Abstract
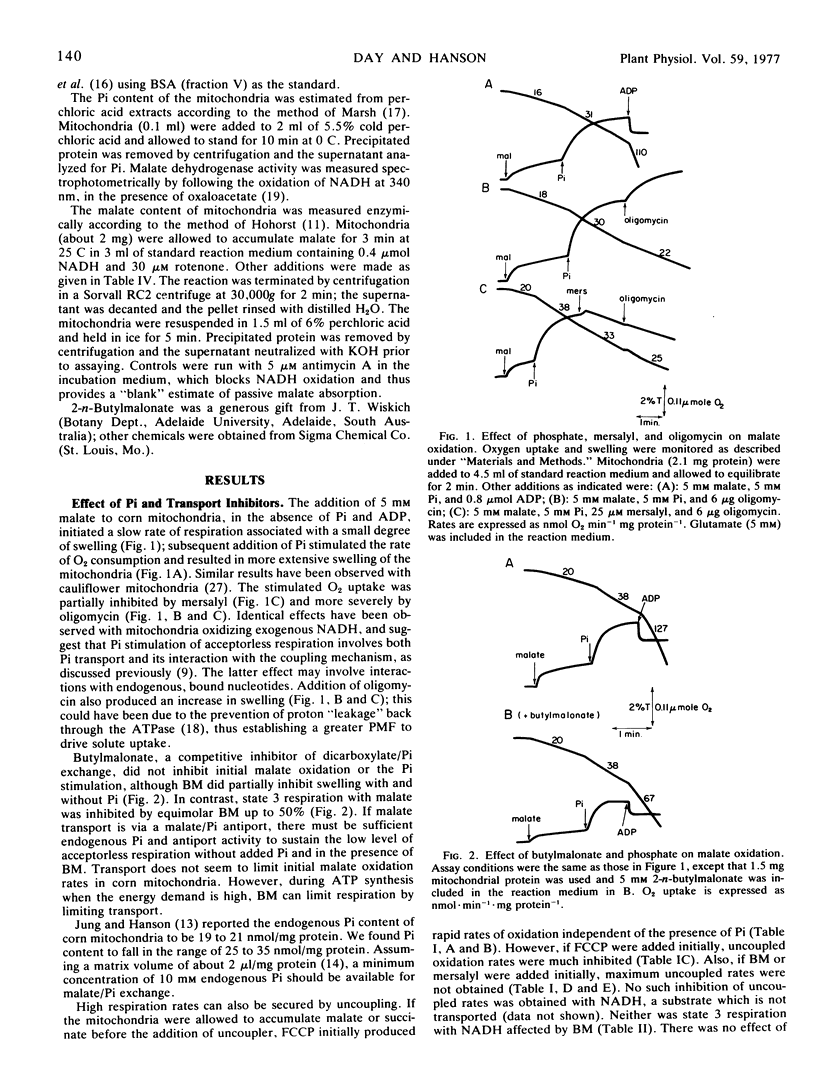
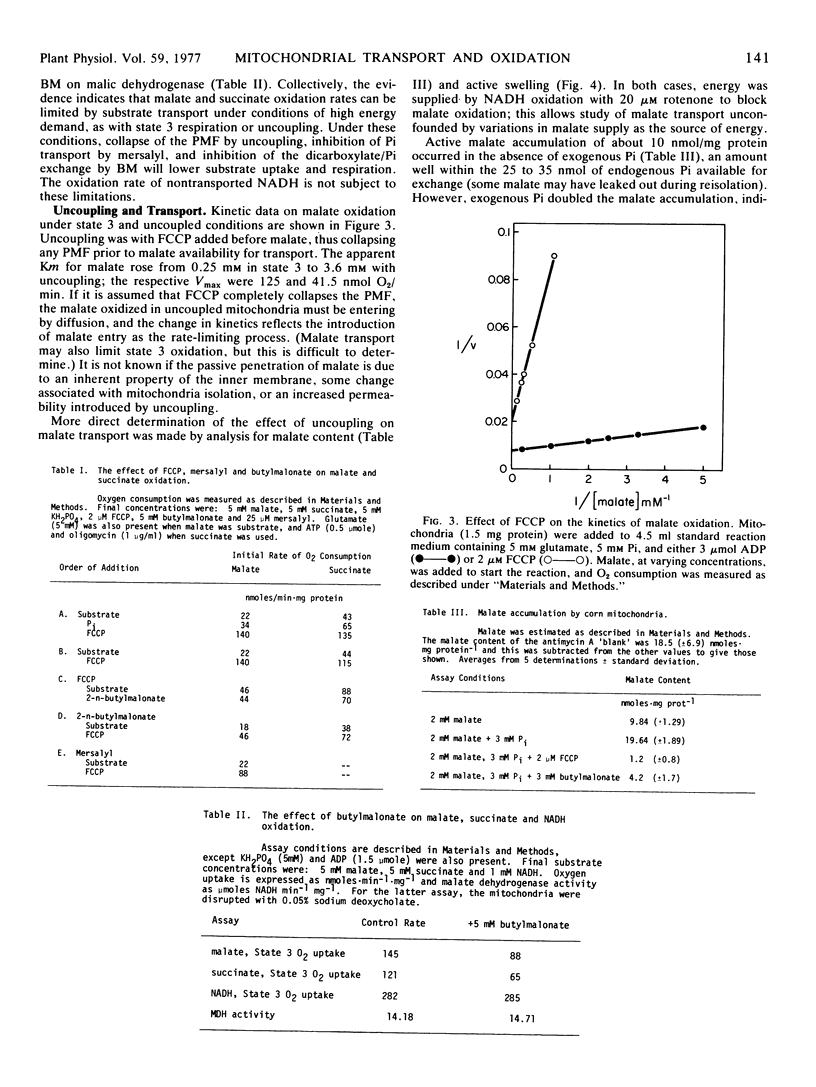
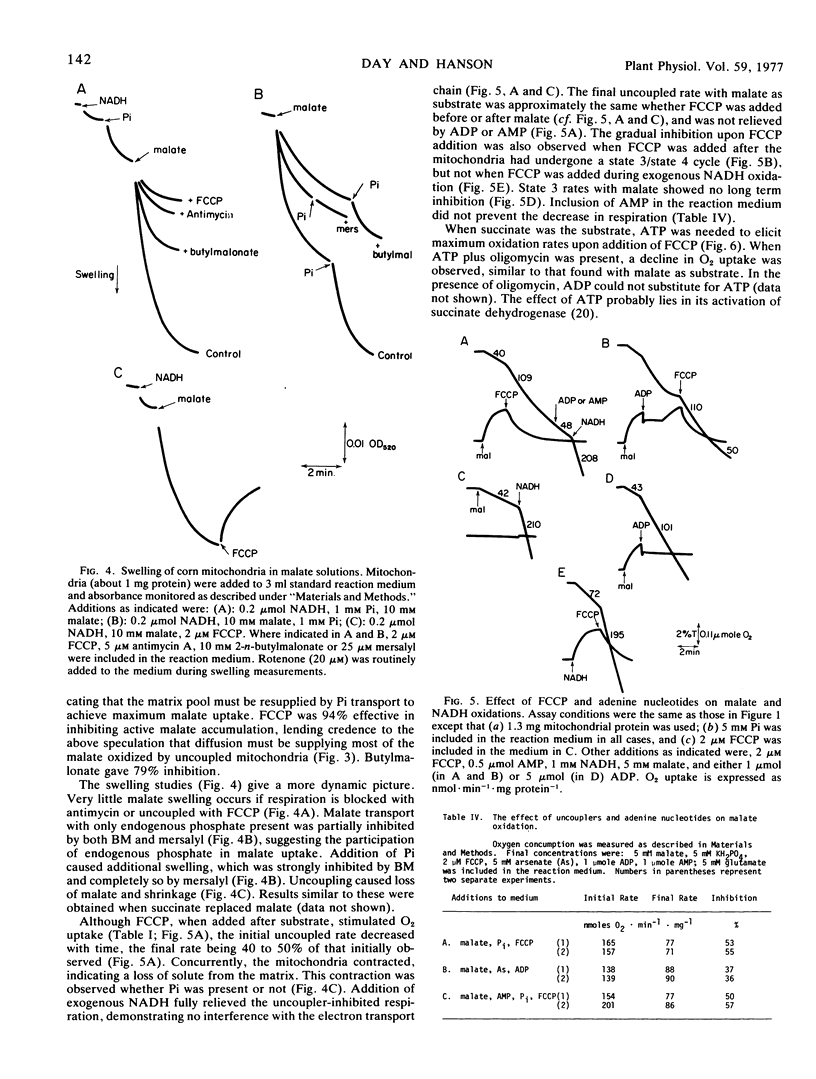
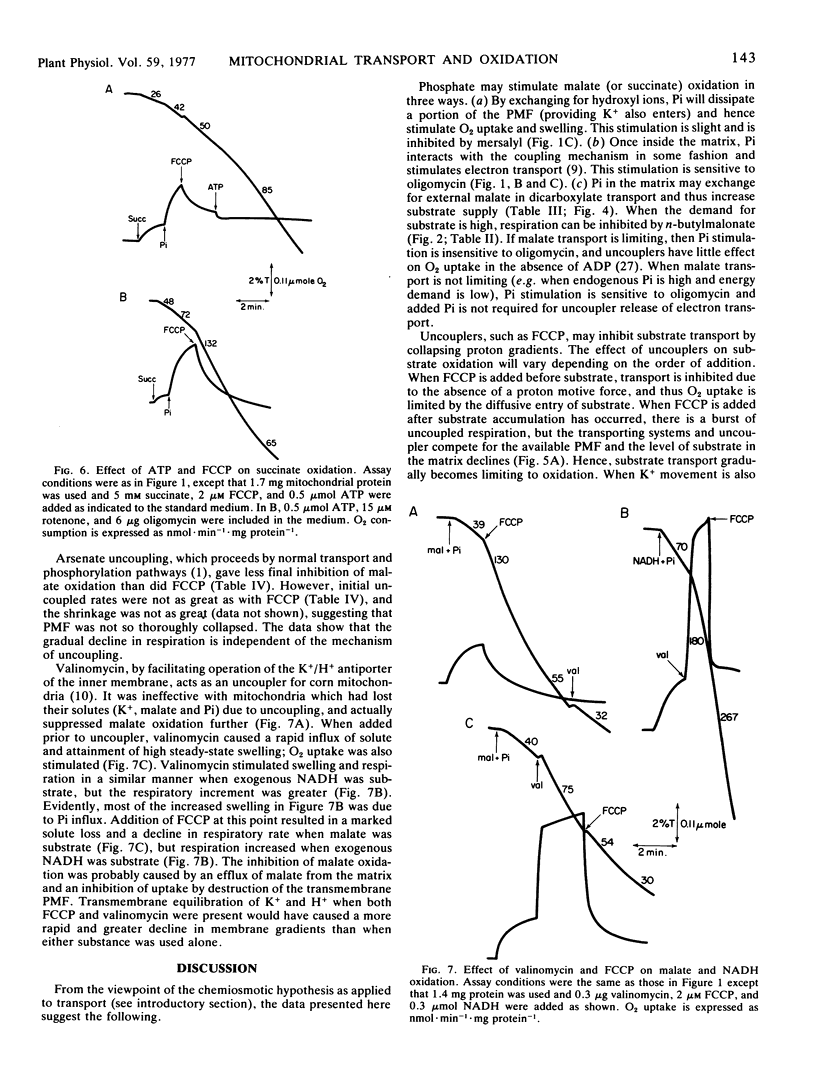
A study was made to determine conditions under which malate oxidation rates in corn (Zea mays L.) mitochondria are limited by transport processes. In the absence of added ADP, inorganic phosphate increased malate oxidation rates by processes inhibited by mersalyl and oligomycin, but phosphate did not stimulate uncoupled respiration. However, the uncoupled oxidation rates were inhibited by butylmalonate and mersalyl. When uncoupler was added prior to substrate, subsequent O2 uptake rates were reduced when malate and succinate, but not exogenous NADH, were used. Uncoupler and butylmalonate also inhibited swelling in malate solutions and malate accumulation by these mitochondria, which were found to have a high endogenous phosphate content. Addition of uncoupler after malate or succinate produced an initial rapid oxidation which declined as the mitochondria lost solute and contracted. This decline was not affected by addition of ADP or AMP, and was not observed when exogenous NADH was substrate. Increasing K+ permeability with valinomycin increased the P-trifluoromethoxy (carboxylcyanide)phenyl hydrazone inhibition. Kinetic studies showed the slow rate of malate oxidation in the presence of uncoupler to be characterized by a high Km and a low Vmax, probably reflecting a diffusion-limited process.
The results indicate that rapid malate and succinate oxidation require the operation of both the phosphate and dicarboxylate transporters, which in turn depend on maintenance of a proton motive force across the inner membrane. In addition, phosphate can stimulate acceptorless malate oxidation by reaction with the coupling mechanism, and in uncoupled mitochondria which are depleted of substrate there is a slow rate of oxidation which appears to be limited by diffusive entry.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bertagnolli B. L., Hanson J. B. Functioning of the adenine nucleotide transporter in the arsenate uncoupling of corn mitochondria. Plant Physiol. 1973 Nov;52(5):431–435. doi: 10.1104/pp.52.5.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappell J. B. Systems used for the transport of substrates into mitochondria. Br Med Bull. 1968 May;24(2):150–157. doi: 10.1093/oxfordjournals.bmb.a070618. [DOI] [PubMed] [Google Scholar]
- Day D. A., Wiskich J. T. The oxidation of malate and exogenous reduced nicotinamide adenine dinucleotide by isolated plant mitochondria. Plant Physiol. 1974 Jan;53(1):104–109. doi: 10.1104/pp.53.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douce R., Christensen E. L., Bonner W. D., Jr Preparation of intaintact plant mitochondria. Biochim Biophys Acta. 1972 Aug 17;275(2):148–160. doi: 10.1016/0005-2728(72)90035-7. [DOI] [PubMed] [Google Scholar]
- Douce R., Mannella C. A., Bonner W. D., Jr The external NADH dehydrogenases of intact plant mitochondria. Biochim Biophys Acta. 1973 Jan 18;292(1):105–116. doi: 10.1016/0005-2728(73)90255-7. [DOI] [PubMed] [Google Scholar]
- Hanson J. B., Bertagnolli B. L., Shepherd W. D. Phosphate-induced Stimulation of Acceptorless Respiration in Corn Mitochondria. Plant Physiol. 1972 Sep;50(3):347–354. doi: 10.1104/pp.50.3.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson J. B. Ion transport induced by polycations and its relationship to loose coupling of corn mitochondria. Plant Physiol. 1972 May;49(5):707–715. doi: 10.1104/pp.49.5.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensley J. R., Hanson J. B. The action of valinomycin in uncoupling corn mitochondria. Plant Physiol. 1975 Jul;56(1):13–18. doi: 10.1104/pp.56.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikuma H., Bonner W. D. Properties of Higher Plant Mitochondria. I. Isolation and Some Characteristics of Tightly-coupled Mitochondria from Dark-grown Mung Bean Hypocotyls. Plant Physiol. 1967 Jan;42(1):67–75. doi: 10.1104/pp.42.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung D. W., Hanson J. B. Activation of 2,4-dinitrophenol-stimulated ATPase activity in cauliflower and corn mitochondria. Arch Biochem Biophys. 1975 Jun;168(2):358–368. doi: 10.1016/0003-9861(75)90264-7. [DOI] [PubMed] [Google Scholar]
- Kirk B. I., Hanson J. B. The Stoichiometry of Respiration-driven Potassium Transport in Corn Mitochondria. Plant Physiol. 1973 Feb;51(2):357–362. doi: 10.1104/pp.51.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laties G. G. The potentiating effect of adenosine diphosphate in the uncoupling of oxidative phosphorylation in potato mitochondria. Biochemistry. 1973 Aug 14;12(17):3350–3355. doi: 10.1021/bi00741a032. [DOI] [PubMed] [Google Scholar]
- MARSH B. B. The estimation of inorganic phosphate in the presence of adenosine triphosphate. Biochim Biophys Acta. 1959 Apr;32:357–361. doi: 10.1016/0006-3002(59)90607-9. [DOI] [PubMed] [Google Scholar]
- Oestreicher G., Hogue P., Singer T. P. Regulation of Succinate Dehydrogenase in Higher Plants: II. Activation by Substrates, Reduced Coenzyme Q, Nucleotides, and Anions. Plant Physiol. 1973 Dec;52(6):622–626. doi: 10.1104/pp.52.6.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overman A. R., Lorimer G. H., Miller R. J. Diffusion and osmotic transfer in corn mitochondria. Plant Physiol. 1970 Feb;45(2):126–132. doi: 10.1104/pp.45.2.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips M. L., Williams G. R. Effects of 2-Butylmalonate, 2-Phenylsuccinate, Benzylmalonate, and p-Iodobenzylmalonate on the Oxidation of Substrates by Mung Bean Mitochondria. Plant Physiol. 1973 Feb;51(2):225–228. doi: 10.1104/pp.51.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson B. H., Chappell J. B. The inhibition of malate, tricarboxylate and oxoglutarate entry into mitochondria by 2-n-butylmalonate. Biochem Biophys Res Commun. 1967 Jul 21;28(2):249–255. doi: 10.1016/0006-291x(67)90437-8. [DOI] [PubMed] [Google Scholar]
- Sauer L. A., Park R. A stimulation by phosphate of malate transport and oxidation in rat adrenal mitochondria. Biochemistry. 1973 Feb;12(4):643–649. doi: 10.1021/bi00728a012. [DOI] [PubMed] [Google Scholar]
- Sotthibandhu R., Palmer J. M. The activation of non-phosphorylating electron transport by adenine nucleotides in Jerusalem-artichoke (Helianthus tuberosus) mitochondria. Biochem J. 1975 Dec;152(3):637–645. doi: 10.1042/bj1520637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiskich J. T. Phosphate-dependent Substrate Transport into Mitochondria: Oxidative Studies. Plant Physiol. 1975 Jul;56(1):121–125. doi: 10.1104/pp.56.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]


