This study is an evaluation of the effect of gastrointestinal cancer chemotherapy with short‐term periodic steroid premedication on bone metabolism. Primary endpoints were changes in bone mineral densities and metabolic bone turnover 16 weeks after initiation of chemotherapy.
Keywords: bone alkaline phosphatase, bone mineral density, chemotherapy, gastrointestinal cancer, steroid-induced osteoporosis, glucocorticoid premedication
Abstract
Background.
A multicenter prospective observational study evaluated the effect of gastrointestinal cancer chemotherapy with short‐term periodic steroid premedication on bone metabolism.
Patients and Methods.
Seventy‐four patients undergoing chemotherapy for gastrointestinal cancer were studied. The primary endpoints were changes in bone mineral densities (BMDs) and metabolic bone turnover 16 weeks after initiation of chemotherapy. BMDs, measured by dual‐energy x‐ray absorptiometry, and serum cross‐linked N‐telopeptides of type I collagen (sNTX), and bone alkaline phosphatase (sBAP) were assessed for evaluation of bone resorption and formation, respectively.
Results.
In 74.3% (55/74) of the patients, BMDs were significantly reduced at 16 weeks relative to baseline. The percent changes of BMD were −1.89% (95% confidence interval [CI], −2.67% to −1.11%: p < .0001) in the lumbar spine, −2.24% (95% CI, −3.59% to −0.89%: p = .002) in the total hip, and −2.05% (95% CI, −3.11% to −0.99%: p < .0001) in the femoral neck. Although there was no significant difference in sNTX levels during 16 weeks (p = .136), there was a significant increase in sBAP levels (p = .010). Decreased BMD was significantly linked to number of chemotherapy cycles (p = .02). There were no significant correlations between changes in BMDs and the primary site of malignancy, chemotherapy regimens, total cumulative steroid dose, steroid dose intensity, and additive steroid usage.
Conclusion.
Gastrointestinal cancer chemotherapy with periodic glucocorticoid premedication was associated with reduced BMD and increased sBAP levels, which were linked to number of chemotherapy cycles but independent of primary site, chemotherapy regimen, duration, and additive steroid usage. The Oncologist 2017;22:592–600
Implications for Practice.
Bone health and the management of treatment‐related bone loss are important for cancer care. The present study showed that a significant decrease in bone mineral density (BMD) and an increase in serum bone alkaline phosphatase levels occurred in gastrointestinal cancer patients receiving chemotherapy, which were linked to number of chemotherapy cycles but were independent of primary site, chemotherapy regimen, total steroid dose, and steroid dose intensity. Surprisingly, it seems that the decreasing BMD levels after only 16 weeks of chemotherapy for gastrointestinal cancer were comparable to that of 12‐month adjuvant aromatase inhibitor therapy for early‐stage breast cancer patients.
Introduction
Glucocorticoids (GCs) are one of the most important drugs used as premedication against chemotherapy‐induced nausea and vomiting (CINV) and are recommended for patients receiving not only highly emetogenic chemotherapy (HEC) but also moderately emetogenic chemotherapy (MEC) or low emetogenic chemotherapy (LEC) [1]. In HEC (e.g., cisplatin‐including regimens), which indicates risk in over 90% of patients, 20 mg of dexamethasone on day 1 for prophylaxis of acute emesis (within 24 h of chemotherapy administration) and 8 mg of dexamethasone orally or intravenously twice daily on days 2–5 for delayed emesis (more than 24 h post‐administration) are recommended. In MEC with a known potential for delayed emesis (e.g. oxaliplatin, irinotecan), which indicates risk in 30%–90% of patients, 8 mg of dexamethasone intravenously on day 1 against acute emesis as well as 8 mg of dexamethasone orally or intravenously for days 2–3 is recommended. Even in LEC (e.g., gemcitabine, docetaxel, paclitaxel), 4–8 mg of dexamethasone once for acute emesis is considered [2]. Furthermore, GCs are also used for the prevention of hypersensitivity reactions/infusion reactions linked to drugs such as paclitaxel or cetuximab [3], [4]. As a typical premedication schedule for hypersensitivity reactions, a single dose of dexamethasone (20 mg intravenously) given 30 min before chemotherapy including paclitaxel is employed [5], [6]. Therefore, in the case of gastrointestinal cancer chemotherapy, significant amounts of GC administration are periodically repeated; for example, the FOLFOX regimen, which is one of the standard chemotherapies for colorectal cancer and is categorized as MEC, has repeated biweekly administration [7], [8], and paclitaxel, which is also widely used as second‐line chemotherapy for advanced or recurrent gastric cancer and is categorized as LEC, is administered in a three‐weeks‐on and one‐week‐off schedule [9]. Furthermore, as in taxanes, anti‐epidermal growth factor antibodies (e.g., cetuximab), which often cause hypersensitivity or infusion‐related reactions, also require routine 20 mg of dexamethasone premedication per cycle [10], [11], [12].
However, GCs also have numerous systemic side effects, including diabetes mellitus type 2, psychosis, infection, insomnia, indigestion/epigastric discomfort, agitation, increased appetite, weight gain, acne, secondary adrenal suppression, and osteoporosis [13], [14]. Persistent GC use is especially associated with bone loss and increased fracture risk [15], [16], [17], [18], [19]. Currently, bone health and the management of treatment‐related bone loss are important for cancer care, as bone loss may lead to osteoporosis and its complications, including fractures, pain, and diminished quality of life (QOL) [20], [21], [22]. With regard to cancer treatment‐induced bone loss, for example, it is reported that endocrine treatments for breast cancer [23], [24], long‐term androgen deprivation therapies for prostate cancer [25], and anticancer therapies for premenopausal women with gynecological malignancies [26] cause the secondary osteoporosis. The use of GCs for palliative care in terminal cancer patients was also reported to cause osteoporosis [27]. Although interest in bone health in cancer care has gradually increased in recent years, data are still insufficient, and there is little evidence whether periodic or intravenous GC premedication for preventing CINV and/or hypersensitivity reactions causes secondary osteoporosis.
In the current study, we aimed to assess the influence of periodic GC premedication in gastrointestinal cancer chemotherapy on bone metabolism. We used dual‐energy x‐ray absorptiometry (DXA) scans to investigate bone mineral density (BMD) changes at the spine, which is widely used to diagnose osteoporosis [28], [29], [30]. In addition to these measurements, we also investigated the variation of two serum bone turnover markers (BTMs), serum cross‐linked N‐telopeptides of type I collagen (sNTX), which are a known bone resorption (osteoclast) marker [31], [32], and serum bone alkaline phosphatase (sBAP), which is a known bone formation (osteoblast) marker [33]. Because of the known hormonal influences in breast or prostate cancer patients, we elected to conduct the present study in patients with gastrointestinal cancer. As median progression‐free survival time of gemcitabine treatment against pancreatic cancer, which has the worst prognosis among gastrointestinal cancers, was reported to be approximately 4 months in a previous study [34], 16 weeks was selected as the follow‐up time for assessing BMD changes to minimize the bias caused by cancer treatment failure.
Materials and Methods
Study Design
This was a multicenter, prospective, observational study, named Evaluation of Steroid PREmedication for cancer chemotherapy aSSociated Osteoporosis (ESPRESSO‐01) study, to evaluate the effect of periodic GC premedication in gastrointestinal cancer patients on bone metabolism. The enrollment period was from June 2013 to May 2015. The protocol was performed in accordance with the Declaration of Helsinki, the Japanese ethical guidelines on clinical research, and the Ethical Guidelines for Clinical Studies and was registered with the University Hospital Medical Information Network (UMIN) Clinical Trials Registry (protocol ID UMIN000011054). Written informed consent was obtained from all patients, and protocol approval was obtained from the clinical research ethics review board of all participating institutions.
Participants
All patients fulfilled the following criteria: histologically confirmed adenocarcinoma in colorectal or non‐colorectal cancers, including esophageal, gastric, pancreatic, and biliary cancer; no prior treatment (e.g., radiation therapy, chemotherapy) for these cancers; a schedule of periodic intravenous steroid administration as a premedication for preventing CINV or allergic reactions that was weekly, biweekly, or triweekly and in which >4‐week steroid‐free intervals were not allowed; and age >20 years. The following exclusion criteria were used: previous or current regular steroid use; regular bisphosphonate or other drug use affecting the skeleton; regular calcium use; regimens with steroid‐free intervals of >4 weeks; patients considered inappropriate for steroid treatment, such as those with hepatitis virus infection or diabetes mellitus; and other factors making patients ineligible for participating according to the investigator.
BMD Measurement
DXA is recognized as the reference method to measure BMD with acceptable accuracy errors and good precision and reproducibility [35]. BMD was measured by DXA at the lumbar spine (L2, L3, and L4 posteroanterior view) and proximal left femur using two densitometers, Hologic Discovery A (Hologic, Inc., Waltham, MA) or Lunar Prodigy (General Electric, Madison, WI), using each manufacturer's standard scan and positioning protocols. Before performing each DXA measurements, a daily quality control test was performed using the manufacturer‐recommended phantom to ensure that observed density changes were not due to machine and/or operator variability. All scanners always performed within the tolerances set by manufacturer quality assurance programs. Furthermore, because BMD data from these DXA systems were slightly different, data at 16 weeks were always measured using the same DXA system used at baseline. All technicians who were responsible for measuring BMD were blinded to the details of this study. T‐scores were calculated by dividing the difference between a patient's measured BMD and the mean BMD of healthy young adults, matched for sex and ethnic group, and by expressing the difference relative to the young adult population standard deviation (SD). Z‐scores were calculated by dividing the difference between a patient's measured BMD and the age‐matched mean BMD expected for the patient's peers (a healthy normal subject matched for age, sex, and ethnic group) by the age‐matched population SD.
BTMs
Two serum BTMs, sBAP and sNTX, were measured; all assays were performed by SRL Inc. (Tokyo, Japan). All blood samples were collected in the morning after fasting for ≥8 h. sBAP levels were measured by a completely automated chemiluminescent enzyme immunoassay using a Beckman Coulter Access Ostase (Beckman Coulter, Inc., Pasadena, CA) with a reference range of 3.7–20.9 µg/L for men, 2.9–14.5 µg/L for premenopausal women, and 3.8–22.6 µg/L for postmenopausal women. sNTX levels were measured using a completely automated enzyme‐linked immunosorbent assay (ELISA) with Osteomark NTx Serum ELISA Test Kits (Alere Inc., Seattle, WA); the reference ranges for men and premenopausal and postmenopausal women were 9.5–17.7, 7.5–16.5, and 10.7–24.0 nmol bone collagen equivalent per L, respectively.
Outcome Assessment
The primary endpoints were BMD variation in the lumbar spine (BMD‐LS; L2–L4) measured by DXA and percent changes in sNTX and sBAP levels between baseline and 16 weeks after starting chemotherapy. Secondary endpoints included the influence of GC dose intensity, treatment duration, regimens, primary site, and differences in DXA‐measured BMD changes in the total hip (TH) and femoral neck (FN). Doses and schedules for each GC premedication were not prespecified and were at the doctors’ discretion. The protocol allowed additive oral steroidal usage or rescue for allergic reactions.
Statistical Analysis
There are no previous data on BMD variation in cancer chemotherapy. Assuming that the effect size to assess variations in BMD between baseline and 16 weeks was 0.35, a required sample size was estimated as 76, with a two‐sided alpha value of 0.05 and a power of 85%. We estimated that 95 cases would be needed to achieve required cases during patient accrual (2 years) and follow‐up periods (3 years), assuming 20% attrition [36]. Patient characteristics were summarized using descriptive statistics. Steroid dose intensity was calculated by dividing total dose by number of treatment weeks. Changes in BMDs and BTM levels between baseline and 16 weeks were examined using the paired samples t test. Correlation analysis between BMD percent changes among 16‐week LS, TH, and FN values and several baseline variables (sBAP, sNTX, calcium, corrected calcium, and albumin levels) or other variables (percent change among 16‐week sBAP/sNTX/calcium/corrected calcium/albumin levels, age, total steroid dosage, steroid dose intensity, chemotherapy cycles, administration days of steroid, and steroid dose per cycle) was estimated using Pearson's correlation coefficients. Associations of different clinical items (age, sex, Eastern Cooperative Oncology Group performance status [ECOG PS], primary site, treatment cycles, treatment setting, steroid administration style, days of steroid administration, steroid dose per cycle, baseline T‐score, and the history of gastrectomy) with BMD divided into high decreasing or low decreasing groups as categorical variables were analyzed using the chi‐square test. Fisher's exact test was used when the frequency of any cell of the contingency table was ≤5. Statistical significance was set at p < .05. Statistical calculations were performed using SPSS for Macintosh (release 22.0; SPSS Inc., Chicago, IL).
Results
Patient Characteristics
There were a total of 98 cases enrolled for the study from June 2013 to April 2015. As shown in Figure 1, three patients were excluded, and 21 discontinued treatment or missed follow‐up assessment. The full analysis set (FAS) for assessing primary outcome included 74 patients with a median age of 66 years (range, 28–87 years). Almost all female patients were postmenopausal except for one patient.
Figure 1.
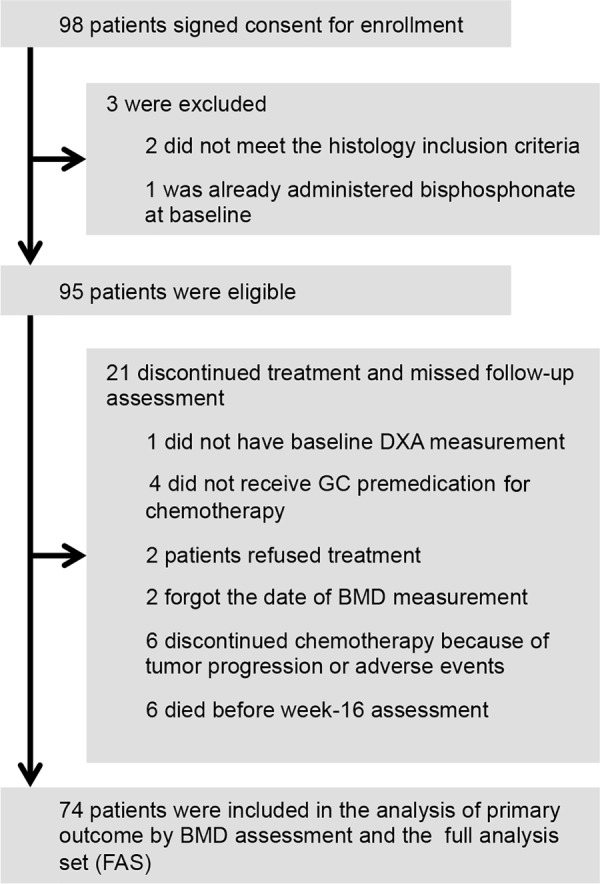
CONSORT diagram.
Abbreviations: BMD, bone mineral density; DXA, dual energy x‐ray absorptiometry; FAS, full analysis set GC, glucocorticoid.
Changes in BMD and BTM Between Baseline and 16 Weeks
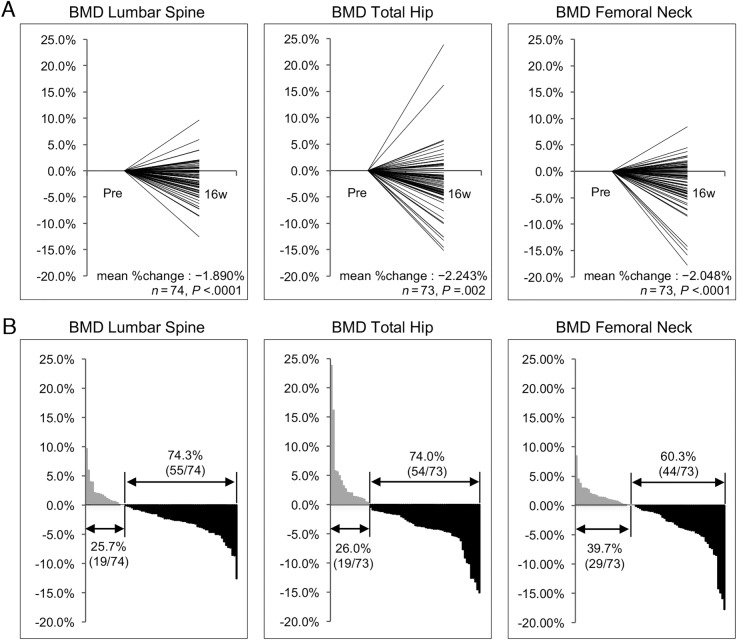
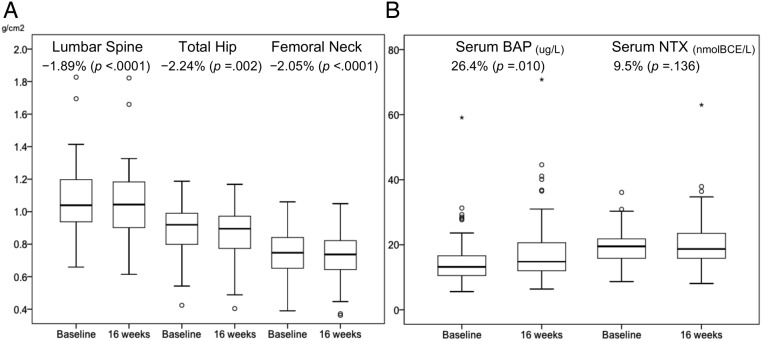
There were statistically significant decreases in mean BMDs between baseline and 16 weeks in all sites: LS (p < .0001), TH (p = .002), and FN (p < .0001; Table 2). Mean BMD percent changes at 16 weeks were −1.890% (95% CI, −2.668 to −1.112%), −2.243% (95% CI, −3.593 to −0.893%), and −2.048% (95% CI, −3.112 to −0.985%) in LS, TH, and FN, respectively. Decreased BMDs were observed in 55 patients (74.3%) in LS, 54 (74.0%) in TH, and 44 (60.3%) in FN (Fig. 2). There was no significant difference in sNTX levels between baseline and 16 weeks (p = .136). However, there was a statistically significant increase in sBAP levels (p = .010; Fig. 3).
Table 2. Variations in BMD and BTMs between baseline and 16 weeks.
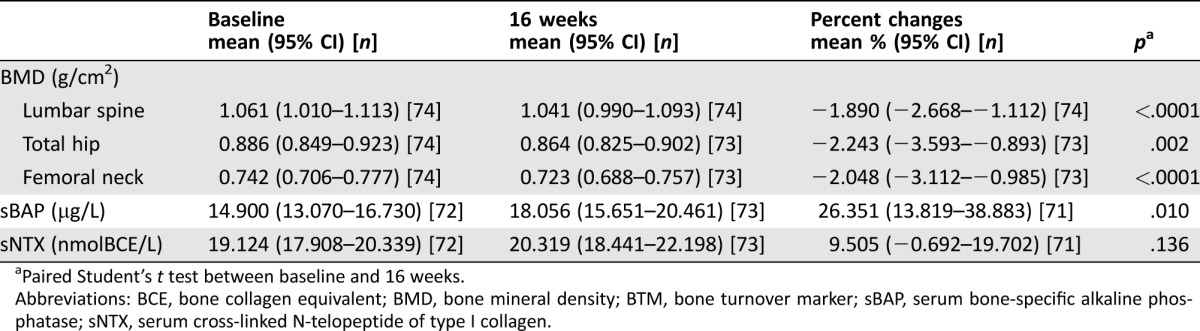
Paired Student's t test between baseline and 16 weeks.
Abbreviations: BCE, bone collagen equivalent; BMD, bone mineral density; BTM, bone turnover marker; sBAP, serum bone‐specific alkaline phosphatase; sNTX, serum cross‐linked N‐telopeptide of type I collagen.
Figure 2.
Percent changes in BMD of lumbar spine, total hip, and femoral neck from baseline to 16 weeks. (A) Plot of each data point. (B) Waterfall plot T‐axis representing % changes.
Abbreviations: BMD, bone mineral density.
Figure 3.
Data of BMDs and BTMs at baseline and 16 weeks. (A) BMD of lumbar spine, total hip, and femoral neck. (B) BTMs (sBAP and sNTX).
Abbreviations: BMD, bone mineral density; BTM, bone turnover marker; sBAP, bone alkaline phosphatase; sNTX, serum cross‐linked N‐telopeptides of type I collagen
Table 1. Baseline patient characteristics.
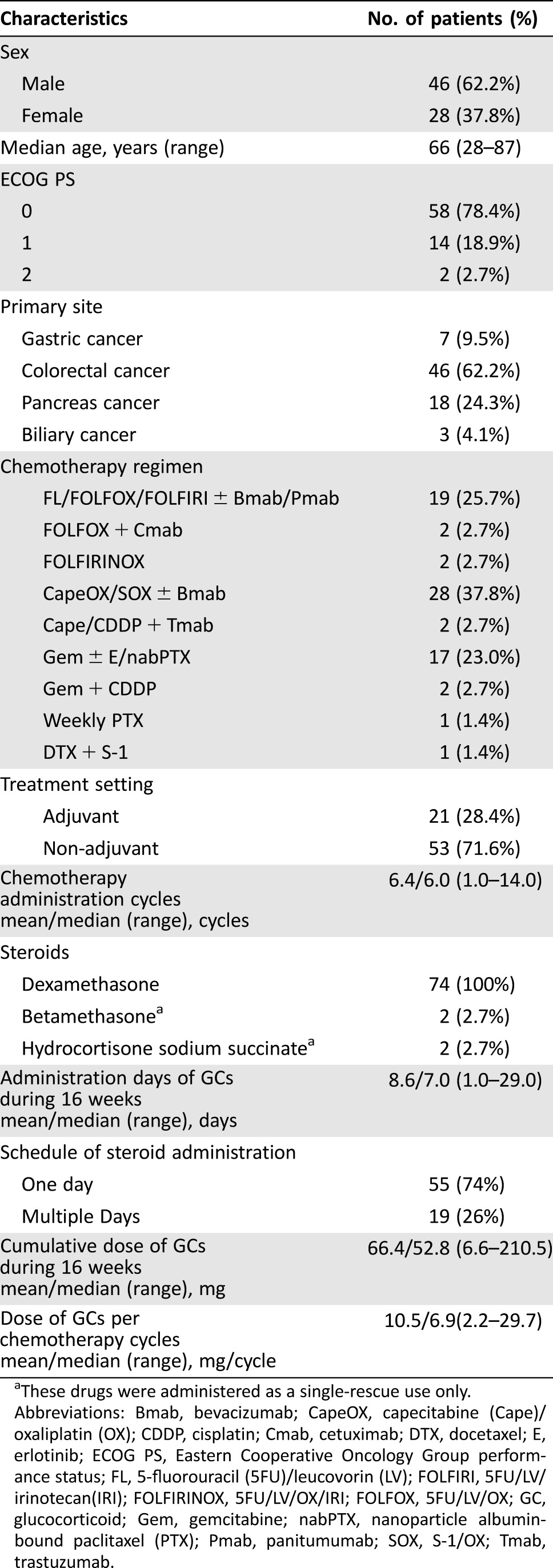
These drugs were administered as a single‐rescue use only.
Abbreviations: Bmab, bevacizumab; CapeOX, capecitabine (Cape)/oxaliplatin (OX); CDDP, cisplatin; Cmab, cetuximab; DTX, docetaxel; E, erlotinib; ECOG PS, Eastern Cooperative Oncology Group performance status; FL, 5‐fluorouracil (5FU)/leucovorin (LV); FOLFIRI, 5FU/LV/irinotecan(IRI); FOLFIRINOX, 5FU/LV/OX/IRI; FOLFOX, 5FU/LV/OX; GC, glucocorticoid; Gem, gemcitabine; nabPTX, nanoparticle albumin‐bound paclitaxel (PTX); Pmab, panitumumab; SOX, S‐1/OX; Tmab, trastuzumab.
Correlations Between BMD Percent Change Among 16 Weeks and Variables
Table 3 shows that there were negative correlations between the percent change of BMD‐LS and that of sBAP (r = −0.322, p = .006) or sNTX levels (r = −0.274, p = .021). Neither total steroid dosage nor steroid dose intensity correlated with BMD change. Furthermore, other variables, such as age, sex, ECOG PS, primary tumor site, treatment setting (i.e., adjuvant or non‐adjuvant treatment), days of steroid administration, steroid dose per cycle, and T‐score at the baseline, were also not associated with the degree of BMD change (Table 4). Although no influences of steroid administration schedules and doses were indicated, there was a statistical correlation between the number of chemotherapy treatment cycles and the degree of BMD decrease (p = .02). The World Health Organization defined osteoporosis patients as having BMD of ≥2.5 SDs below the mean value for young adults (T‐score ≤ −2.5) [30], [37]. Therefore, we investigated the BMD change at baseline in osteoporosis patients with T‐scores of ≤−2.5. Regardless of the baseline T‐score, BMD‐LS tended to be lower at 16 weeks. Because metabolic bone disorders developing after gastrectomy influence calcium homeostasis [38], [39], we investigated the influence of gastrectomy on BMD change. Only six FAS cases had previous gastrectomy, and we could not find a consistent relationship between BMD changes and gastrectomy (data not shown).
Table 3. Correlation of the percent change of each variable at week 16.
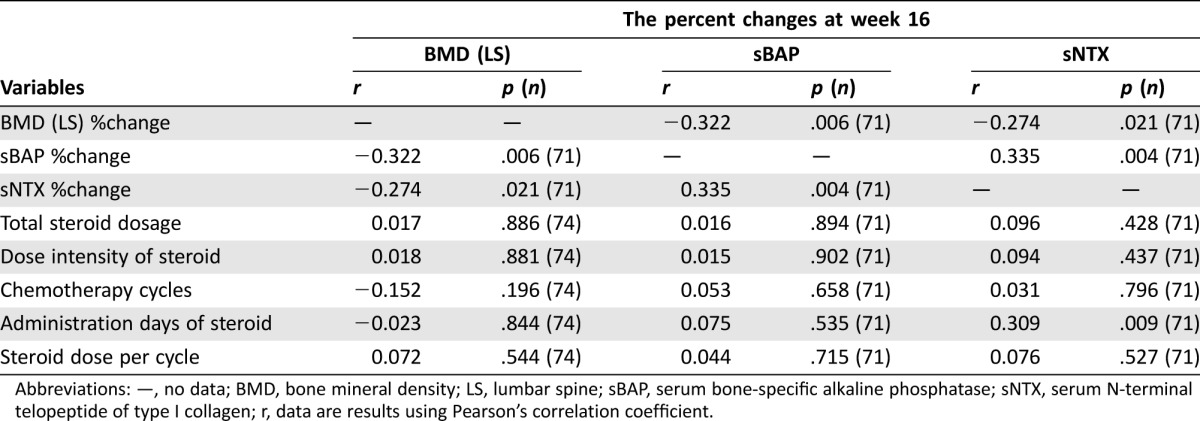
Abbreviations: —, no data; BMD, bone mineral density; LS, lumbar spine; sBAP, serum bone‐specific alkaline phosphatase; sNTX, serum N‐terminal telopeptide of type I collagen; r, data are results using Pearson's correlation coefficient.
Table 4. Associations of BMD decreasing levels with various variables.
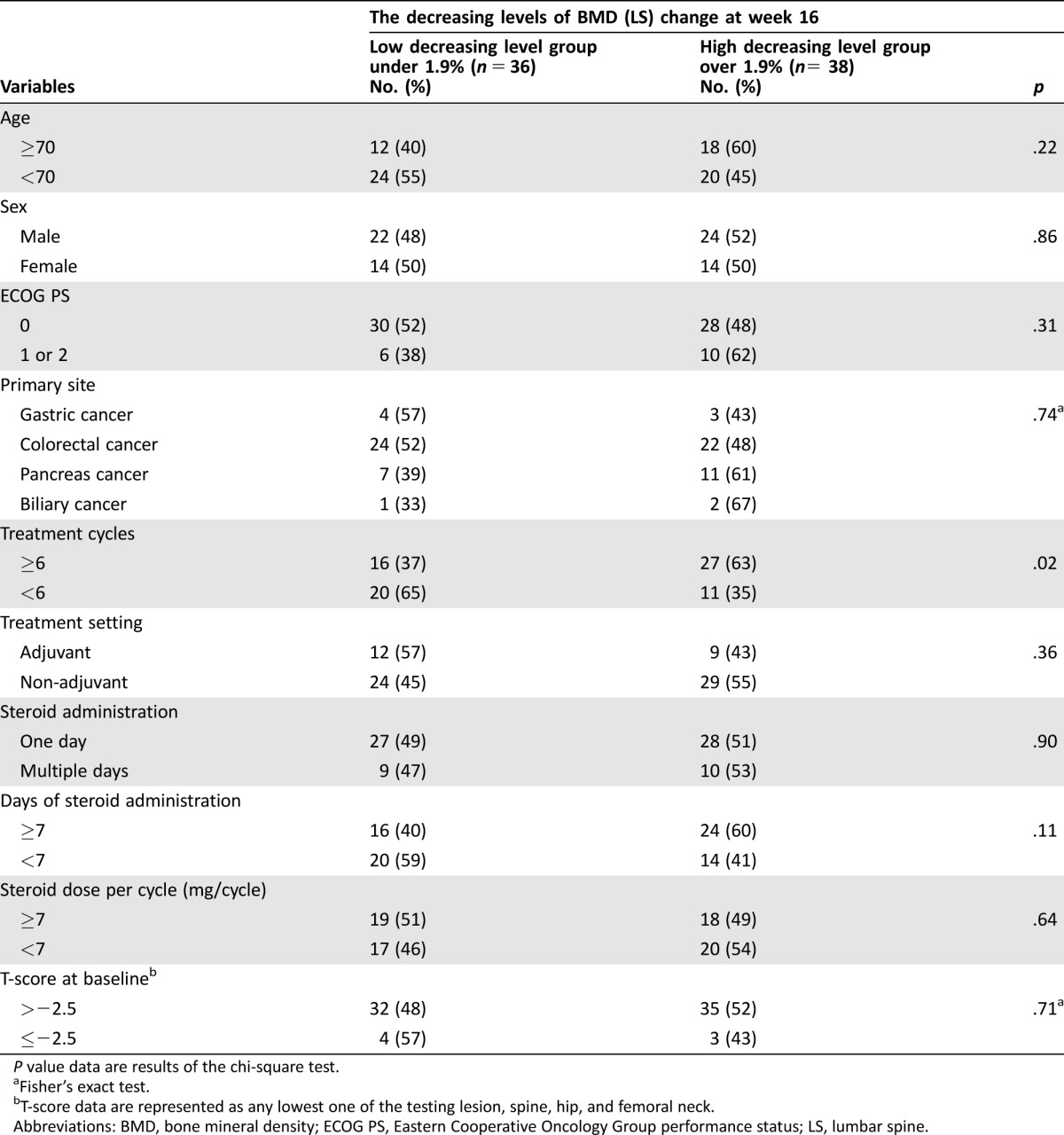
P value data are results of the chi‐square test.
Fisher's exact test.
T‐score data are represented as any lowest one of the testing lesion, spine, hip, and femoral neck.
Abbreviations: BMD, bone mineral density; ECOG PS, Eastern Cooperative Oncology Group performance status; LS, lumbar spine.
Discussion
This study showed that significant BMD decreases occurred in patients with gastrointestinal cancer who received 16 weeks of chemotherapy. In the pivotal trial to investigate the effects of the anti‐receptor activator of nuclear factor kappa‐B ligand antibody denosumab in postmenopausal breast cancer patients receiving adjuvant aromatase inhibitor therapy, patients in the placebo group had a relative decrease in BMDs of −1.81% at LS, −1.20% at TH, and −1.08% at FN during 12 months [40]. Furthermore, in the trial to investigate the effects of zoledronic acid in non‐metastatic prostate cancer undergoing androgen deprivation therapy, patients in the placebo group had a relative decrease in BMDs of −2.2% at LS, −2.8% at TH, and −2.1% at FN during 12 months [41]. Based on these data, it seems that the decreasing BMD levels in our study due to the 16‐week GC usage in gastrointestinal cancer chemotherapy were comparable to that after 12 months of adjuvant aromatase inhibitor therapy or androgen deprivation therapy. Although it is well known that GC‐induced BMD change depends on cumulative GC dose administered [15], [18], [42], contrary to expectation, there was no relationship between the cumulative GC dose, GC dose intensity, GC dose per chemotherapy cycles, administration days of GCs, and BMD changes of any anatomic sites in this study. The BTM changes in this study were also interesting. Generally, in an early rapid phase of GC‐induced osteoporosis, BMD is rapidly reduced due to increased bone resorption and is then followed by a slower phase characterized by decreased bone formation [43], [44]. However, in the case of sNTX, which was evaluated as a bone resorption marker, there was no statistically significant difference between baseline and 16 weeks. In the case of sBAP, which is a known bone formation marker, there was a significant increase at 16 weeks compared with that of baseline. Also, there were significant inverse correlations between sBAP or sNTX percent changes and BMD‐LS percent changes over 16 weeks (Table 3). Although BTMs are often used as markers to assess the effect of anti‐osteoporosis treatments, the trend of these BTMs at 16 weeks could be used as a surrogate marker for decreased BMD‐LS. Some studies report that cytotoxic agents such as doxorubicin and methotrexate reduced bone formation by reducing osteoblastic activity in rats, and cancer treatment itself carries the risk of causing the development of osteoporosis both directly and indirectly [45], [46]. We have no data on the trend of BTM changes over time or how chemotherapy and/or GCs would impact bone metabolism, such as osteoclastic or osteoblastic effects. However, because we could not show the dose dependency of steroids on the decreasing BMD over 16 weeks chemotherapy and because there was a statistical correlation between BMD decrease and the number of chemotherapy treatment cycles, cancer chemotherapy itself, and not premedication GCs, might directly cause the bone loss detected in this study. Changes in BMD and BTMs are very complicated, and assessing both changes of these BTMs over time as well as the relationship with the timing of interventions such as chemotherapy and GC administration is essential.
Although there were four studies that had demonstrated decreases in BMD in the lumbar spine of 1%–4% during a 6‐month period after induction of breast cancer chemotherapy [47], [48], [49], [50], to the best of our knowledge, the present study provides the first report of a prospective observational study to evaluate bone metabolism in gastrointestinal cancer chemotherapy. In contrast to these four breast cancer reports, recently, Christensen et al. reported the opposite result in 97 Danish early‐stage breast cancer patients; in that study, an increase in BMD in the spine of 1.36% was seen approximately 4 months after the start of adjuvant chemotherapy [51]. All participants in this study were advised to take daily calcium (800 mg) and vitamin D (20 µg) supplements while undergoing chemotherapy. In contrast, in our study, no participants took calcium or vitamin D supplements, which is a decidedly different approach of our study. Intake of these supplements might cancel out the effect of decreasing BMD due to breast cancer chemotherapy or GC premedication, which might be a potential bias. Furthermore, both the type and dose of steroid used in each study were different. Generally, GC premedication is repeated usage of dexamethasone, which shows almost six times more GC activity than prednisolone (PSL), and dexamethasone is usually dosed weekly, biweekly, or triweekly, but not daily. In the study by Christensen et al., PSL was used as anti‐emetic prophylaxis with a typical dose of 175 mg for one cycle of epirubicin and cyclophosphamide combination regimen or 300 mg for one cycle of docetaxel regimen, and the median total cumulative PSL dose the participants received was 1,325 mg. On the other hand, the median cumulative dose of dexamethasone in our study was 52.8 mg, which is equal to almost 316 mg of PSL, and total steroid dose was lower that than used in the Christensen et al. study. Because calcium and vitamin D were not used in our study, the BMD decrease might be detected.
Although we could not accurately investigate risk of fracture in this study because the follow‐up time was too short for the assessment of bone fracture incidence in the entire study population, bone fracture occurred in two cases in the FAS within the study time. If bone fractures occur in cancer patients during chemotherapy, chemotherapy must be temporarily stopped and QOL markedly worsens; therefore, fracture prevention is important to continue comfortable chemotherapy. Furthermore, it is more important to eliminate the unnecessary steroid administration, and, if its usage is necessary for supporting cancer treatment, we have to be mindful of secondary osteoporosis. Further prospective interventional study is needed to investigate not only whether the prevention of secondary osteoporosis can decrease the risk and incidence of bone fracture caused by chemotherapy and/or GC premedication‐induced osteoporosis but also whether it can contribute to the amelioration of patients’ QOL or cancer prognosis.
There were notable limitations in this study. First, the sample size was small. Although the effect size assumed to appropriately assess the variation of BMD and used to calculate a sample size was 0.35, the actual effect size was 0.40, and the result of this study met the assumption. We have planned further studies to assess the preventive effect of anti‐osteoporosis agents, and we are going to use the same sample size for comparison with the present study. Second, the dosage of GCs was not prespecified in the protocol. It was difficult to predetermine GC premedication because patients with many different types of cancers and regimens were enrolled in the study. Additionally, several patients required additive or rescue GCs to treat breakthrough CINV or allergic reactions. However, even when patients who received unscheduled additive steroids were excluded from the analysis, there were significant decreases in BMD percent changes in LS, TH, and FN (supplemental online Table 1). In the daily practical setting, the addition of GCs occurs frequently, and it is difficult to decide the cut‐off value that causes secondary osteoporosis. Third, although chemotherapy cycles and BMD change were significantly correlated in this study, it is still uncertain what is most responsible for the decrease in BMD, whether it is due to GC premedication, chemotherapy, or cancer itself, or these factors’ complicated interaction. It is a very important and interesting point but also a complicated issue to prove clearly. To investigate whether BMD changes would be caused by steroid or chemotherapy alone, we have to conduct a randomized prospective study with an experimental arm that prohibits use of any steroid administration during treatment, which is ethically unacceptable. In this study, however, there were no significant difference in levels of BMD change with respect to type of cancer, regimen used, and treatment setting (adjuvant or non‐adjuvant). However, cancer type or the difference among chemotherapy regimens is unlikely to have a significant, direct impact on the BMD decrease. Fourth, we had not pre‐planned to collect follow‐up data of BMD and BTMs after the 16‐week evaluation, and we could not know whether the bone loss that occurred during chemotherapy could be recovered without an active support. There is evidence that bone loss resulting from GC therapy in rheumatoid arthritis may be partially reversible [52], and, therefore, there is a possibility to recover decreased BMD in patients receiving adjuvant cancer chemotherapy. Another important issue is whether intervention for chemotherapy and/or GC premedication‐induced decrease in BMD in all patients receiving adjuvant chemotherapy should be performed. Further studies are required to determine whether continuous anti‐osteoporotic treatment beyond completion of chemotherapy cycles in patients receiving adjuvant chemotherapy is useful.
Conclusion
This is the first report to focus on bone health in gastrointestinal cancer patients receiving chemotherapy. We showed that BMD levels decreased and sBAP levels increased during 16 weeks of chemotherapy, independent of primary site, chemotherapy treatment regimens, and additive steroid usage. Further study is important to assess what factors most contributed to this bone loss in gastrointestinal cancer patients and whether the prevention of BMD decrease is required, as well as to determine type and length of intervention.
See http://www.TheOncologist.com for supplemental material available online.
Supplementary Material
Acknowledgments
We thank all of the participants and their families and the investigators of the Hokkaido Gastrointestinal Cancer Study Group (HGCSG) for their patient recruitment and technical advice. Michio Nakamura and Yoshito Komatsu had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. This study is a registered clinical trial: University Hospital Medical Information Network (UMIN) Clinical Trials Registry (protocol ID UMIN000011054).
Author Contributions
Conception/Design: M. Nakamura, Y. Komatsu
Provision of study material or patients: M. Nakamura, A. Ishiguro, T. Muranaka, H. Fukushima, S. Yuki, K. Ono, T. Murai, C. Matsuda, A. Oba, K. Itaya, T. Sone, M. Yagisawa, Y. Koike, A. Endo, Y. Tsukuda, Y. Ono, T. Kudo, A. Nagasaka, S. Nishikawa, Y. Komatsu
Collection and/or assembly of data: M. Nakamura, A. Ishiguro, T. Muranaka, H. Fukushima, S. Yuki, K. Ono, T. Murai, C. Matsuda, A. Oba, K. Itaya, T. Sone, M. Yagisawa, Y. Koike, A. Endo, Y. Tsukuda, Y. Ono, T. Kudo, A. Nagasaka, S. Nishikawa, Y. Komatsu
Data analysis and interpretation: M. Nakamura, A. Ishiguro, T. Muranaka, S. Yuki, K. Ono, A. Oba, M. Yagisawa, Y. Komatsu
Manuscript writing: M. Nakamura, A. Ishiguro, T. Muranaka, H. Fukushima, S. Yuki, K. Ono, T. Murai, C. Matsuda, A. Oba, K. Itaya, T. Sone, M. Yagisawa, Y. Koike, A. Endo, Y. Tsukuda, Y. Ono, T. Kudo, A. Nagasaka, S. Nishikawa, Y. Komatsu
Final approval of manuscript: M. Nakamura, A. Ishiguro, T. Muranaka, H. Fukushima, S. Yuki, K. Ono, T. Murai, C. Matsuda, A. Oba, K. Itaya, T. Sone, M. Yagisawa, Y. Koike, A. Endo, Y. Tsukuda, Y. Ono, T. Kudo, A. Nagasaka, S. Nishikawa, Y. Komatsu
Disclosures
Michio Nakamura: Chugai, Daiichi Sankyo (H); Atsushi Ishiguro: Chugai, Merck Serono, Yakult (H); Satoshi Yuki: Chugai, Taiho, Takeda (H); Yoshito Komatsu: Chugai, Ono, Pfizer (H), Kureha, Lilly (RF), Daiichi Sankyo, Merck Serono, Novartis, Taiho, Takeda, Yakult (H, RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1. Basch E, Prestrud AA, Hesketh PJ et al. Antiemetics: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 2011;29:4189–4198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Roila F, Molassiotis A, Herrstedt J et al. 2016 MASCC and ESMO guideline update for the prevention of chemotherapy‐ and radiotherapy‐induced nausea and vomiting and of nausea and vomiting in advanced cancer patients. Ann Oncol 2016;27:v119–v133. [DOI] [PubMed] [Google Scholar]
- 3. Lenz HJ. Management and preparedness for infusion and hypersensitivity reactions. The Oncologist 2007;12:601–609. [DOI] [PubMed] [Google Scholar]
- 4. Peereboom DM, Donehower RC, Eisenhauer EA et al. Successful re‐treatment with taxol after major hypersensitivity reactions. J Clin Oncol 1993;11:885–890. [DOI] [PubMed] [Google Scholar]
- 5. Bonomi P, Kim K, Fairclough D et al. Comparison of survival and quality of life in advanced non‐small‐cell lung cancer patients treated with two dose levels of paclitaxel combined with cisplatin versus etoposide with cisplatin: Results of an Eastern Cooperative Oncology Group trial. J Clin Oncol 2000;18:623–631. [DOI] [PubMed] [Google Scholar]
- 6. Bookman MA, Kloth DD, Kover PE et al. Short‐course intravenous prophylaxis for paclitaxel‐related hypersensitivity reactions. Ann Oncol 1997;8:611–614. [DOI] [PubMed] [Google Scholar]
- 7. de Gramont A, Figer A, Seymour M et al. Leucovorin and fluorouracil with or without oxaliplatin as first‐line treatment in advanced colorectal cancer. J Clin Oncol 2000;18:2938–2947. [DOI] [PubMed] [Google Scholar]
- 8. Douillard JY, Cunningham D, Roth AD et al. Irinotecan combined with fluorouracil compared with fluorouracil alone as first‐line treatment for metastatic colorectal cancer: A multicentre randomised trial. Lancet 2000;355:1041–1047. [DOI] [PubMed] [Google Scholar]
- 9. Hironaka S, Ueda S, Yasui H et al. Randomized, open‐label, phase III study comparing irinotecan with paclitaxel in patients with advanced gastric cancer without severe peritoneal metastasis after failure of prior combination chemotherapy using fluoropyrimidine plus platinum: WJOG 4007 trial. J Clin Oncol 2013;31:4438–4444. [DOI] [PubMed] [Google Scholar]
- 10. Kwon JS, Elit L, Finn M et al. A comparison of two prophylactic regimens for hypersensitivity reactions to paclitaxel. Gynecol Oncol 2002;84:420–425. [DOI] [PubMed] [Google Scholar]
- 11. Chouhan JD, Herrington JD. Single premedication dose of dexamethasone 20 mg IV before docetaxel administration. J Oncol Pharm Pract 2011;17:155–159. [DOI] [PubMed] [Google Scholar]
- 12. Siena S, Glynne‐Jones R, Adenis A et al. Reduced incidence of infusion‐related reactions in metastatic colorectal cancer during treatment with cetuximab plus irinotecan with combined corticosteroid and antihistamine premedication. Cancer 2010;116:1827–1837. [DOI] [PubMed] [Google Scholar]
- 13. Vardy J, Chiew KS, Galica J et al. Side effects associated with the use of dexamethasone for prophylaxis of delayed emesis after moderately emetogenic chemotherapy. Br J Cancer 2006;94:1011–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Han HS, Park JC, Park SY et al. A prospective multicenter study evaluating secondary adrenal suppression after antiemetic dexamethasone therapy in cancer patients receiving chemotherapy: A Korean South West Oncology Group study. The Oncologist 2015;20:1432–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. van Staa TP, Leufkens HG, Abenhaim L et al. Oral corticosteroids and fracture risk: Relationship to daily and cumulative doses. Rheumatology (Oxford) 2000;39:1383–1389. [DOI] [PubMed] [Google Scholar]
- 16. van Staa TP, Leufkens HG, Abenhaim L et al. Use of oral corticosteroids and risk of fractures. J Bone Miner Res 2000;15:993–1000. [DOI] [PubMed] [Google Scholar]
- 17.Recommendations for the prevention and treatment of glucocorticoid‐induced osteoporosis: 2001 update. American College of Rheumatology Ad Hoc Committee on Glucocorticoid‐Induced Osteoporosis. Arthritis Rheum 2001;44:1496–1503. [DOI] [PubMed] [Google Scholar]
- 18. van Staa TP, Leufkens HG, Cooper C. The epidemiology of corticosteroid‐induced osteoporosis: A meta‐analysis. Osteoporos Int 2002;13:777–787. [DOI] [PubMed] [Google Scholar]
- 19. Devogelaer JP, Goemaere S, Boonen S et al. Evidence‐based guidelines for the prevention and treatment of glucocorticoid‐induced osteoporosis: A consensus document of the Belgian Bone Club. Osteoporos Int 2006;17:8–19 [DOI] [PubMed] [Google Scholar]
- 20. Gralow JR, Biermann JS, Farooki A et al. NCCN Task Force Report: Bone health in cancer care. J Natl Compr Canc Netw 2013;11 Suppl 3:S1–S50;quiz S51. [DOI] [PubMed] [Google Scholar]
- 21. Khan MN, Khan AA. Cancer treatment‐related bone loss: A review and synthesis of the literature. Curr Oncol 2008;15(Suppl 1):S30–S40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lipton A, Uzzo R, Amato RJ et al. The science and practice of bone health in oncology: Managing bone loss and metastasis in patients with solid tumors. J Natl Compr Canc Netw 2009;7 Suppl 7:S1–S29;quiz S30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Coleman RE, Banks LM, Girgis SI et al. Reversal of skeletal effects of endocrine treatments in the Intergroup Exemestane Study. Breast Cancer Res Treat 2010;124:153–161. [DOI] [PubMed] [Google Scholar]
- 24. Eastell R, Adams J, Clack G et al. Long‐term effects of anastrozole on bone mineral density: 7‐year results from the ATAC trial. Ann Oncol 2011;22:857–862. [DOI] [PubMed] [Google Scholar]
- 25. Brown JE, Sherriff JM, James ND. Osteoporosis in patients with prostate cancer on long‐term androgen deprivation therapy: An increasing, but under‐recognized problem. BJU Int 2010;105:1042–1043. [DOI] [PubMed] [Google Scholar]
- 26. Nishio K, Tanabe A, Maruoka R et al. Bone mineral loss induced by anticancer treatment for gynecological malignancies in premenopausal women. Endocr Connect 2013;2:11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hanks GW, Trueman T, Twycross RG. Corticosteroids in terminal cancer–A prospective analysis of current practice. Postgrad Med J 1983;59:702–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Genant HK, Engelke K, Fuerst T et al. Noninvasive assessment of bone mineral and structure: State of the art. J Bone Miner Res 1996;11:707–730. [DOI] [PubMed] [Google Scholar]
- 29. Kanis JA, Delmas P, Burckhardt P et al. Guidelines for diagnosis and management of osteoporosis. The European Foundation for Osteoporosis and Bone Disease. Osteoporos Int 1997;7:390–406. [DOI] [PubMed] [Google Scholar]
- 30. Kanis JA, Glüer CC. An update on the diagnosis and assessment of osteoporosis with densitometry. Committee of Scientific Advisors, International Osteoporosis Foundation. Osteoporos Int 2000;11:192–202. [DOI] [PubMed] [Google Scholar]
- 31. Clemens JD, Herrick MV, Singer FR et al. Evidence that serum NTx (collagen‐type I N‐telopeptides) can act as an immunochemical marker of bone resorption. Clin Chem 1997;43:2058–2063. [PubMed] [Google Scholar]
- 32. Hanson DA, Weis MA, Bollen AM et al. A specific immunoassay for monitoring human bone resorption: Quantitation of type I collagen cross‐linked N‐telopeptides in urine. J Bone Miner Res 1992;7:1251–1258. [DOI] [PubMed] [Google Scholar]
- 33. Ross PD, Kress BC, Parson RE et al. Serum bone alkaline phosphatase and calcaneus bone density predict fractures: A prospective study. Osteoporos Int 2000;11:76–82. [DOI] [PubMed] [Google Scholar]
- 34. Ueno H, Ioka T, Ikeda M et al. Randomized phase III study of gemcitabine plus S‐1, S‐1 alone, or gemcitabine alone in patients with locally advanced and metastatic pancreatic cancer in Japan and Taiwan: GEST study. J Clin Oncol 2013;31:1640–1648. [DOI] [PubMed] [Google Scholar]
- 35. Blake GM, Fogelman I. The role of DXA bone density scans in the diagnosis and treatment of osteoporosis. Postgrad Med J 2007;83:509–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Song JW, Chung KC. Observational studies: Cohort and case‐control studies. Plast Reconstr Surg 2010;126:2234–2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.World Health Organization . Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Report of a WHO Study Group. World Health Organ Tech Rep Ser 1994;843:1–129. [PubMed] [Google Scholar]
- 38. Zittel TT, Zeeb B, Maier GW et al. High prevalence of bone disorders after gastrectomy. Am J Surg 1997;174:431–438. [DOI] [PubMed] [Google Scholar]
- 39. Tovey FI, Hall ML, Ell PJ et al. Postgastrectomy osteoporosis. Br J Surg 1991;78:1335–1337. [DOI] [PubMed] [Google Scholar]
- 40. Gnant M, Pfeiler G, Dubsky PC et al. Adjuvant denosumab in breast cancer (ABCSG‐18): A multicentre, randomised, double‐blind, placebo‐controlled trial. Lancet 2015;386:433–443. [DOI] [PubMed] [Google Scholar]
- 41. Smith MR, Eastham J, Gleason DM et al. Randomized controlled trial of zoledronic acid to prevent bone loss in men receiving androgen deprivation therapy for nonmetastatic prostate cancer. J Urol 2003;169:2008–2012. [DOI] [PubMed] [Google Scholar]
- 42. De Vries F, Bracke M, Leufkens HG et al. Fracture risk with intermittent high‐dose oral glucocorticoid therapy. Arthritis Rheum 2007;56:208–214. [DOI] [PubMed] [Google Scholar]
- 43. Hofbauer LC, Gori F, Riggs BL et al. Stimulation of osteoprotegerin ligand and inhibition of osteoprotegerin production by glucocorticoids in human osteoblastic lineage cells: Potential paracrine mechanisms of glucocorticoid‐induced osteoporosis. Endocrinology 1999;140:4382–4389. [DOI] [PubMed] [Google Scholar]
- 44. Epstein S, Inzerillo AM, Caminis J et al. Disorders associated with acute rapid and severe bone loss. J Bone Miner Res 2003;18:2083–2094. [DOI] [PubMed] [Google Scholar]
- 45. Pfeilschifter J, Diel IJ. Osteoporosis due to cancer treatment: Pathogenesis and management. J Clin Oncol 2000;18:1570–1593. [DOI] [PubMed] [Google Scholar]
- 46. Wissing MD. Chemotherapy‐ and irradiation‐induced bone loss in adults with solid tumors. Curr Osteoporos Rep 2015;13:140–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Shapiro CL, Manola J, Leboff M. Ovarian failure after adjuvant chemotherapy is associated with rapid bone loss in women with early‐stage breast cancer. J Clin Oncol 2001;19:3306–3311. [DOI] [PubMed] [Google Scholar]
- 48. Vehmanen L, Saarto T, Risteli J et al. Short‐term intermittent intravenous clodronate in the prevention of bone loss related to chemotherapy‐induced ovarian failure. Breast Cancer Res Treat 2004;87:181–188. [DOI] [PubMed] [Google Scholar]
- 49. Hershman DL, McMahon DJ, Crew KD et al. Zoledronic acid prevents bone loss in premenopausal women undergoing adjuvant chemotherapy for early‐stage breast cancer. J Clin Oncol 2008;26:4739–4745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Cameron DA, Douglas S, Brown JE et al. Bone mineral density loss during adjuvant chemotherapy in pre‐menopausal women with early breast cancer: Is it dependent on oestrogen deficiency? Breast Cancer Res Treat 2010;123:805–814. [DOI] [PubMed] [Google Scholar]
- 51. Christensen CØ, Cronin‐Fenton D, Frøslev T et al. Change in bone mineral density during adjuvant chemotherapy for early‐stage breast cancer. Support Care Cancer 2016;24:4229–4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Laan RF, van Riel PL, van de Putte LB et al. Low‐dose prednisone induces rapid reversible axial bone loss in patients with rheumatoid arthritis. A randomized, controlled study. Ann Intern Med 1993;119:963–968. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.