Abstract
Lessons Learned.
The combination of standard dose abiraterone acetate and BEZ235, a pan‐class I PI3K and mTORC1/2 inhibitor, was poorly tolerated in men with progressive mCRPC.
Although the clinical development of BEZ235 has been discontinued in prostate cancer, agents that more selectively target PI3K‐AKT‐mTOR signaling may have a more favorable therapeutic index and should continue to be explored.
Background.
Androgen receptor (AR) and phosphatidylinositol‐3 kinase (PI3K) signaling are two commonly perturbed pathways in prostate cancer. Preclinical data have shown that the two pathways compensate for each other when one is inhibited, and combined inhibition of AR and PI3K signaling may be a viable strategy to prevent or overcome castration resistance.
Methods.
This phase I study evaluated the safety and tolerability of abiraterone acetate and prednisone combined with BEZ235, a dual PI3K and mTORC1/2 inhibitor, in men with progressive metastatic castration resistant prostate cancer (mCRPC) who have not received prior chemotherapy.
Results.
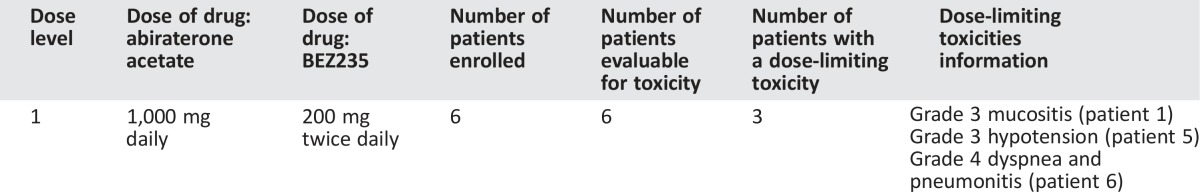
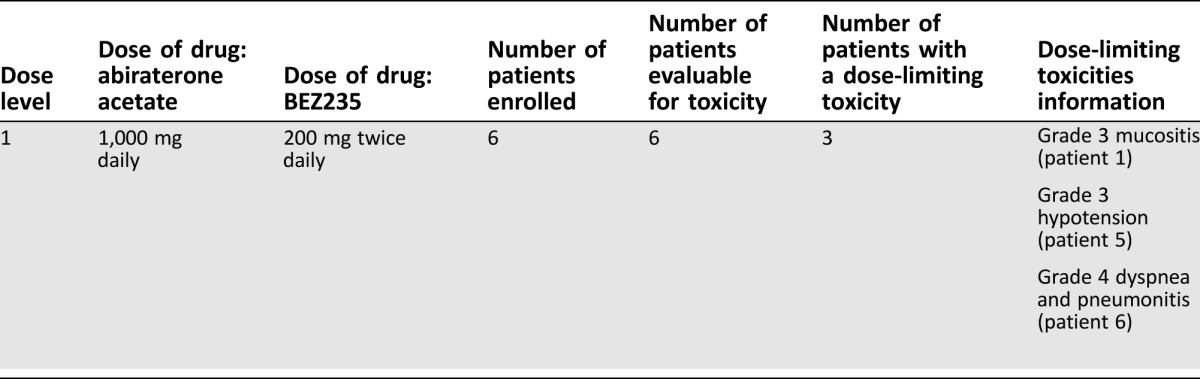
Six patients (n = 6) were treated at the starting dose level of abiraterone acetate 1,000 mg with prednisone 5 mg twice daily and BEZ235 200 mg twice daily in a 3 + 3 dose escalation design. The study was terminated early because three of the six patients (50%) experienced dose‐limiting toxicities: grade 3 mucositis, grade 3 hypotension, and grade 4 dyspnea and pneumonitis. All six patients had previously progressed on abiraterone/prednisone. The median treatment duration was 27 days (range: 3–130 days). No prostate‐specific antigen (PSA) decline or objective response were observed.
Conclusion.
The combination of standard‐dose abiraterone/prednisone with BEZ235 200 mg twice daily was poorly tolerated in patients with mCRPC. The on‐target and off‐target effects of dual PI3K and mTORC inhibition likely contributed to the unacceptable toxicity profile. The Oncologist 2017;22:503–e43
Abstract
经验总结
• 在疾病进展的mCRPC患者中, 标准剂量的醋酸阿比特龙与泛I类PI3K和mTORC1/2抑制剂BEZ235联合用药的耐受性较差。
• 虽然BEZ235治疗前列腺癌的临床开发现已终止, 但以PI3K‐AKT‐mTOR信号通路为靶点的高度选择性药物可能具有更为理想的治疗指数, 应继续进行探索。
摘要
背景. 雄激素受体(AR)和磷脂酰肌醇‐3激酶(PI3K)信号通路是前列腺癌中常发生紊乱的两条通路。临床前数据表明, 如果两条通路的其中之一受到抑制, 二者可相互补偿;因此, 同时抑制AR和PI3K信号通路可能是防止或克服去势抵抗性的一种可行策略。
方法. 本项I期研究在既往未接受过化疗的转移性去势抵抗性前列腺癌(mCRPC)进展患者中评价了醋酸阿比特龙和泼尼松与PI3K和mTORC1/2双重抑制剂BEZ235联用的安全性和耐受性。
结果. 6例患者(n=6)按照3 + 3剂量递增设计接受起始剂量水平的醋酸阿比特龙(1 000 mg)、泼尼松(5 mg每日两次)和BEZ235(200 mg每日两次)联合治疗。6例患者中有3例(50%)出现剂量限制性毒性(3级黏膜炎、3级低血压以及4级呼吸困难和非感染性肺炎), 故本研究被提前终止。所有6例患者既往接受阿比特龙/泼尼松治疗时均出现疾病进展。中位治疗持续时间为27天(范围:3‐130天)。未观察到前列腺特异性抗原(PSA)降低或客观缓解。
结论. 标准剂量的阿比特龙/泼尼松与BEZ235 200 mg每日两次联合给药在mCRPC患者中耐受性较差。PI3K和mTORC双重抑制的靶向和脱靶效应可能会产生不可接受的毒性。The Oncologist 2017;22:503–e43
Discussion
AR signaling and PI3K‐AKT‐mTOR signaling are among the most common aberrant pathways found in advanced prostate cancer and are implicated in the development and maintenance of castration resistant disease. Preclinical prostate cancer models have shown crosstalk and cross‐regulation between the two pathways, and enhanced tumor control with combination strategies that co‐inhibit AR and PI3K‐AKT‐mTOR signaling.
We report results of a phase I study evaluating the safety and tolerability of standard dose abiraterone acetate (1,000 mg daily with prednisone 5 mg b.i.d.) combined with BEZ235, a potent dual pan‐class I PI3K and mTORC1/2 inhibitor, in patients with progressive mCRPC. The original study design planned to determine the maximum tolerated dose (MTD) of the combination during 3 + 3 dose escalation, followed by a dose expansion phase to assess efficacy. The study protocol specified that if >1 of 3 or >2 of 6 patients experience a dose‐limiting toxicity (DLT) at dose level 1, the study would be terminated. One of the first three patients accrued experienced a DLT at dose level 1 (abiraterone/prednisone plus BEZ235 200 mg b.i.d.), and three more patients were accrued at dose level 1 (Table 1). Two of the last three patients also experienced DLT, and the study was terminated due to lack of safety as specified by study protocol. The median age of the patients was 71 years (range: 59–75 years). The majority of patients (83.3%) had Gleason 8–10 disease. All patients had bone metastases with or without nodal metastasis; no patients had visceral metastases. All patients had previously progressed on abiraterone.
Table 1. Dose‐limiting toxicities.
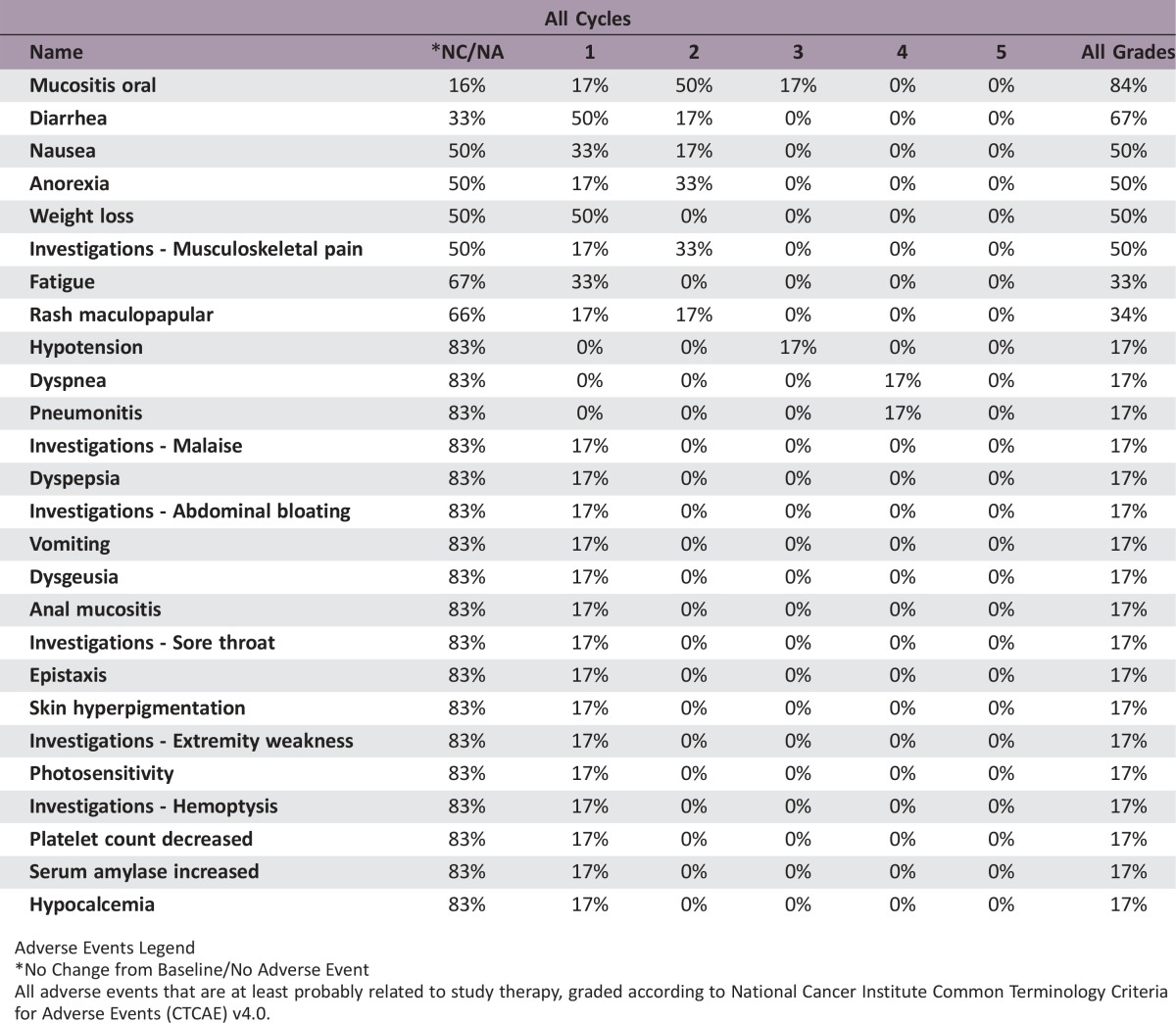
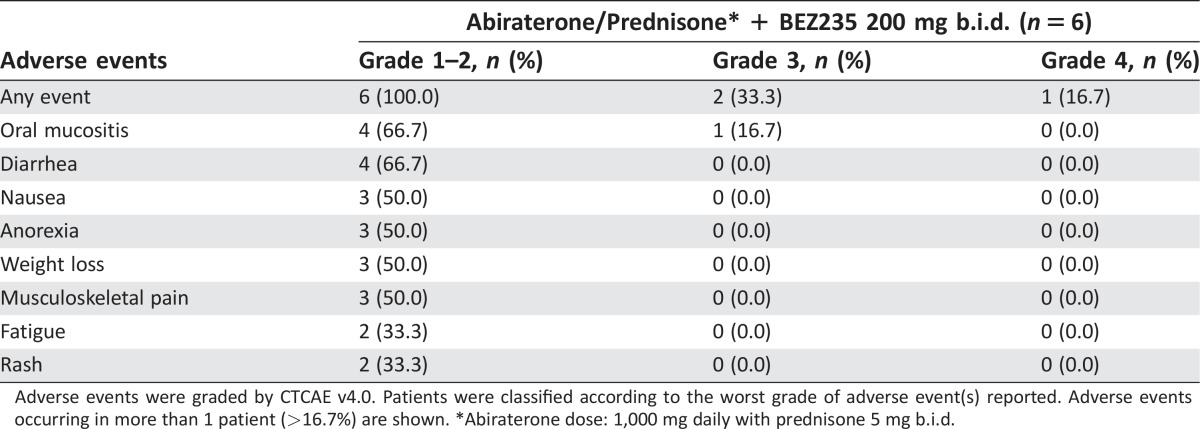
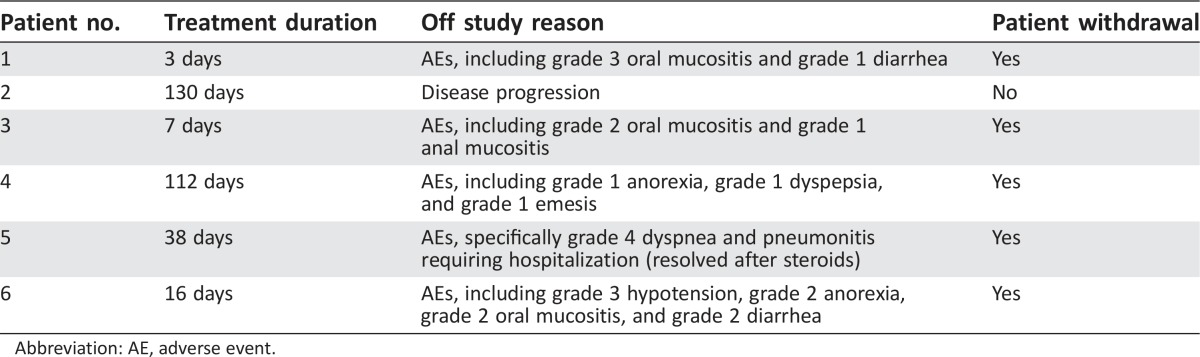
The combination of standard dose abiraterone acetate and BEZ235 200 mg b.i.d. was poorly tolerated. The median treatment duration was 27 days (range: 3–130 days). The most common adverse events were oral mucositis (83.4%), diarrhea (66.7%), nausea (50.0%), anorexia (50.0%), weight loss (50.0%), and musculoskeletal pain (50.0%). The DLTs experienced by patients (n = 3, 50%) were grade 3 mucositis, grade 3 hypotension, and grade 4 dyspnea and pneumonitis. Five patients (83%) came off study because of study‐related adverse events, and one patient came off study due to disease progression. No patient achieved any level of PSA decline (Fig. 1). The best radiographic response in two patients was stable disease. The clinical development of BEZ235 as a potential therapy for prostate cancer has been discontinued.
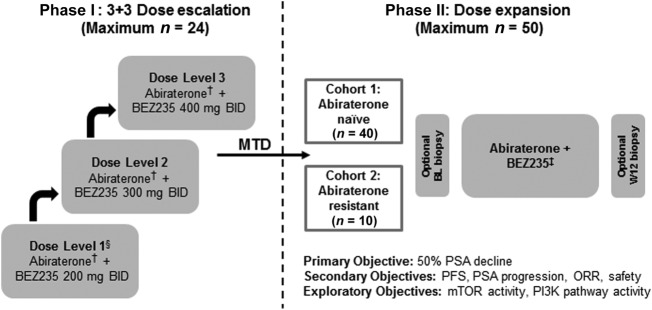
Figure 1.
Study schema. †, Phase 1 abiraterone dose was 1,000 mg daily with prednisone 5 mg b.i.d. and BEZ235 at MTD. ‡, Phase 2 starting dose was abiraterone 1,000 mg daily with prednisone 5 mg b.i.d. and BEZ235 at MTD. §, If >1 of 3 or ≥2 of 6 patients experienced a dose‐limiting toxicity at dose level 1, the study would be terminated.
Abbreviations: BL, baseline; BID, twice daily; MTD, maximum tolerated dose; mTOR, mechanistic target of rapamycin; ORR, objective response rate; PFS, progression‐free survival; PI3K, phosphoinositide 3‐kinase; PSA, prostate‐specific antigen; W12, week 12.
Trial Information
- Disease
Prostate Cancer
- Stage of disease/treatment
Metastatic/Advanced
- Prior Therapy
No designated number of regimens
- Type of study
Phase I/II
- Primary Endpoint
Safety and feasibility
- Secondary Endpoint
Pharmacokinetics studies were not performed because the study was terminated early.
- Additional Details of Endpoints or Study Design
- The original study design planned to determine the MTD of the combination of abiraterone acetate and BEZ235 during 3 + 3 dose escalation (phase I), followed by a dose expansion phase to assess efficacy (phase II). The primary endpoint of the phase I study was to determine the safety and feasibility of combining BEZ235 and abiraterone acetate. The primary endpoint of the phase II study was PSA response rate, by >50%, at 12 weeks. The study protocol specified that if >1 of 3 patients or >2 of 6 patients experience a dose‐limiting toxicity (DLT) at dose level 1, the study would be terminated (Fig. 2).
- Investigator's Analysis
Poorly tolerated/not feasible
Figure 2.
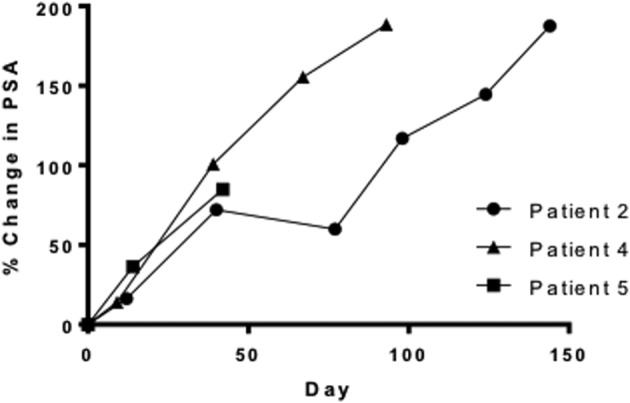
Percentage PSA change from baseline during study period for patients who received at least 1 month of study treatment. The longest treatment break was 12 days in patient 2.
Abbreviation: PSA, prostate‐specific antigen.
Drug Information
- Drug 1
- Generic/Working name
Abiraterone acetate
- Trade name
Zytiga
- Company name
Janssen Biotech
- Drug type
Small molecule
- Dose
milligrams (mg) per flat dose
- Route
oral (p.o.)
- Schedule of Administration
1,000 mg daily (given with prednisone 5 mg twice daily)
- Drug 2
- Generic/Working name
BEZ235
- Trade name
N/A
- Company name
Novartis
- Drug type
Small molecule
- Drug class
PI3 kinase
- Dose
milligrams (mg) per flat dose
- Route
oral (p.o.)
- Schedule of Administration
200 mg twice daily
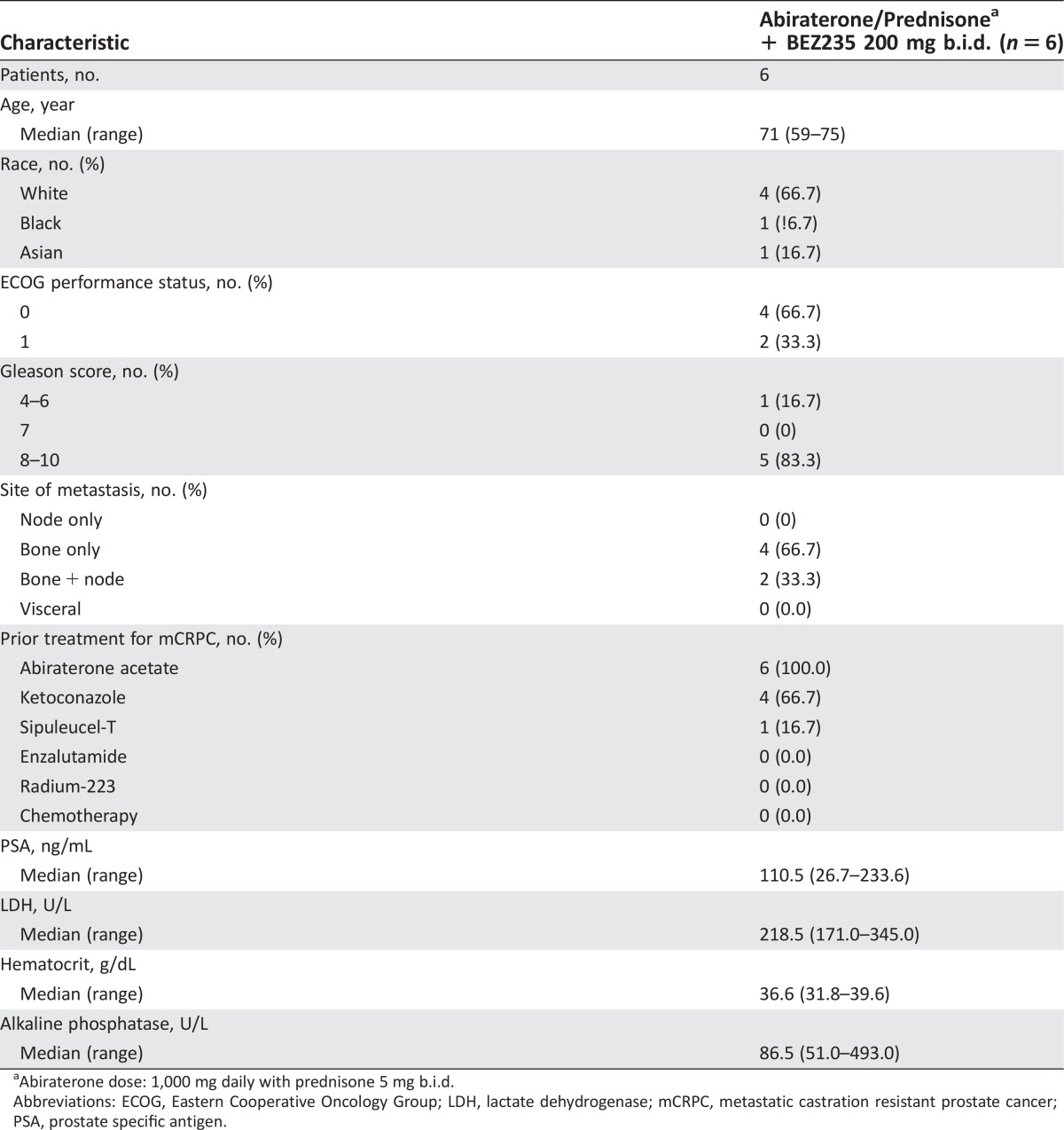
Patient Characteristics
- Number of patients, male
6
- Number of patients, female
0
- Stage
Metastatic, castration resistant
- Age
Median (range): 71 years (59–75 years)
- Number of prior systemic therapies
Median (range): 2 (1–3)
- Performance status: ECOG
-
0 — 4
1 — 2
2 —
3 —
unknown —
- Cancer types or histologic subtypes
Prostate 6
Primary Assessment Method
- Control Arm: Prostate
- Number of patients screened
13
- Number of patients enrolled
6
- Number of patients evaluable for toxicity
6
- Number of patients evaluated for efficacy
2
- Evaluation method
RECIST v1.1 and PCWG2
- Response assessment SD
n = 2 (100%)
- (Median) duration assessments duration of treatment
27 days
Adverse Events
Adverse Events Legend
No Change from Baseline/No Adverse Event
All adverse events that are at least probably related to study therapy, graded according to National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) v4.0.
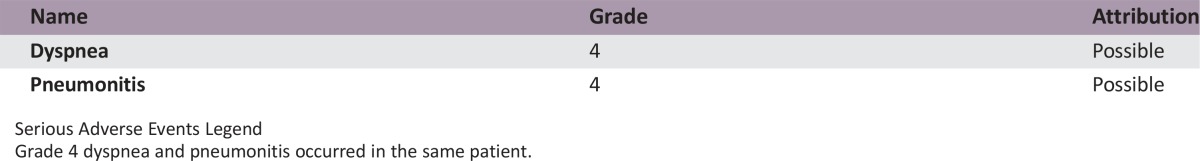
Serious Adverse Events
Serious Adverse Events Legend
Grade 4 dyspnea and pneumonitis occurred in the same patient.
Dose‐Limiting Toxicities
Assessment, Analysis, and Discussion
- Completion
Study terminated before completion
- Terminated reason
Toxicity
- Pharmacokinetics/Pharmacodynamics
Not collected—pharmacokinetics studies were not performed because the study was terminated early.
- Investigator's Assessment
Poorly tolerated/not feasible
In this phase I study of the combination of abiraterone/prednisone plus BEZ235, combination therapy was found to be intolerable. The PI3K (phosphoinositide 3‐kinase) pathway is a major driver of cancer progression in different malignancies. Genomic aberrancy in the PI3K pathway is present in approximately 60% of mCRPC, and PI3K signaling has been identified as a major mechanism of resistance in mCRPC [1], [2]. Preclinical work from different groups has shown cross‐regulation between AR signaling and PI3K‐AKT‐mTOR signaling [3], [4], [5]. Furthermore, combined inhibition of AR signaling and PI3K‐AKT‐mTOR signaling has been shown to augment anti‐cancer activity, particularly in models of prostate cancer with PTEN‐loss [3], [4], [5]. This led to the hypothesis that co‐inhibition of these two interconnected pathways may be a viable strategy to prevent or overcome resistance in patients with CRPC.
Clinical trials of allosteric mTOR inhibitors in men with advanced prostate cancer have demonstrated limited clinical activity [6], [7]. This was thought to be due in part to the activation of AKT and led to the development of strategies that target upstream of PI3K‐AKT‐mTOR signaling. Here, we report the first study designed specifically to target androgen synthesis inhibition in combination with targeting PI3K signaling in mCRPC. In this study, the combination of standard dose abiraterone acetate (1,000 mg daily with prednisone 5 mg b.i.d.) with BEZ235, a potent dual pan‐class I PI3K and mTORC1/2 inhibitor was evaluated (Figure 1). The combination was found to be poorly tolerated, and dose escalation and cohort expansion was not possible.
Six patients were accrued at dose level 1. The median age of the patients was 71 years. The majority of patients (83.3%) had Gleason 8–10 disease (Table 2). All patients had bone metastasis with or without nodal metastasis; no patients had visceral metastasis. The median number of prior therapy for mCRPC was 2 (range: 1–3). All patients had previously progressed on abiraterone and were abiraterone‐resistant. All patients were chemotherapy‐naive (Table 1).
Table 2. Baseline demographic and disease characteristics.
Abiraterone dose: 1,000 mg daily with prednisone 5 mg b.i.d.
Abbreviations: ECOG, Eastern Cooperative Oncology Group; LDH, lactate dehydrogenase; mCRPC, metastatic castration resistant prostate cancer; PSA, prostate specific antigen.
The median treatment duration was 27 days (range: 3–130 days). The most common adverse events were grade 1–2 oral mucositis (66.7%), diarrhea (66.7%), nausea (50.0%), anorexia (50.0%), weight loss (50.0%), and musculoskeletal pain (50.0%). Three patients (50%) experienced DLT: grade 3 mucositis, grade 3 hypotension, and grade 4 dyspnea and pneumonitis (Table 3). Five patients (83%) came off study due to study‐related adverse events, and one patient came off study due to disease progression (Table 4). Of the three patients who received study therapy for at least 1 month (range: 38–130 days), none achieved any level of PSA decline (Figure 2). The best radiographic response in two patients was stable disease.
Table 3. Treatment‐related adverse events.
Adverse events were graded by CTCAE v4.0. Patients were classified according to the worst grade of adverse event(s) reported. Adverse events occurring in more than 1 patient (>16.7%) are shown. *Abiraterone dose: 1,000 mg daily with prednisone 5 mg b.i.d.
Table 4. Treatment duration and reason off study.
Abbreviation: AE, adverse event.
Mucositis, anorexia, and weight loss are adverse events previously observed with PI3K‐AKT‐mTOR pathway inhibition and may represent a class effect of targeting the PI3K pathway. These adverse events were observed to a greater extent than anticipated. BEZ235 was administered in suspension, after sachets of the drug were dissolved in approximately 200 mL of water; therefore, the increased rate of mucositis observed may be related to higher drug exposure and absorption in the oral mucosa. All patients enrolled received maximal supportive care; therefore, our findings demonstrate an extremely low likelihood that pan‐PI3K and mTORC1/2 inhibition can be achieved at the dose administered. It is likely that adverse events were contributed by off‐target effects as well as on‐target effects from inhibiting all class I PI3K isoforms in addition to mTORC1/2 inhibition, irrespective of their role in tumorigenesis. Based on these findings as well as findings from another study with this agent (NCT01634061, unpublished, but registered at ClinicalTrials.gov), clinical development of BEZ235 as a potential therapy for prostate cancer was discontinued. Of note, the development of BEZ235 has also been discontinued for renal cell carcinoma (RCC) due to high incidence of DLTs seen in a phase Ib study of BEZ235 monotherapy in advanced RCC patients [8].
Metastatic castration resistant prostate cancer that has progressed despite abiraterone therapy is a challenge for drug development as no agent other than docetaxel chemotherapy has shown consistent clinical activity in this setting [9]. The rationale for targeting the PI3K‐AKT‐mTOR pathway in mCRPC remains sound, however, and distinct agents that may have a more favorable therapeutic index should continue to be explored. Agents targeting specific PI3K isoforms may be better tolerated and achieve greater therapeutic efficacy [10], and several isoform‐specific PI3K inhibitors are currently under investigation as monotherapy or as combination therapy in advanced solid tumors in early phase clinical trials [11]. Furthermore, mTOR inhibitors that compete with the ATP‐binding site are currently under clinical development, including the combination of enzalutamide with a dual ATP‐competitive mTOR and DNA‐PK inhibitor, CC‐115 in men with abiraterone‐ and enzalutamide‐naive progressive mCRPC (NCT02833883) [12].
It remains to be determined what combination of AR and PI3K pathway inhibition will be both tolerable and clinically active. The combination of enzalutamide with BKM120, a pan‐class I PI3 kinase inhibitor, was investigated in a phase II nonrandomized study of BKM120 with or without enzalutamide in men with progressive mCRPC who failed or were not candidates for docetaxel chemotherapy [13]. Thirteen of 30 (43%) patients received BKM120 while continuing enzalutamide. Of all patients treated, treatment was not well tolerated (47% of patients experienced grade 3 adverse events), and no patient achieved >50% PSA response or objective response [13]. More recently, results of a randomized phase II study of ipatasertib (GDC‐0068), an AKT inhibitor, combined with abiraterone/prednisone in men with docetaxel‐pretreated mCRPC, were presented [14). In this study, 253 patients were randomized to ipatasertib 200 mg daily, ipatasertib 400 mg daily, or placebo in combination with standard dose abiraterone. A trend toward improved radiographic progression‐free survival (hazard ratio (HR) = 0.75, p = .17) and overall survival (HR = 0.72, p = .22) was observed with ipatasertib 400 mg daily plus abiraterone, but not ipatasertib 200 mg daily plus abiraterone, indicating a dose response. Importantly, adverse events were also dose dependent, and this combination was well tolerated with manageable side effects. AKT inhibitors lie at an important signaling junction—downstream of PI3K and upstream of mTOR—and may have a better therapeutic index compared with agents such as BEZ235.
Figures and Tables
Acknowledgments
Novartis Pharmaceuticals provided financial support for this study. The authors thank all patients who participated in this study and their families.
Footnotes
ClinicalTrials.gov Identifier: NCT01717898
Sponsor(s): Charles J. Ryan
Principal Investigator: Charles J. Ryan
IRB Approved: Yes
Disclosures
Andrew C. Hsieh: Revolution Medicines (C/A); Won Kim: Genentech (C/A); Terence Friedlander: MedBioGene (E), Genentech, AstraZeneca, Clovis Oncology, Pfizer (C/A), Dendreon, Astellas, Sanofi‐Genzyme (H), Janssen, Novartis (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1. Robinson D, Van Allen EM, Wu YM et al. Integrative clinical genomics of advanced prostate cancer. Cell 2015;161:1215–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Taylor BS, Schultz N, Hieronymus H et al. Integrative genomic profiling of human prostate cancer. Cancer Cell 2010;18:11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Carver BS, Chapinski C, Wongvipat J et al. Reciprocal feedback regulation of PI3K and androgen receptor signaling in PTEN‐deficient prostate cancer. Cancer Cell 2011;19:575–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Thomas C, Lamoureux F, Crafter C et al. Synergistic targeting of PI3K/AKT pathway and androgen receptor axis significantly delays castration‐resistant prostate cancer progression in vivo. Mol Cancer Ther 2013;12:2342–2355. [DOI] [PubMed] [Google Scholar]
- 5. Marques RB, Aghai A, de Ridder CM et al. High efficacy of combination therapy using PI3K/AKT inhibitors with androgen deprivation in prostate cancer preclinical models. Eur Urol 2015;67:1177–1185. [DOI] [PubMed] [Google Scholar]
- 6. Nakabayashi M, Werner L, Courtney KD et al. Phase II trial of RAD001 and bicalutamide for castration‐resistant prostate cancer. BJU Int 2012;110:1729–1735. [DOI] [PubMed] [Google Scholar]
- 7. Templeton AJ, Dutoit V, Cathomas R et al. Phase 2 trial of single‐agent everolimus in chemotherapy‐naïve patients with castration‐resistant prostate cancer (SAKK 08/08). Eur Urol 2013;64:150–158. [DOI] [PubMed] [Google Scholar]
- 8. Carlo MI, Molina AM, Lakhman Y et al. A phase Ib study of BEZ235, a dual inhibitor of phosphatidylinositol 3‐kinase (PI3K) and mammalian target of rapamycin (mTOR), in patients with advanced renal cell carcinoma. The Oncologist 2016;21:787–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. de Bono JS, Smith MR, Saad F et al. Subsequent chemotherapy and treatment patterns after abiraterone acetate in patients with metastatic castration‐resistant prostate cancer: Post hoc analysis of COU‐AA‐302. Eur Urol: 2016. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Thorpe LM, Yuzugullu H, Zhao JJ et al. PI3K in cancer: Divergent roles of isoforms, modes of activation and therapeutic targeting. Nat Rev Cancer 2015;15:7–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Edlind MP and Hsieh AC. PI3K‐AKT‐mTOR signaling in prostate cancer progression and androgen deprivation therapy resistance. Asian J Androl 2014;16:378–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Munster PN, Mahipal A, Nemunaitis JJ et al. Phase I trial of dual TOR kinase and DNA‐PK inhibitor (CC‐115) in advanced solid and hematologic cancers. J Clin Oncol 2016;34(suppl):abstract 2505. [Google Scholar]
- 13. Armstrong AJ, Halabi S, Healy P et al. Phase II trial of the PI3 kinase inhibitor BKM120 with or without enzalutamide in men with metastatic castration resistant prostate cancer (mCRPC). J Clin Oncol 2015:33(suppl):abstract 5025. [Google Scholar]
- 14. De Bono JS, Giorgi UD, Massard C et al. Randomized phase II study of AKT blockade with ipatasertib (GDC‐0068) and abiraterone (Abi) vs. abi alone in patients with metastatic castration‐resistant prostate cancer (mCRPC) after docetaxel chemotherapy (A. MARTIN Study). J Clin Oncol 2016;34(suppl);abstract 5017. [Google Scholar]