined as months on specific ART regimen used at the time of screening visit.
- Cancer types or histologic subtypes
-
Kaposi sarcoma, HIV‐associated: 9
Classic Kaposi sarcoma, HIV‐negative: 1
Primary Assessment Method
- Control Arm: Total Patient Population
- Number of patients screened
29
- Number of patients enrolled
10
- Number of patients evaluable for toxicity
10
- Number of patients evaluated for efficacy
7
- Evaluation method
Modified AIDS Clinical Trial Group Criteria
- Response assessment CR
n = 0
- Response assessment PR
n = 2
- Response assessment SD
n = 4
- Response assessment PD
n = 1
- (Median) duration assessments response duration
3 months
- Note:
Seven patients (five in Cohort R1, two in Cohort NR1) were evaluable for response. Best responses were PR in two patients (R1), stable disease (SD) in four (three in R1, one in NR1), and progressive disease (PD) in one (NR1). The ORR was 2/10 (20%; 95% CI 3%–56%) in all patients and 2/7 (29%; 95% CI 4%–71%) in patients evaluable for response. Duration of PR was 3 months in the two responding patients. Median duration of SD was 4 cycles (range 1–5). Of six patients with tumor‐associated edema, five showed objective improvement with ≥2 cm decrease (range 2–5 cm) in circumference of affected limbs at the end of treatment, and one of these obtained a PR. One with severe tumor‐associated edema had improved range of motion in affected limbs, decreased weight, and decreased serous ooze after 1 cycle.
Adverse Events
Adverse events represent the worst grade for each patient that was possibly, probably, or definitely related to sorafenib during the entire course of treatment.
Abbreviations: NA, no adverse event; NC, no change from baseline.
Dose‐Limiting Toxicity
Pharmacokinetics/Pharmacodynamics
Assessment, Analysis, and Discussion
- Completion
Study terminated before completion
- Investigator's Assessment
Active but too toxic as administered in this study
KS is an angioproliferative tumor caused by Kaposi sarcoma herpesvirus (KSHV), also known as human herpesvirus‐8 [11], [12], [13]. HIV infection substantially increases KS risk [14] and accounts for more than 80% of KS in the U.S. High prevalence of HIV and KSHV coinfection has led to a high incidence of KS in areas of sub‐Saharan Africa [16]. In AIDS‐associated KS, combination ART is indicated but often insufficient. Current therapies for KS are limited by cumulative toxicities. Effective and less toxic approaches are needed. Oral agents are particularly desirable for resource‐limited settings.
Paracrine stimulation by pro‐angiogenic factors produced in part by KSHV‐infected cells contributes to KS pathogenesis. KS spindle cells express vascular epithelial growth factor (VEGF) receptors (R) types 2 and 3 (VEGFR‐2, VEGF‐R3), platelet‐derived growth factor (PDGF)‐R [17], [18], [19], [20], and c‐kit [21]. In vitro, spindle cells derived from KS patients proliferate in response to VEGF, VEGF‐C (a ligand for VEGF‐R3), and PDGF [17], [19], [20]. Sorafenib is a tyrosine kinase inhibitor (TKI) of VEGFR2, VEGFR3, PDGFR, and c‐kit [17], [18], [19], [20], [22], making it a rational agent to treat KS. However, prospective evaluation of novel cancer therapies in people with HIV for safety and PK interactions with antiretroviral agents is important [23].
Our primary objective in this phase Ib study was to evaluate the safety and tolerability of sorafenib in KS patients and the effect of ritonavir on levels of sorafenib [1]. Overall, sorafenib was poorly tolerated, with two patients experiencing DLTs at the first dose level (one in R1 and one in NR1). Additionally, five patients had grade 3 toxicities that did not meet DLT criteria and found the drug difficult to tolerate. The ORR in seven evaluable patients was 29% (95% CI 4%–71%). Although the MTD was not determined, accrual was terminated after review of Cohort R1 safety and efficacy data. Our safety and PK data suggest ritonavir, a strong CYP3A4 inhibitor, affects sorafenib metabolism by decreasing production of sorafenib‐N‐oxide and shunting the metabolism towards more toxic metabolites.
Although small, this phase Ib study provides valuable information to help inform treatment decisions for medical oncologists treating HIV‐associated tumors. Sorafenib [24] is indicated for the treatment of hepatocellular carcinoma (HCC) and other tumors in people with HIV [25], [26]. Increased toxicity has been described in a limited number of patients co‐administered ritonavir [27], [28], while other studies report that sorafenib was relatively well tolerated [29], [30], [31]. The largest retrospective series included 27 patients with HIV and HCC treated with 400 mg sorafenib twice daily. In that study, 93% were co‐administered ART. No information on number of patients on ritonavir was available, although protease inhibitor‐based therapy was common during that study time period (2007–2010). AEs were graded retrospectively, a source of bias and underreporting. Nonetheless, diarrhea, palmar‐plantar erythrodysesthesia syndrome, and hypertension were the most common grade 3–4 AEs, observed in 15%, 15%, and 11% of patients, respectively, higher than reported in the phase III trial that helped establish approval of sorafenib in HCC (8%, 8% and 2%) [24] and consistent with potential drug–drug interactions.
Despite our inability to escalate to standard doses, the ORR of 29% with sorafenib was comparable to observed response rates with other anti‐angiogenic agents and TKIs for KS. For example, ORRs in studies evaluating imatinib and bevacizumab in KS were 33% and 31%, respectively [21], [32]. Interestingly, there was evidence of a clinical effect related to decreased tumor‐associated edema in most patients with edema at baseline [32]. However, it is unclear why only modest tumor regression is observed, given the strong rationale. One possibility is redundancy of angiogenic pathways in KS. Better results may require combination with agents that target KS through other mechanisms.
KSHV‐infected dendritic cells overproduce IL‐12p40, a common subunit for IL‐12 and IL‐23. Despite modest antitumor effect, evaluating 14 serum factors associated with KS pathogenesis, we found a statistically significant decrease in the amount of IL‐12p40 between baseline and the end of cycle 1 (p = .002), suggesting that sorafenib has some effect on KSHV‐induced signaling [33]. Signal transducer and activator of transcription 3 (STAT3) activation by KSHV in endothelial and dendritic cells [34], [35] has been implicated in increased immunosuppressive cytokines, including IL‐23 [35], and indirect downregulation of phospho‐STAT3 by sorafenib [36] is a potential mechanism for our observed IL‐12p40 findings. Further evaluation of STAT3 inhibition in KSHV‐associated diseases is warranted [37]. We also noted a potential trend towards decreased bFGF (p = .018), a growth factor implicated in KS pathogenesis [18]. Our results are similar to findings in non‐small‐cell lung cancer [38] and consistent with a potential role for bFGF downregulation by sorafenib in the treatment of HCC [39], [40].
In summary, sorafenib is relatively poorly tolerated in patients with KS when co‐administered with ritonavir and has modest activity. Although these results do not support its further study or use in KS, findings from this study inform treatment of patients with HIV and cancers for which sorafenib is indicated, particularly those with HCC, a tumor with increasing incidence [10]. Prospective data on co‐administration of ART and cancer therapeutics are important, as concerns regarding toxicity contribute to treatment disparities in patients with HIV and cancer [10]. Caution in using sorafenib in patients with HIV and cancers for which it is approved is advised. Although this study did not conclusively show that ritonavir affected sorafenib metabolism, the results are suggestive, and concurrent ritonavir or other strong CYP3A4 inhibitors should be avoided. Antiretroviral agents without predicted strong CYP3A4 interactions are available and preferred for concurrent treatment of HIV in patients with cancers best treated by sorafenib. Sorafenib dose modification may be required even if an alternate ART regimen is used.
Figures and Tables
Table 1. Select pharmacokinetic parameters for sorafenib and sorafenib N‐oxide.
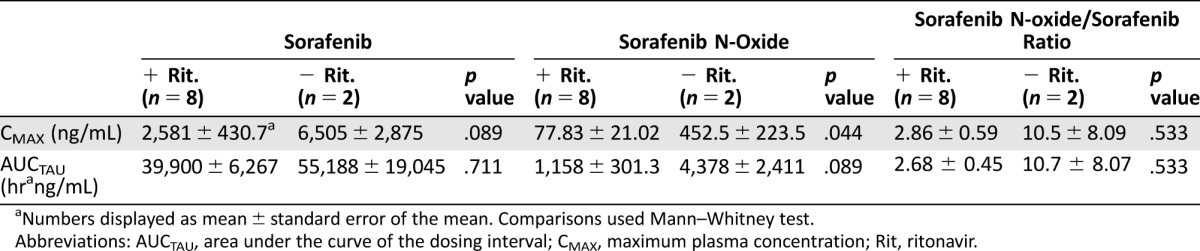
Numbers displayed as mean ± standard error of the mean. Comparisons used Mann–Whitney test.
Abbreviations: AUCTAU, area under the curve of the dosing interval; CMAX, maximum plasma concentration; Rit, ritonavir.
Table 2. Baseline characteristics.
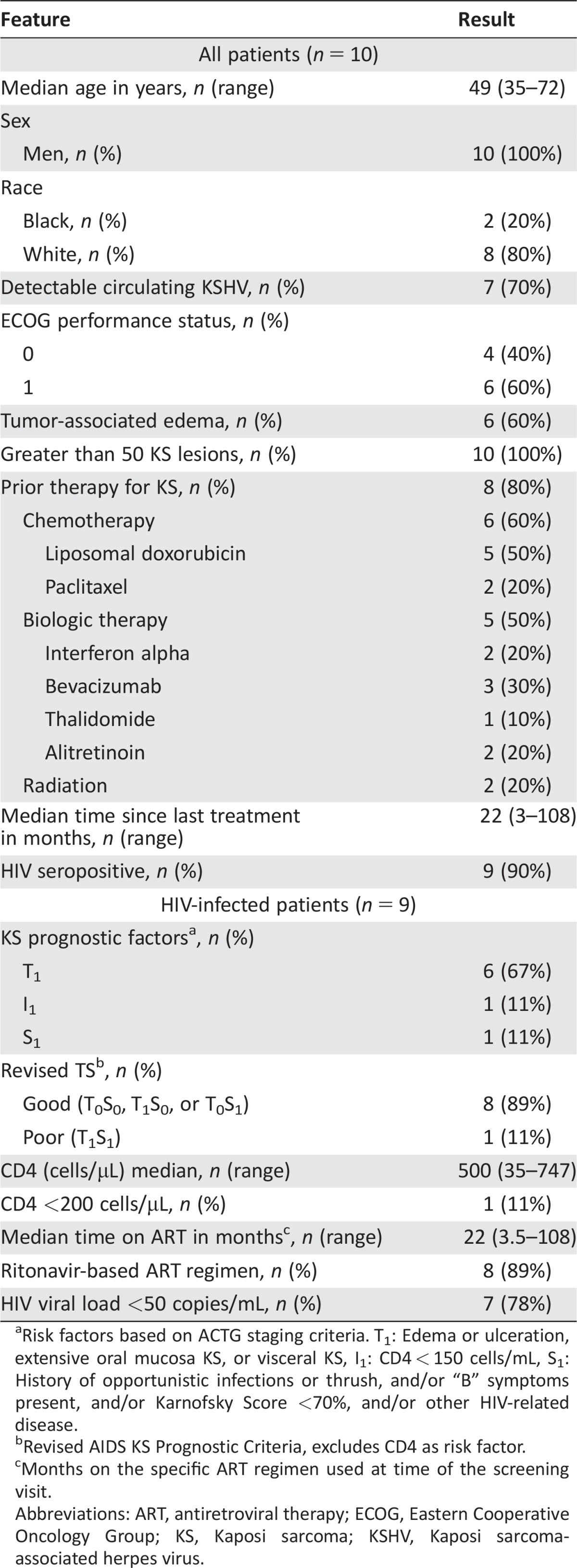
Risk factors based on ACTG staging criteria. T1: Edema or ulceration, extensive oral mucosa KS, or visceral KS, I1: CD4 < 150 cells/mL, S1: History of opportunistic infections or thrush, and/or “B” symptoms present, and/or Karnofsky Score <70%, and/or other HIV‐related disease.
Revised AIDS KS Prognostic Criteria, excludes CD4 as risk factor.
Months on the specific ART regimen used at time of the screening visit.
Abbreviations: ART, antiretroviral therapy; ECOG, Eastern Cooperative Oncology Group; KS, Kaposi sarcoma; KSHV, Kaposi sarcoma‐associated herpes virus.
Acknowledgments
This research was supported in part by the Intramural Research Program of the NIH, National Cancer Institute and gift funds from Johns Hopkins University. Further support was provided by a Clinical Trials Agreement between Bayer Health Care Corporation and the NCI Cancer Therapy Evaluation Program and by NCI Contract No. HHSN261200800001E. We thank the patients who volunteered to participate; the medical, nursing, and support staffs of the HIV and AIDS Malignancy Branch, the Medical Oncology Service, and the NIH Clinical Center; and Adam Rupert and Randy Stevens and colleagues in Leidos, NCI‐Frederick, Frederick, Maryland.
Footnotes
ClinicalTrials.gov Identifier: NCT00287495
Sponsor(s): Bayer Health Care Corporation and NCI Cancer Therapy Evaluation Program
Principal Investigator(s): Robert Yarchoan
IRB Approved: Yes
Disclosures
The authors indicated no financial relationships.
References
- 1. Sevrioukova IF, Poulos TL. Structure and mechanism of the complex between cytochrome p4503a4 and ritonavir. Proc Natl Acad Sci USA 2010;107:18422–18427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shacham‐Shmueli E, Geva R, Figer A et al. Phase I trial of sorafenib in combination with 5‐fluorouracil/leucovorin in advanced solid tumors. J Clin Pharmacol 2012;52:656–669. [DOI] [PubMed] [Google Scholar]
- 3. Desar IM, Timmer‐Bonte JN, Burger DM et al. A phase I dose‐escalation study to evaluate safety and tolerability of sorafenib combined with sirolimus in patients with advanced solid cancer. Br J Cancer 2010;103:1637–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Azad N, Dasari A, Arcaroli J et al. Phase I pharmacokinetic and pharmacodynamic study of cetuximab, irinotecan and sorafenib in advanced colorectal cancer. Invest New Drugs 2013;31:345–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Peer CJ, Sissung TM, Kim A et al. Sorafenib is an inhibitor of UGT1a1 but is metabolized by UGT1a9: Implications of genetic variants on pharmacokinetics and hyperbilirubinemia. Clin Cancer Res 2012;18:2099–2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Antoniou T, Tseng AL. Interactions between antiretrovirals and antineoplastic drug therapy. Clin Pharmacokinet 2005;44:111–145. [DOI] [PubMed] [Google Scholar]
- 7. Rudek MA, Moore PC, Mitsuyasu RT et al. A phase 1/pharmacokinetic study of sunitinib in combination with highly active antiretroviral therapy in human immunodeficiency virus‐positive patients with cancer: AIDS malignancy consortium trial AMC 061. Cancer 2014;120:1194–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lathia C, Lettieri J, Cihon F et al. Lack of effect of ketoconazole‐mediated CYP3a inhibition on sorafenib clinical pharmacokinetics. Cancer Chemother Pharmacol 2006;57:685–692. [DOI] [PubMed] [Google Scholar]
- 9. Keating GM, Santoro A. Sorafenib: A review of its use in advanced hepatocellular carcinoma. Drugs 2009;69:223–240. [DOI] [PubMed] [Google Scholar]
- 10. Suneja G, Boyer M, Yehia BR et al. Cancer treatment in patients with HIV infection and non‐AIDS‐defining cancers: A survey of US oncologists. J Oncol Pract 2015;11:e380–e387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chang Y, Cesarman E, Pessin MS et al. Identification of herpesvirus‐like DNA sequences in AIDS‐associated Kaposi's sarcoma. Science 1994;266:1865–1869. [DOI] [PubMed] [Google Scholar]
- 12. Gao SJ, Kingsley L, Li M et al. KSHV antibodies among Americans, Italians, and Ugandans with and without Kaposi's sarcoma. Nat Med 1996;2:925–928. [DOI] [PubMed] [Google Scholar]
- 13. Biggar RJ, Chaturvedi AK, Goedert JJ et al. AIDS‐related cancer and severity of immunosuppression in persons with AIDS. J Natl Cancer Inst 2007;99:962–972. [DOI] [PubMed] [Google Scholar]
- 14. Engels EA, Biggar RJ, Hall HI et al. Cancer risk in people infected with human immunodeficiency virus in the United States. Int J Cancer 2008;123:187–194. [DOI] [PubMed] [Google Scholar]
- 15. Shiels MS, Pfeiffer RM, Gail MH et al. Cancer burden in the HIV‐infected population in the United States. J Natl Cancer Inst 2011;103:753–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mosam A, Carrara H, Shaik F et al. Increasing incidence of Kaposi's sarcoma in black South Africans in KwaZulu‐Natal, South Africa (1983–2006). Int J STD AIDS 2009;20:553–556. [DOI] [PubMed] [Google Scholar]
- 17. Skobe M, Brown LF, Tognazzi K et al. Vascular endothelial growth factor‐C (VEGF‐C) and its receptors KDR and flt‐4 are expressed in AIDS‐associated Kaposi's sarcoma. J Invest Dermatol 1999;113:1047–1053. [DOI] [PubMed] [Google Scholar]
- 18. Ensoli B, Gendelman R, Markham P et al. Synergy between basic fibroblast growth factor and HIV‐1 Tat protein in induction of Kaposi's sarcoma. Nature 1994;371:674–680. [DOI] [PubMed] [Google Scholar]
- 19. Marchiò S, Primo L, Pagano M et al. Vascular endothelial growth factor‐C stimulates the migration and proliferation of Kaposi's sarcoma cells. J Biol Chem 1999;274:27617–27622. [DOI] [PubMed] [Google Scholar]
- 20. Stürzl M, Brandstetter H, Zietz C et al. Identification of interleukin‐1 and platelet‐derived growth factor‐B as major mitogens for the spindle cells of Kaposi's sarcoma: A combined in vitro and in vivo analysis. Oncogene 1995;10:2007–2016. [PubMed] [Google Scholar]
- 21. Koon HB, Krown SE, Lee JY et al. Phase II trial of imatinib in AIDS‐associated Kaposi's sarcoma: AIDS Malignancy Consortium Protocol 042. J Clin Oncol 2014;10:402–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Masood R, Cai J, Zheng T et al. Vascular endothelial growth factor/vascular permeability factor is an autocrine growth factor for AIDS‐Kaposi sarcoma. Proc Natl Acad Sci USA 1997;94:979–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Persad GC, Little RF, Grady C. Including persons with HIV infection in cancer clinical trials. J Clin Oncol 2008;26:1027–1032. [DOI] [PubMed] [Google Scholar]
- 24. Llovet JM, Ricci S, Mazzaferro V et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008;359:378–390. [DOI] [PubMed] [Google Scholar]
- 25. Sulkowski MS. Viral hepatitis and HIV coinfection. J Hepatol 2008;48:353–367. [DOI] [PubMed] [Google Scholar]
- 26. Rosenthal E, Roussillon C, Salmon‐Céron D et al. Liver‐related deaths in HIV‐infected patients between 1995 and 2010 in France: The Mortavic 2010 study in collaboration with the Agence Nationale de Recherche sur le SIDA (ANRS) EN 20 Mortalité 2010 survey. HIV Med 2015;16:230–239. [DOI] [PubMed] [Google Scholar]
- 27. Chelis L, Ntinos N, Souftas V et al. Complete response after sorafenib therapy for hepatocellular carcinoma in an HIV‐HBV co infected patient: Possible synergy with HAART? A case report. Med Oncol 2011;28(suppl 1):S165–S168. [DOI] [PubMed] [Google Scholar]
- 28. Ozenne V, Gervais A, Peytavin G et al. Suspected interaction between sorafenib and HAART in an HIV‐1 infected patient: A case report. Hepatogastroenterology 2011;58:161–162. [PubMed] [Google Scholar]
- 29. Berretta M, Di Benedetto F, Dal Maso L et al. Sorafenib for the treatment of unresectable hepatocellular carcinoma in HIV‐positive patients. Anticancer Drugs 2013;24:212–218. [DOI] [PubMed] [Google Scholar]
- 30. De Nardo P, Viscione M, Corpolongo A et al. Treatment of recurrent hepatocellular carcinoma with sorafenib in a HIV/HCV co‐infected patient in HAART: A case report. Infect Agent Cancer 2012;7:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Perboni G, Costa P, Fibbia GC et al. Sorafenib therapy for hepatocellular carcinoma in an HIV‐HCV coinfected patient: A case report. The Oncologist 2010;15:142–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Uldrick TS, Wyvill KM, Kumar P et al. Phase II study of bevacizumab in patients with HIV‐associated Kaposi's sarcoma receiving antiretroviral therapy. J Clin Oncol 2012;30:1476–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hensler HR, Rappocciolo G, Rinaldo CR et al. Cytokine production by human herpesvirus 8‐infected dendritic cells. J Gen Virol 2009;90:79–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Punjabi AS, Carroll PA, Chen L et al. Persistent activation of STAT3 by latent Kaposi's sarcoma‐associated herpesvirus infection of endothelial cells. J Virol 2007;81:2449–2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Santarelli R, Gonnella R, Di Giovenale G et al. STAT3 activation by KSHV correlates with IL‐10, IL‐6 and IL‐23 release and an autophagic block in dendritic cells. Sci Rep 2014;4:4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shiels MS, Pfeiffer RM, Hall HI et al. Proportions of Kaposi sarcoma, selected non‐Hodgkin lymphomas, and cervical cancer in the United States occurring in persons with AIDS, 1980–2007. JAMA 2011;305:1450–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Aoki Y, Feldman GM, Tosato G. Inhibition of STAT3 signaling induces apoptosis and decreases survivin expression in primary effusion lymphoma. Blood 2003;101:1535–1542. [DOI] [PubMed] [Google Scholar]
- 38. Kelly RJ, Rajan A, Force J et al. Evaluation of KRAS mutations, angiogenic biomarkers, and DCE‐MRI in patients with advanced non‐small‐cell lung cancer receiving sorafenib. Clin Cancer Res 2011;17:1190–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wang L, Park H, Chhim S et al. A novel monoclonal antibody to fibroblast growth factor 2 effectively inhibits growth of hepatocellular carcinoma xenografts. Mol Cancer Ther 2012;11:864–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cheng AL, Shen YC, Zhu AX. Targeting fibroblast growth factor receptor signaling in hepatocellular carcinoma. Oncology 2011;81:372–380. [DOI] [PubMed] [Google Scholar]






