R‐CHOP has improved the clinical outcome of diffuse large B‐cell lymphoma compared with CHOP alone; however, patients with unfavorable prognostic factors have less change of benefitting from such standard treatments. Advanced age is a crucial risk factor for unfavorable prognosis, as is hypoalbuminemia and medical comorbidities. This study measured the effects of these three biomarkers on clinical outcomes and developed a decision‐making treatment model that can serve as a guide for optimal personalized therapy for elderly patients treated with R‐CHOP.
Keywords: Comorbidity, Diffuse large B‐cell lymphoma, Geriatric assessment, Hypoalbuminemia, Personalized medicine, R‐CHOP chemotherapy
Abstract
Background.
Decision‐making models for elderly patients with diffuse large B‐cell lymphoma (DLBCL) treated with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R‐CHOP) are in great demand.
Patients and Methods.
The Society of Lymphoma Treatment in Japan (SoLT‐J), in collaboration with the West‐Japan Hematology and Oncology Group (West‐JHOG), collected and retrospectively analyzed the clinical records of ≥65‐year‐old patients with DLBCL treated with R‐CHOP from 19 sites across Japan to build an algorithm that can stratify adherence to R‐CHOP.
Results.
A total of 836 patients with a median age of 74 years (range, 65–96 years) were analyzed. In the SoLT‐J cohort (n = 555), age >75 years, serum albumin level <3.7 g/dL, and Charlson Comorbidity Index score ≥3 were independent adverse risk factors and were defined as the Age, Comorbidities, and Albumin (ACA) index. Based on their ACA index score, patients were categorized into “excellent” (0 points), “good” (1 point), “moderate” (2 points), and “poor” (3 points) groups. This grouping effectively discriminated the 3‐year overall survival rates, mean relative total doses (or relative dose intensity) of anthracycline and cyclophosphamide, unanticipated R‐CHOP discontinuance rates, febrile neutropenia rates, and treatment‐related death rates. Additionally, the ACA index showed comparable results for these clinical parameters when it was applied to the West‐JHOG cohort (n = 281).
Conclusion.
The ACA index has the ability to stratify the prognosis, tolerability to cytotoxic drugs, and adherence to treatment of elderly patients with DLBCL treated with R‐CHOP. The Oncologist 2017;22:554–560
Implications for Practice.
Currently, little is known regarding how to identify elderly patients with diffuse large B‐cell lymphoma who may tolerate a full dose of chemotherapy or to what extent cytotoxic drugs should be reduced in some specific conditions. The Society of Lymphoma Treatment in Japan developed a host‐dependent prognostic model consisting of higher age (>75 years), hypoalbuminemia (<3.7 g/dL), and higher Charlson Comorbidity Index score (≥3) for such elderly patients. This model can stratify the prognosis, tolerability to cytotoxic drugs, and adherence to treatment of these patients and thus help clinicians in formulating personalized treatment strategies for this growing patient population.
Introduction
The wide use of rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R‐CHOP) has improved the clinical outcome of diffuse large B‐cell lymphoma (DLBCL) compared with CHOP alone. Nevertheless, although a major subset of patients can be cured by receiving standard immunochemotherapy with R‐CHOP, patients with unfavorable prognostic factors have a lower chance of benefitting from such standard treatments. The International Prognostic Index (IPI), which was established before the advent of rituximab, has been widely used to predict the outcome of patients with DLBCL, and their prognoses are also well stratified by the IPI in the rituximab era [1], [2]. In particular, advanced age, that is, >60 years, is a crucial risk factor for unfavorable prognosis in patients with DLBCL. A recent study revealed that more advanced age, that is, >75 years, was associated with even poorer prognosis among patients with DLBCL treated with standard R‐CHOP [3]. Considering the fact that the median age of DLBCL patients exceeds 65 years in developed countries, the management of elderly patients is increasingly important [4].
In general, elderly patients have specific characteristics, such as multiple clinical comorbidities, malnutrition, impaired organ function, weakened immunocompetence, and/or limitations to activities of daily living. These conditions seriously affect the metabolism and toxicities of anticancer drugs [5]. In particular, hypoalbuminemia is a well‐known adverse risk factor among patients with cancers, including DLBCL [6], [7]. Hypoalbuminemia has been hypothesized to reflect poor nutrition status and inflammatory reaction to disease. In particular, recent studies reported that conditions associated with low serum protein concentration had adverse prognostic impact in elderly patients with DLBCL [8], [9].
Regarding the health status of elderly patients, the Charlson Comorbidity Index (CCI)—a geriatric assessment for clinical comorbidities in elderly patients with cancer [10]—was found to be a poor prognostic indicator in 80 elderly DLBCL patients with a median age of 73 years independent of the IPI [11]. Thus, a higher prevalence of coexisting disorders in elderly patients is one of the primary reasons for the failure of treatments for DLBCL.
Insufficient dose intensity of the treatment is another concern in elderly patients with DLBCL. They are more likely to face severe adverse events when treated with R‐CHOP, and thus the dose intensity of R‐CHOP is commonly reduced in clinical practice. However, an unnecessarily lower dose of key drugs may result in disease progression in potentially curable patients [12], [13]. Thus, there is an inevitable trade‐off between the ideal dose intensity of R‐CHOP and the toxicities confronting elderly patients with DLBCL in practice.
Based on the results of previous studies referenced above, we hypothesized that very old age, hypoalbuminemia, and medical comorbidities would reduce the tolerance to cytotoxic chemotherapy and thus lead to poor clinical outcomes of elderly DLBCL patients with such conditions. Therefore, we measured the effects of these three biomarkers on clinical outcomes and developed a decision‐making model; this may serve as a guide for optimal therapy personalization for elderly DLBCL patients treated with R‐CHOP.
Materials and Methods
Patient Selection
This multicenter, retrospective study was conducted by the Society of Lymphoma Treatment in Japan (SoLT‐J) in collaboration with the West‐Japan Hematology and Oncology Group (West‐JHOG). The inclusion criteria of the study were (a) patients ≥65 years of age who had been diagnosed with de novo DLBCL between January 2001 and December 2012; (b) patients who had received at least one cycle of R‐CHOP; and (c) patients who had not been affected with disease involving central nervous system lesions. Other specific sites of the disease, such as testis, were irrespectively included in the study. DLBCL was pathologically diagnosed according to the diagnostic procedure at each site. Pathological subtypes of DLBCL, such as Epstein‐Barr virus (EBV)‐positive DLBCL of the elderly, were also included in the study. Patients treated with tetrahydropyranyl‐adriamycin, a less cardiotoxic anthracycline widely used instead of doxorubicin in Japan for elderly patients with cancer, were also included [14].
Data Collection
Clinical features and treatment records of patients were electronically collected at the data center at Nihon University. Clinical data included age, sex, height, weight, serum lactate dehydrogenase concentration, performance status defined by Eastern Cooperative Oncology Group, Ann Arbor stage, number of extranodal sites, serum albumin concentration, and comorbidities. Treatment records consisted of the initial treatment plan (i.e., the number of R‐CHOP cycles and administration of radiation therapy); actual dose of cyclophosphamide, doxorubicin, vincristine, and prednisone per body surface area administered in every cycle; date of CHOP administration in every cycle; actual dose and site of radiation therapy, if any; prophylactic use of granulocyte‐colony stimulating factor (G‐CSF); any reasons for unexpected treatment discontinuance; occurrence or absence of febrile neutropenia; date of the last follow‐up; and any cause of death. This study, including the electronic data collection system, was approved by the institutional review board at Nihon University Itabashi Hospital where the data center was located and at each participating institution in accordance with its policy.
Clinical Parameters
The IPI score was determined as previously defined [1]. For the calculation of a total CCI score, medical comorbidities of each patient were scored 1–6 point(s) according to their impact on life expectancy, as previously defined by Charlson et al. [10]. Relative total dose (RTD) and relative dose intensity (RDI) of the average of cyclophosphamide and anthracycline throughout all cycles was calculated. RTD was defined as the percentage of total delivered dose per planned dose. RDI was defined as the percentage of actual dose administered per protocol‐specified dose per unit time. The RTD and RDI were calculated based on 6 cycles of a standard dose of CHOP‐21. Thus, if a patient received 750 mg/m2 of cyclophosphamide and 50 mg/m2 of doxorubicin 6 times with just 14‐day intervals, the RTD and RDI of this patient equaled 100% and 150%, respectively.
Statistical Analysis
First, we performed exploratory analyses to build a prognostic model that could stratify adherence to R‐CHOP by using data from the SoLT‐J cohort (training set). Cutoff values of age, serum albumin concentration, and CCI score were determined by the receiver operating characteristic curve using the logistic regression analysis for death. Prognostic factors that could affect clinical outcomes were analyzed by using the univariate and multivariate Cox proportional hazard regression model. Overall survival (OS) of each group was calculated as previously described [15]. The OS of each risk category was estimated by using the Kaplan‐Meier method and evaluated by using the log‐rank test. Then, we evaluated the RTD, RDI, and frequencies of unplanned discontinuation of the treatment according to risk stratification. Intergroup differences for categorical and continuous variables were assessed by using the chi‐square test and one‐way analysis of variance test, respectively. Finally, we applied the prognostic model to the data from the West‐JHOG cohort (validation set). A p value <.05 was considered statistically significant. Statistical analysis was performed with JMP software version 8.0.1 (SAS Institute Inc., Cary, NC, USA, https://www.sas.com/en_us/home.html).
Results
Patient Characteristics
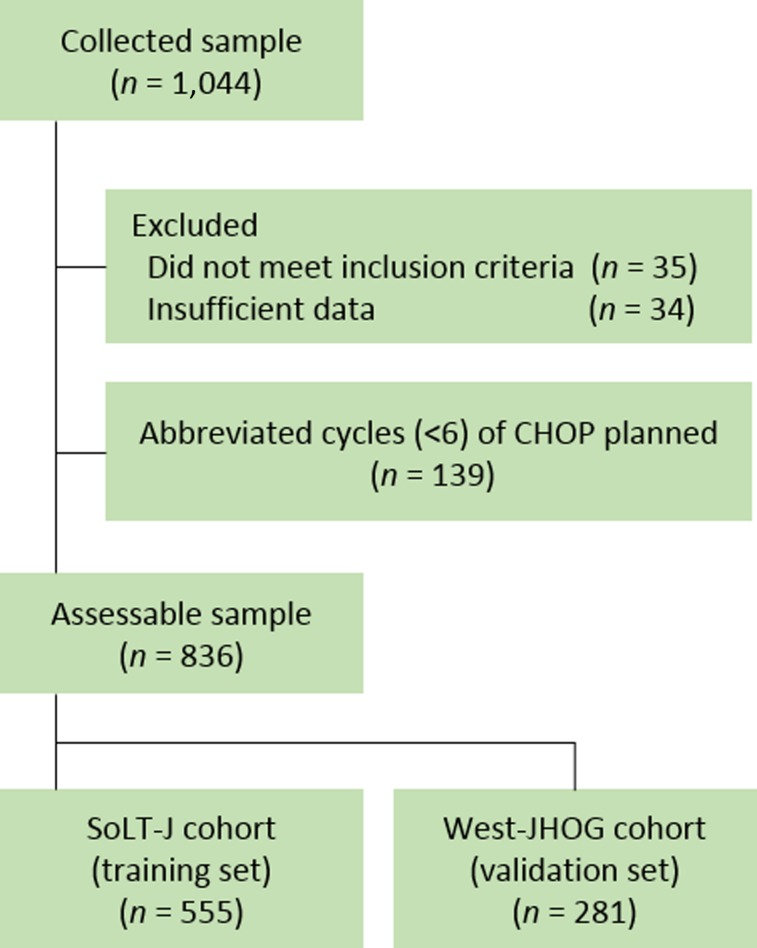
Clinical features and treatment records of 1,044 patients were collected from 19 institutions across Japan that participated or collaborated with either SoLT‐J or West‐JHOG. Among these records, 28 patients who did not meet inclusion criteria and 34 patients who did not have sufficient clinical data to calculate the clinical parameters were excluded from analysis. To minimize the selection bias against the RTD and RDI, we further excluded 139 patients for whom abbreviated cycles (<6) of CHOP with or without radiation therapy were planned before the treatment. Consequently, a total of 836 patients, 19 of whom had testis involvement, from the SoLT‐J (n = 555) and the West‐JHOG (n = 281) cohorts, with a median age of 74 years (range, 65–96 years) who had been diagnosed with de novo DLBCL, were assessable (Fig. 1). The baseline characteristics of patients in each cohort are summarized in Table 1. Among these patients, more than half had at least one comorbidity (Table 2).
Figure 1.
CONSORT diagram of the data collection. A total of 836 patients from the SoLT‐J (n = 555) and the West‐JHOG (n = 281) were evaluated as the training set and validation set, respectively.
Abbreviations: CHOP, cyclophosphamide, doxorubicin, vincristine, and prednisone; SoLT‐J, Society of Lymphoma Treatment in Japan; West‐JHOG, West‐Japan Hematology and Oncology Group.
Table 1. Baseline characteristics of the patients in the SoLT‐J cohort and the West‐JHOG cohort.
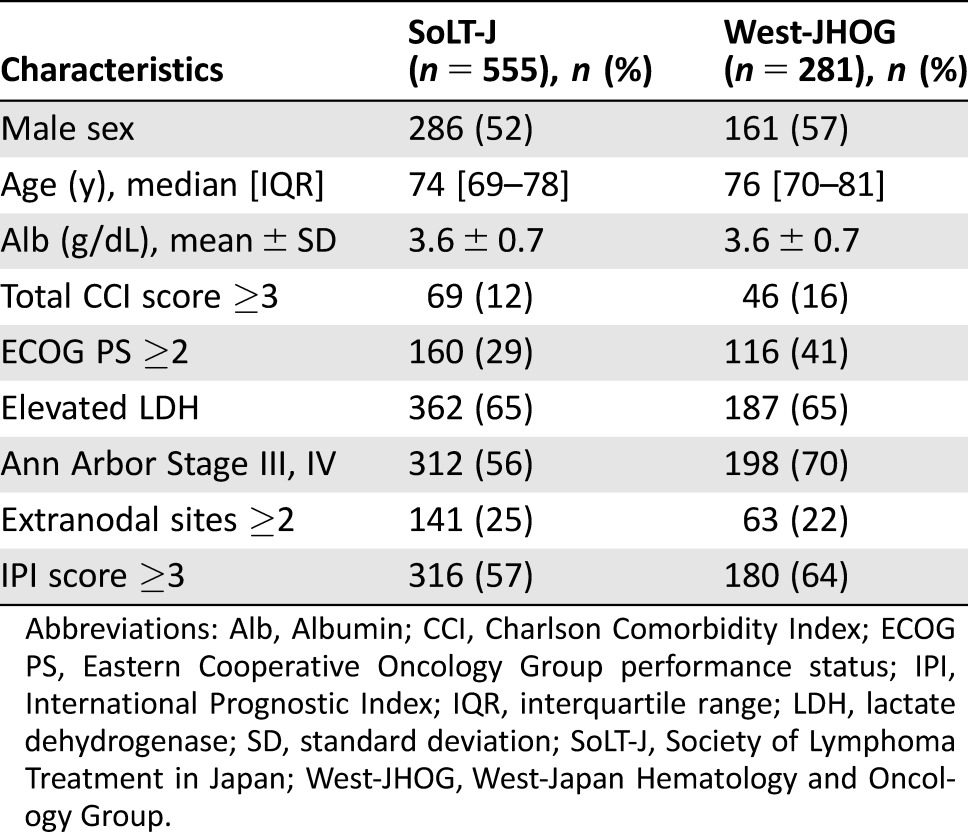
Abbreviations: Alb, Albumin; CCI, Charlson Comorbidity Index; ECOG PS, Eastern Cooperative Oncology Group performance status; IPI, International Prognostic Index; IQR, interquartile range; LDH, lactate dehydrogenase; SD, standard deviation; SoLT‐J, Society of Lymphoma Treatment in Japan; West‐JHOG, West‐Japan Hematology and Oncology Group.
Table 2. Medical comorbidities according to the Charlson Comorbidity Index of the patients in the SoLT‐J cohort and the West‐JHOG cohort.
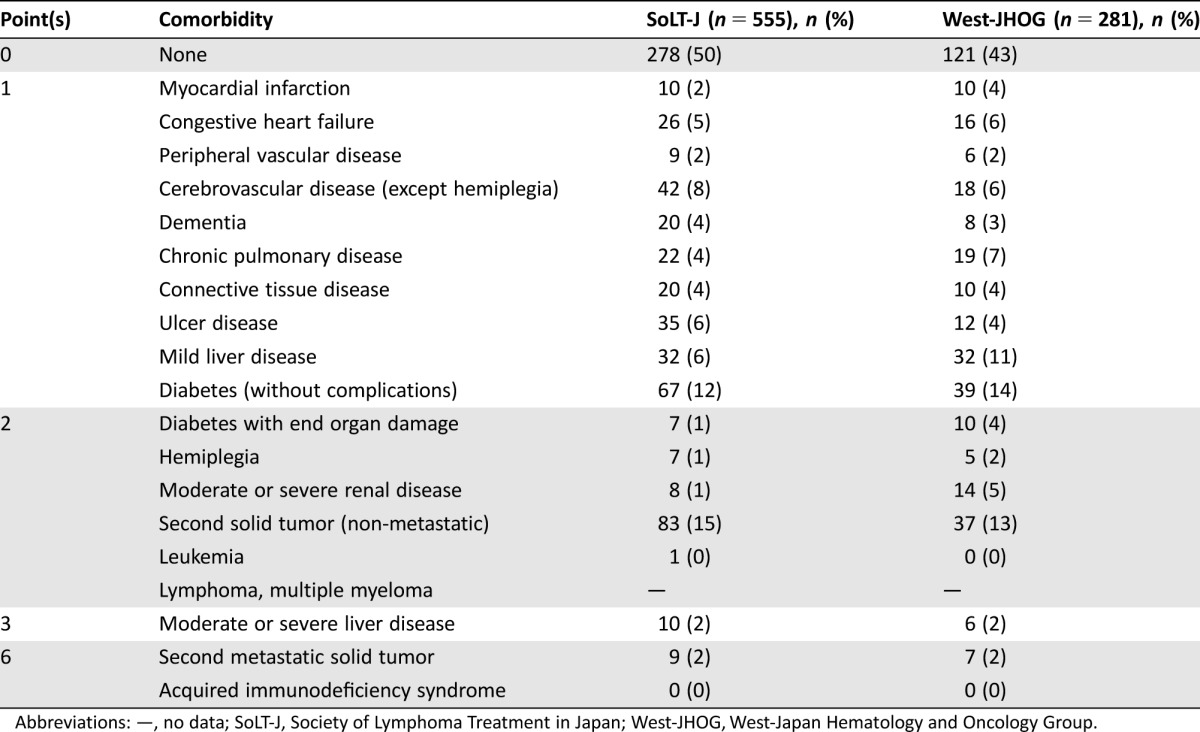
Abbreviations: —, no data; SoLT‐J, Society of Lymphoma Treatment in Japan; West‐JHOG, West‐Japan Hematology and Oncology Group.
Treatment
Before the treatment, 6–8 cycles of R‐CHOP were planned in all 836 assessable patients. Among them, a total of 672 patients (80%) actually received 6–8 cycles of R‐CHOP. Tetrahydropyranyl‐adriamycin was substituted for doxorubicin in 326 patients (39%). The initial average dose of cyclophosphamide and anthracyclines was reduced to less than 80% in 428 patients (51%), according to physicians' preference and/or institutional policies. Radiation therapy was applied in combination with R‐CHOP in 69 patients (8%). Primary or secondary prophylaxis using G‐CSF was applied in 592 patients (71%). R‐CHOP treatment was unexpectedly discontinued in a total of 164 patients (20%) owing to toxicities (n = 88), disease progression (n = 38), death (n = 16), patient request (n = 12), development of other malignant diseases (n = 6), or unknown reasons (n = 4). Febrile neutropenia was reported in 267 patients (32%). During the median 37‐month observation period, 194 patients (35%) died, and 34 of these deaths were treatment related. The 3‐year OS rate for all patients was 68%.
Development of the ACA Index
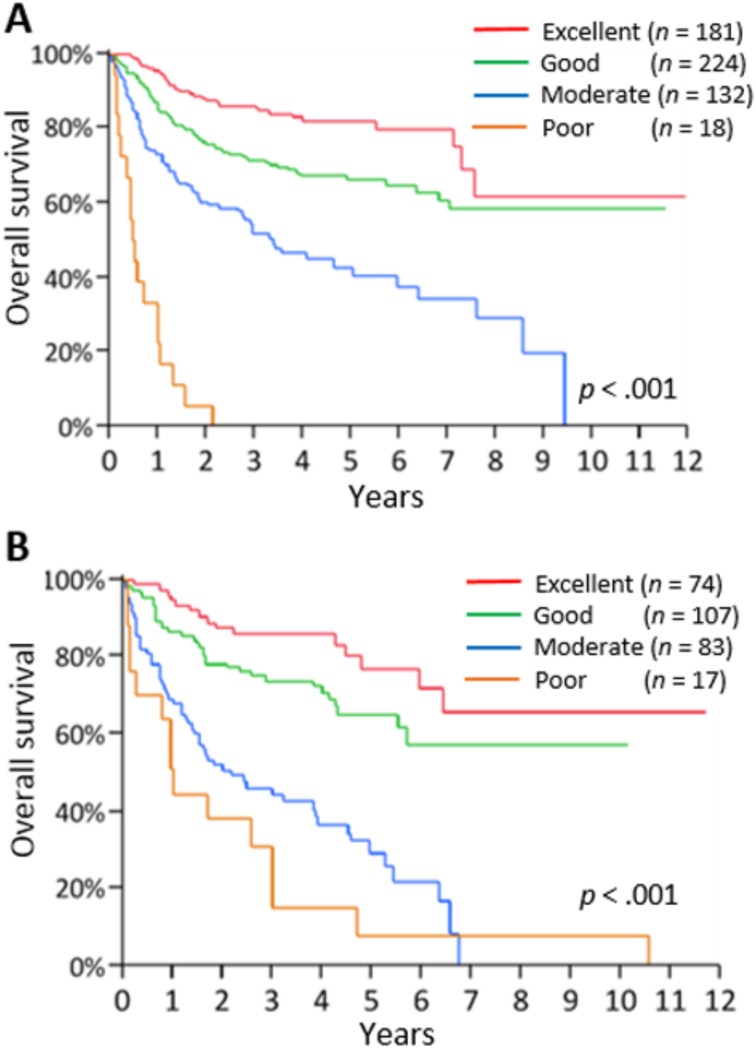
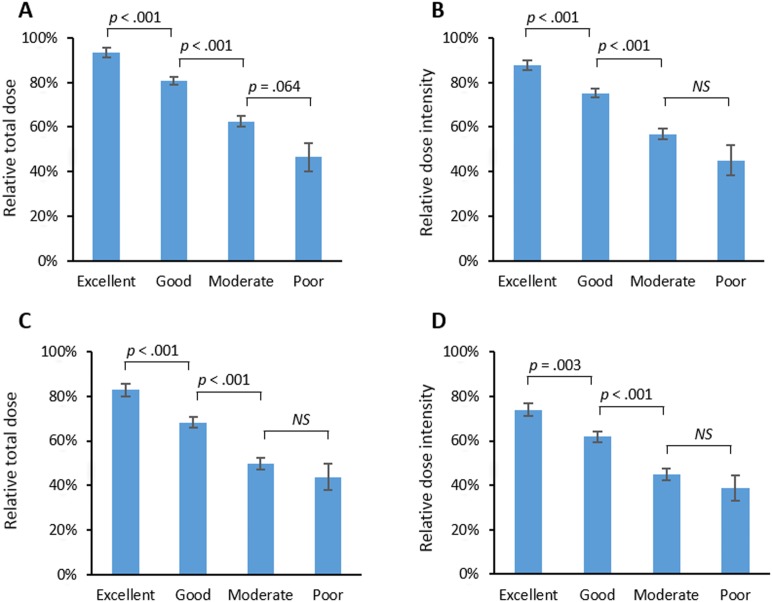
As the training set, 555 patients from the SoLT‐J cohort were used to measure the effect of advanced age, hypoalbuminemia, and higher CCI score on survival. The logistic regression analysis revealed that all of these factors were significantly associated with death (p < .001), and thus cutoff values of age, serum albumin, and CCI score were determined to be >75 years, <3.7 g/dL, and ≥3, respectively. The univariate and multivariate Cox regression analysis for OS revealed that advanced age (>75 years), hypoalbuminemia (<3.7 g/dL), and high CCI score (≥3) were significantly associated with poor OS independently of the other risk factors consisting of the IPI (Table 3). The index, comprising these three new risk factors, was defined as the “Age, Comorbidities, and Albumin (ACA) index” and categorized as “excellent,” “good,” “moderate,” or “poor” according to the scores of 0–3, respectively (Table 4). Consequently, 181, 224, 132, and 18 patients were categorized into the excellent, good, moderate, and poor groups, respectively. The 3‐year OS rates of these groups were 86%, 72%, 51%, and 0%, respectively (p < .001; Fig. 2A). Regarding the RTD and RDI, a higher ACA index score was associated with lower mean dose of chemotherapy (94%, 81%, 63%, and 46%, p < .001; and 88%, 75%, 57%, and 45%, p < .001, respectively; Fig. 3A and 3B). The probabilities of unanticipated discontinuation of R‐CHOP treatment, febrile neutropenia, and treatment‐related death among these groups were also significantly different (7%, 19%, 27%, and 61%, χ2 = 42, p < .001; 11%, 28%, 39%, and 61%, χ2 = 47, p < .001; and 0%, 3%, 5%, and 22%, χ2 = 23, p < .001, respectively).
Table 3. Cox regression analysis of each risk factor for overall survival of the SoLT‐J cohort.
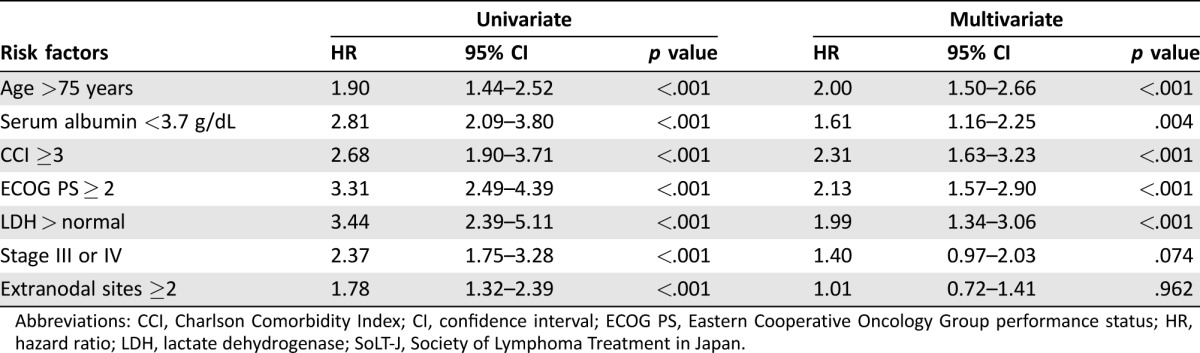
Abbreviations: CCI, Charlson Comorbidity Index; CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status; HR, hazard ratio; LDH, lactate dehydrogenase; SoLT‐J, Society of Lymphoma Treatment in Japan.
Table 4. The ACA index.
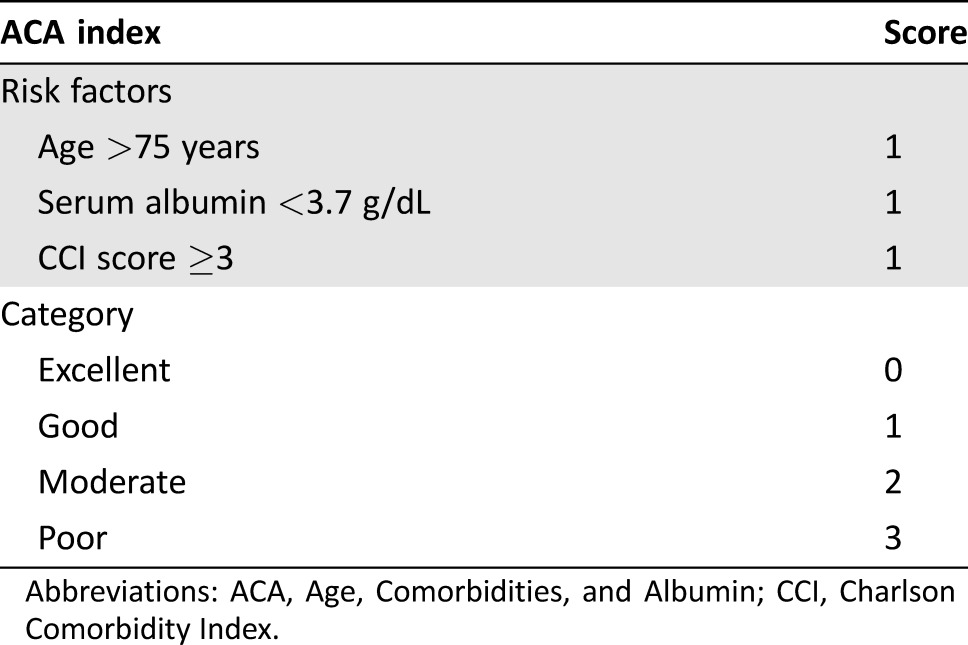
Abbreviations: ACA, Age, Comorbidities, and Albumin; CCI, Charlson Comorbidity Index.
Figure 2.
Overall survival according to the risk groups stratified by the ACA index in the SoLT‐J cohort (A) and West‐JHOG cohort (B).
Abbreviations: ACA index, Age, Comorbidities, and Albumin index; SoLT‐J, Society of Lymphoma Treatment in Japan; West‐JHOG, West‐Japan Hematology and Oncology Group.
Figure 3.
Mean relative total dose and relative dose intensity of doxorubicin and cyclophosphamide in each risk group of the ACA index in the Society of Lymphoma Treatment in Japan cohort (A and B) and West‐Japan Hematology and Oncology Group cohort (C and D). Error bars represent standard error. Each p value was calculated by post hoc t test and adjusted by Bonferroni correction.
Abbreviations: ACA index, Age, Comorbidities, and Albumin index; NS, not significant.
Validation of the ACA Index
We applied the ACA index to 281 patients in the West‐JHOG cohort as the validation set. Consequently, 74, 107, 83, and 17 patients were categorized into the excellent, good, moderate, and poor groups, respectively. The 3‐year OS rates of these groups were 86%, 74%, 44%, and 15% (p < .001), respectively (Fig. 2B). A higher ACA index score was associated with lower mean RTD and RDI (83%, 68%, 50%, and 44%, p < .001; 74%, 62%, 45%, and 39%, p < .001, respectively; Fig. 3C and 3D). The probabilities of unanticipated discontinuation of R‐CHOP treatment, febrile neutropenia, and treatment‐related death among these groups were significantly different (12%, 17%, 31%, and 41%, χ2 = 32, p < .001; 31%, 43%, 48%, and 71%, χ2 = 11, p = .014; and 0%, 3%, 13%, and 18%, χ2 = 20, p < .001, respectively).
Discussion
The ACA index, a new prognostic model consisting of higher age (>75 years), hypoalbuminemia (<3.7 g/dL), and higher CCI score (≥3) for elderly DLBCL patients treated with R‐CHOP, is composed of only host factors and is less dependent on the effect of progression or biology of the disease. As a result, the index has an excellent ability to stratify prognosis, tolerability to cytotoxic drugs, and adherence to treatment in elderly patients with DLBCL treated with R‐CHOP.
It is also noteworthy that higher ACA index score was associated with lower RTD and RDI of cyclophosphamide and anthracycline, which serve as key components of R‐CHOP. Reduced initial dose and incompletion of the chemotherapy might be major reasons for the different RTDs and RDIs among the groups. Even after the advent of rituximab, the optimal dose intensity for elderly patients with aggressive B‐cell lymphomas has been discussed without definitive conclusions [16]. Previous investigations, both prospective and retrospective, have evaluated reduced intensities of R‐CHOP, ranging from 50% to 70% doses of the cytotoxic drugs, for elderly patients with DLBCL [9], [17]. However, to optimize R‐CHOP treatment for elderly patients with DLBCL, risk‐oriented individualized therapy rather than universally attenuated treatment should be applied, because the backgrounds of elderly patients with DLBCL are quite heterogeneous [18]. Thus, the results of this study provide a platform to perform risk‐oriented dose adjustment of R‐CHOP for each elderly patient with DLBCL.
Although there are a number of scales to quantify the health status of elderly patients with or without cancer, physicians have limited tools available to aid in deciding whether full‐dose R‐CHOP should be administered to elderly patients with DLBCL [19]. Several prior investigations performed in Italy evaluated the role of the Comprehensive Geriatric Assessment (CGA), a multidisciplinary, objective tool to assess various functions of elderly cancer patients [20]. In prospective studies, CGA effectively discriminated elderly patients with DLBCL between “fit” and “frail” groups, of which the former was treated with R‐CHOP much more favorably [21], [22]. However, the complexity of the CGA remains a barrier to its integration into daily practice, in contrast to the ACA index, which could be scored much more easily.
Although the ACA index showed excellent ability to stratify the adherence to R‐CHOP among elderly patients with DLBCL, several uncertainties regarding its utilization in clinical practice remain to be resolved. First, tetrahydropyranyl‐adriamycin was substituted for doxorubicin in 39% of the patients in this study. Although tetrahydropyranyl‐adriamycin has shown similar efficacy to doxorubicin for elderly DLBCL patients with favorable adherence [14], data from large‐scale, randomized trials are still lacking. Thus, differences between these two drugs probably had some effect on the results. Second, only 35 of 836 patients (4%) were categorized into the poor risk group by the ACA index in this study, resulting in low statistical power. This imbalance might arise from the unlikelihood of R‐CHOP application to such patients in clinical practice. Finally, we did not evaluate the biology of the disease, which has a crucial impact on the outcome of DLBCL treatment. Affected extranodal sites of the disease (e.g., bone marrow, testis, or gastrointestinal tract) are essential information for planning DLBCL treatment. Additionally, immunohistochemical characteristics (e.g., germinal center B‐cell type or not), EBV association, and genetic alterations (e.g. myc and bcl‐2) of the disease should be considered before treatment [23].
Conclusion
We demonstrated that the ACA index is useful for the treatment of elderly patients with DLBCL to support risk‐oriented personalized therapy. The utility of the index needs further evaluation in large‐scale, prospective studies. Finally, our results suggest that this new decision‐making model comprising simple, host‐dependent factors has the potential to be applied to the management of elderly patients with other types of lymphomas or chemosensitive solid tumors.
Acknowledgments
The SoLT‐J members would like to thank all members of the West‐JHOG for their enormous contribution to this work. The authors would like to recognize the dedication of attending physicians, especially Dr. Y. Kumano and Dr. T. Imi at Toyama Prefectural Central Hospital; Dr. K. Kawamoto at Niigata University Faculty of Medicine; Dr. Y. Fujiwara at Himeji Red Cross Hospital; Dr. K. Arita at Toyama University; Dr. F. Ishiyama, Dr. A. Numata, Dr. Y. Hattori, and Dr. H. Takasaki at Kanagawa Cancer Center; Dr. X. Wang, Dr. K. Ono, Dr. A. Satou, Dr. T. Sugawara, and Dr. M. Ise at Chiba Cancer Center; and Dr. Y. Nawa at Ehime Prefectural Central Hospital. The authors also thank Mr. K. Yoneyama at Advanced Clinical Research Organization, and Ms. A. Hirama at Nihon University for their excellent assistance on electronic data management. A part of this study was presented in a poster at the 57th annual meeting of the American Society of Hematology, held in Orlando, FL, in December 2015.
Author Contributions
Conception/Design: Katsuhiro Miura, Takaaki Miyake, Atsuko Hojo, Yasufumi Masaki, Jun Ozaki, Chikamasa Yoshida, Koichi Kitazume, Yoshinobu Maeda, Jun Takizawa, Rika Sakai, Hideki Tsujimura, Yoshihiro Hatta, Masatoshi Kanno
Provision of study material or patients: Katsuhiro Miura, Yasufumi Masaki, Yoshinobu Maeda, Jun Takizawa, Rika Sakai, Hideki Tsujimura, Masatoshi Kanno
Collection and/or assembly of data: Katsuhiro Miura, Jun Konishi, Takaaki Miyake, Masanori Makita, Atsuko Hojo, Yasufumi Masaki, Masatoshi Uno, Jun Ozaki, Chikamasa Yoshida, Daigo Niiya, Koichi Kitazume, Yoshinobu Maeda, Jun Takizawa, Rika Sakai, Tomofumi Yano, Kazuhiko Yamamoto, Kazutaka Sunami, Yasushi Hiramatsu, Kazutoshi Aoyama, Hideki Tsujimura,Jun Murakami
Data analysis and interpretation: Katsuhiro Miura, Yasufumi Masaki, Chikamasa Yoshida, Yoshinobu Maeda, Jun Takizawa, Rika Sakai, Hideki Tsujimura,Masatoshi Kanno
Manuscript writing: Katsuhiro Miura, Jun Konishi, Takaaki Miyake, Masanori Makita, Atsuko Hojo, Yasufumi Masaki, Masatoshi Uno, Jun Ozaki, Chikamasa Yoshida, Daigo Niiya, Koichi Kitazume, Yoshinobu Maeda, Jun Takizawa, Rika Sakai, Tomofumi Yano, Kazuhiko Yamamoto, Kazutaka Sunami, Yasushi Hiramatsu, Kazutoshi Aoyama, Hideki Tsujimura,Jun Murakami, Yoshihiro Hatta, Masatoshi Kanno
Final approval of manuscript: Katsuhiro Miura, Jun Konishi, Takaaki Miyake, Masanori Makita, Atsuko Hojo, Yasufumi Masaki, Masatoshi Uno, Jun Ozaki, Chikamasa Yoshida, Daigo Niiya, Koichi Kitazume, Yoshinobu Maeda, Jun Takizawa, Rika Sakai, Tomofumi Yano, Kazuhiko Yamamoto, Kazutaka Sunami, Yasushi Hiramatsu, Kazutoshi Aoyama, Hideki Tsujimura,Jun Murakami, Yoshihiro Hatta, Masatoshi Kanno
Disclosures
Katsuhiro Miura: Chugai, Kyowa Hakko Kirin (H); Takaaki Miyake: Chugai (H); Atsuko Hojo: Chugai, Kyowa Hakko Kirin (H); Yasufumi Masaki: Shionogi (H), Chugai, Kyowa Hakko Kirin (H, RF); Jun Takizawa: Chugai, Kyowa Hakko Kirin (H); Rika Sakai: Chugai, Kyowa Hakko Kirin (H, RF), Nippon Kayaku (RF); Kazutaka Sunami: Celgene (H, RF), Ono, Takeda, Daiichi Sankyo, Novartis, Sanofi, Janssen, Bristol‐Myers Squibb (RF); Kazutoshi Aoyama: Kyowa Hakko Kirin (H); Yoshihiro Hatta: Chugai, Kyowa Hakko Kirin (H); Masatoshi Kanno: Chugai (H), Nippon Kayaku (H, RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.A predictive model for aggressive non‐Hodgkin's lymphoma . The International Non‐Hodgkin's Lymphoma Prognostic Factors Project. N Engl J Med 1993;329:987–994. [DOI] [PubMed] [Google Scholar]
- 2. Sehn LH, Berry B, Chhanabhai M et al. The revised International Prognostic Index (R‐IPI) is a better predictor of outcome than the standard IPI for patients with diffuse large B‐cell lymphoma treated with R‐CHOP. Blood 2007;109:1857–1861. [DOI] [PubMed] [Google Scholar]
- 3. Zhou Z, Sehn LH, Rademaker AW et al. An enhanced International Prognostic Index (NCCN‐IPI) for patients with diffuse large B‐cell lymphoma treated in the rituximab era. Blood 2014;123:837–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. DeSantis CE, Lin CC, Mariotto AB et al. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin 2014;64:252–271. [DOI] [PubMed] [Google Scholar]
- 5. Lichtman SM. Chemotherapy in the elderly. Semin Oncol 2004;31:160–174. [DOI] [PubMed] [Google Scholar]
- 6. Dalia S, Chavez J, Little B et al. Serum albumin retains independent prognostic significance in diffuse large B‐cell lymphoma in the post‐rituximab era. Ann Hematol 2014;93:1305–1312. [DOI] [PubMed] [Google Scholar]
- 7. Gupta D, Lis CG. Pretreatment serum albumin as a predictor of cancer survival: A systematic review of the epidemiological literature. Nutr J 2010;9:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Miura K, Kobayashi Y, Hojo A et al. Serum total protein level is an alternative prognostic factor among elderly patients with diffuse large B‐cell lymphoma. Ann Oncol 2010;21 (suppl 9):40. 19622588 [Google Scholar]
- 9. Peyrade F, Jardin F, Thieblemont C et al. Attenuated immunochemotherapy regimen (R‐miniCHOP) in elderly patients older than 80 years with diffuse large B‐cell lymphoma: A multicentre, single‐arm, phase 2 trial. Lancet Oncol 2011;12:460–468. [DOI] [PubMed] [Google Scholar]
- 10. Charlson ME, Pompei P, Ales KL et al. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis 1987;40:373–383. [DOI] [PubMed] [Google Scholar]
- 11. Kobayashi Y, Miura K, Hojo A et al. Charlson Comorbidity Index is an independent prognostic factor among elderly patients with diffuse large B‐cell lymphoma. J Cancer Res Clin Oncol 2011;137:1079–1084. [DOI] [PubMed] [Google Scholar]
- 12. Kwak LW, Halpern J, Olshen RA. Prognostic significance of actual dose intensity in diffuse large‐cell lymphoma: Results of a tree‐structured survival analysis. J Clin Oncol 1990;8:963–977. [DOI] [PubMed] [Google Scholar]
- 13. Hirakawa T, Yamaguchi H, Yokose N et al. Importance of maintaining the relative dose intensity of CHOP‐like regimens combined with rituximab in patients with diffuse large B‐cell lymphoma. Ann Hematol 2010;89:897–904. [DOI] [PubMed] [Google Scholar]
- 14. Kasahara S, Hara T, Tsurumi H et al. Phase II study of the tetrahydropyranyl adriamycin‐cyclophosphamide, vincristine, and prednisolone regimen combined with rituximab as first‐line treatment for elderly patients with diffuse large B‐cell lymphoma. Leuk Lymphoma 2011;52:629–634. [DOI] [PubMed] [Google Scholar]
- 15. Cheson BD, Pfistner B, Juweid ME et al. Revised response criteria for malignant lymphoma. J Clin Oncol 2007;25:579–586. [DOI] [PubMed] [Google Scholar]
- 16. Fields PA, Linch DC. Treatment of the elderly patient with diffuse large B cell lymphoma. Br J Haematol 2012;157:159–170. [DOI] [PubMed] [Google Scholar]
- 17. Meguro A, Ozaki K, Sato K et al. Rituximab plus 70% cyclophosphamide, doxorubicin, vincristine and prednisone for Japanese patients with diffuse large B‐cell lymphoma aged 70 years and older. Leuk Lymphoma 2012;53:43–49. [DOI] [PubMed] [Google Scholar]
- 18. Miura K, Kobayashi Y, Hojo A et al. Attenuated immunochemotherapy for diffuse large B‐cell lymphoma. Lancet Oncol 2011;12: 725; author reply 725–726. [DOI] [PubMed] [Google Scholar]
- 19. Martinez‐Tapia C, Canoui‐Poitrine F, Bastuji‐Garin S et al. Optimizing the G8 screening tool for older patients with cancer: Diagnostic performance and validation of a six‐item version. The Oncologist 2016;21:188–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Extermann M, Hurria A et al. Comprehensive geriatric assessment for older patients with cancer. J Clin Oncol 2007;25:1824–1831. [DOI] [PubMed] [Google Scholar]
- 21. Tucci A, Ferrari S, Bottelli C et al. A comprehensive geriatric assessment is more effective than clinical judgment to identify elderly diffuse large cell lymphoma patients who benefit from aggressive therapy. Cancer 2009;115:4547–4553. [DOI] [PubMed] [Google Scholar]
- 22. Merli F, Luminari S, Rossi G et al. Outcome of frail elderly patients with diffuse large B‐cell lymphoma prospectively identified by Comprehensive Geriatric Assessment: Results from a study of the Fondazione Italiana Linfomi. Leuk Lymphoma 2014;55:38–43. [DOI] [PubMed] [Google Scholar]
- 23. Dunleavy K, Roschewski M, Wilson WH. Precision treatment of distinct molecular subtypes of diffuse large B‐cell lymphoma: Ascribing treatment based on the molecular phenotype. Clin Cancer Res 2014;20:5182–5193. [DOI] [PMC free article] [PubMed] [Google Scholar]