Stratification of risk through predictive models is a strategic method for reducing the burden of venous thromboembolism in cancer patients. The objective of this study was to derivate a novel predictive scoring tool for measuring the risk of the individual patient.
Keywords: Thromboembolism, Cancer, Risk stratification, Predictive score
Abstract
Background.
The efficacy of risk model scores to predict venous thromboembolism (VTE) in ambulatory cancer patients is under investigation, aiming to stratify on an individual risk basis the subset of the cancer population that could mostly benefit from primary thromboprophylaxis.
Materials and Methods.
We prospectively assessed 843 patients with active cancers, collecting clinical and laboratory data. We screened all the patients with a duplex ultrasound (B‐mode imaging and Doppler waveform analysis) of the upper and lower limbs to evaluate the right incidence of VTE (both asymptomatic and symptomatic). The efficacy of the existing Khorana risk model in preventing VTE was also explored in our population. Several risk factors associated with VTE were analyzed, leading to the construction of a risk model. The Fine and Gray model was used to account for death as a competing risk in the derivation of the new model.
Results.
The risk factors significantly associated with VTE at univariate analysis and further confirmed in the multivariate analysis, after bootstrap validation, were the presence of metastatic disease, the compression of vascular/lymphatic structures by tumor, a history of previous VTE, and a Khorana score >2. Time‐dependent receiving operating characteristic (ROC) curve analysis showed a significant improvement in the area under the curve of the new score over the Khorana model at 3 months (71.9% vs. 57.9%, p = .001), 6 months (75.4% vs. 58.6%, p < .001), and 12 months (69.8% vs. 58.3%, p = .014).
Conclusion.
ONKOTEV score steps into history of cancer‐related‐VTE as a promising tool to drive the decision about primary prophylaxis in cancer outpatients. The validation represents the goal of the prospective ONKOTEV‐2 study, endorsed and approved by the European Organization for Research and Treatment of Cancer Young Investigators Program. The Oncologist 2017;22:601–608
Implications for Practice.
Preventing venous thromboembolism in cancer outpatients with a risk model score will drive physicians' decision of starting thromboprophylaxis in high‐risk patients.
Introduction
Venous thromboembolism (VTE), which includes both deep vein thrombosis and pulmonary embolism (PE), is a significant source of morbidity in the cancer population [1]. Apart from causing elevated risk of early mortality, high incidence of VTE in the cancer population leads, in some cases, to interruption or delay of potentially life‐saving treatments, worsening of quality of life, and higher utilization of health care resources [2]. Venous thromboembolism affects up to 20% of hospitalized and ambulatory cancer patients prior to death, and this rate tends to double at postmortem examination [3], [4]. The incidence among ambulatory patients, however, is not exactly defined. Data extracted from a large health care database of insured American patients between 2004 and 2009 suggest that VTE occurred in 12.6% of ambulatory cancer patients over 12 months of follow‐up after chemotherapy [5].
As the VTE risk in ambulatory subjects is due to different factors (cancer‐, patient‐, and treatment‐related risk factors), recommendation of primary prevention in all patients has not achieved consensus because of doubts on risk/benefit and cost/efficacy ratios.
Several meta‐analyses and randomized controlled trials exploring the efficacy of thromboprophylaxis with low molecular weight heparins in ambulatory cancer patients have been reported. Overall, they demonstrated a moderate benefit for the thromboprophylaxis group and highlighted the need to select a subgroup of high‐risk patients who could mostly benefit from the primary prophylaxis [6], [7], [8], [9], [10]. However, the best tool to measure the risk of each individual patient still lacks scientific evidence.
In this scenario, stratification of VTE risk through predictive models represents the preferred strategy to reduce the burden of VTE in oncology. The first risk‐scoring method was developed by Khorana in 2008 [11] and further derivated in three separate studies [12], [13], [14]. Currently, the Khorana score is the best known tool available (Table 1). Here, we present a large prospective study in which we aim to validate a novel score and explore its further optimization with the addition of easy‐to‐use clinical covariates.
Table 1. Khorana score and risk categories.
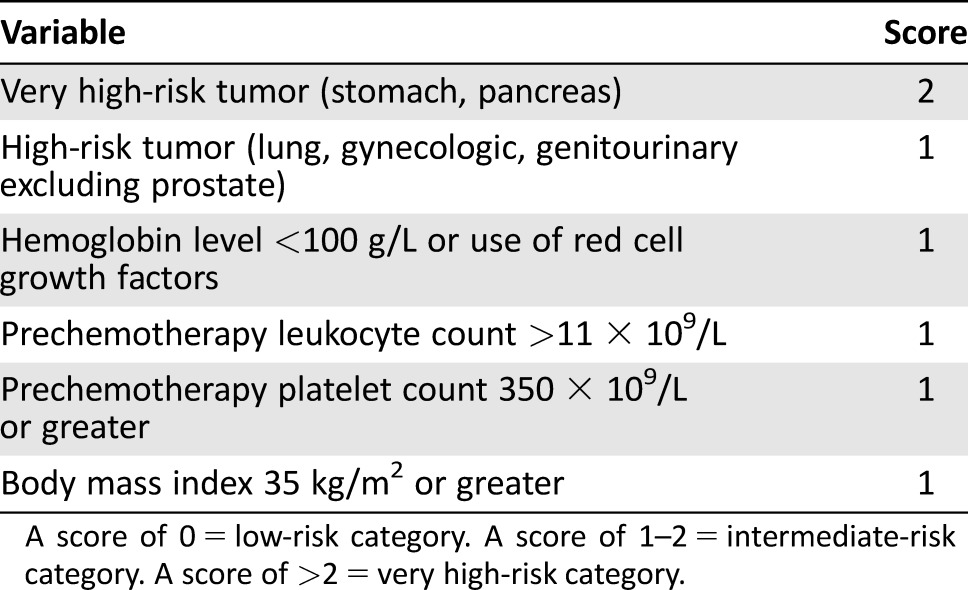
A score of 0 = low‐risk category. A score of 1–2 = intermediate‐risk category. A score of >2 = very high‐risk category.
Methods
Study Population and Design
The present study population included 843 patients enrolled between October 2012 and April 2014 in a prospective observational study. The trial was carried on at Federico II University of Naples (Italy) and at the University Cancer Center Leipzig (Germany). The protocol was approved by the Ethics Committees of Naples (September 2012) and Leipzig (August 2013). Each patient signed a written informed consent at the enrollment visit. Patients ≥18 years of age, with a diagnosis of solid tumors confirmed by cytology/histology at any stage and candidate to receive chemotherapy, endocrine therapy, radiotherapy, target therapy, and/or surgery, alone or in combination. Exclusion criteria were end‐stage renal (eGFR <15 mL/minute) or liver (Child‐Pugh C) disease and disease‐free patients in follow‐up.
Patients with at least 6 months life expectation were included; those with renal or liver failure were excluded because they usually do not receive tumor‐specific treatments. This data could potentially affect the survival outcomes. Finally, we excluded disease‐free patients, in particular those with no active cancer, to avoid complicating the cause and effect relationship between thrombosis and neoplasm in patients without cancer.
The primary endpoint of the ONKOTEV trial was to analyze, in an outpatient cancer setting, risk factors associated with cancer‐related VTE.
At the time of the inclusion visit, clinicians reported data from clinical examination, routine blood tests (complete blood count, electrolytes, renal and hepatic function, coagulation parameters), venous compression ultrasound (B‐mode imaging), duplex ultrasound (B‐mode imaging and Doppler waveform analysis), and color Doppler imaging of the upper and lower limbs and the veins of the neck performed on all patients. Moreover, in addition to Doppler ultrasound, the most recent abdomen ultrasound and/or chest/abdomen/pelvis computerized tomography (CT) scan was also reviewed to detect a silent PE or a deep vein thrombosis and finally collected in basal evaluation form. Moreover, standardized imaging, such as CT scan or magnetic resonance imaging (MRI), can accurately detect macroscopic compression of vascular structure by tumor, discriminating by other forms of vascular involvement (such as direct infiltration), which—in our study—were not included.
Each patient was reassessed after 6 months, collecting data using the same parameters (clinical, laboratory, and imaging data). An extensive reassessment with a clinical examination or telephone contact, up to 12 months after the inclusion visit, was optionally allowed, when applicable.
Statistical Analysis
The number of patients enrolled in the present study have been determined based on previous estimates [15], [16]. Assuming a VTE incidence equal to 6% on an average follow‐up of 12 months and using the criterion of a minimum number of events per predictor equal to 10, a sample size of at least 800 subjects was deemed to be sufficient for the construction of a risk model that includes up to five predictors. Sufficiency is here to be understood in terms of a relative distortion in the estimated coefficients of less than 5% in absolute value.
In the analysis of potential risk factor for the occurrence of VTE, mortality has been considered as a competing risk factor and thus the marginal probability of VTE has been estimated using the cumulative incidence (CI) function. Accordingly, the Fine and Gray model has been used to model the association between prognostic factors and the occurrence of VTE. In particular, those factors that presented a univariate association with the event at a p < .05 have been selected for the development of the risk score model. The model was constructed and internally validated using a bootstrap approach [17]; 999 bootstrap samples (with replacement) were drawn from the original study sample. On each of them, a multivariable regression model for competing risk with all predictors identified in the univariate step was fitted using a backward selection procedure. The backward selection was based on the BICcr criterion as suggested in [18]. Those variables with p values less than .05 in at least 70% of the bootstrap samples were included in the final multivariate model. After selecting these strong predictors, a further screening of covariates was performed to address the correlation pattern between covariates by considering the variables with the larger frequency out of each highly frequent variable pair (>90 %). Although the adopted bootstrap approach can reduce the risk of overfitting, we stress that only by using an external validation cohort, the predictive performance of a model could be correctly estimated and assessed. Results from the Fine and Gray regression models are reported as subdistribution hazard ratio (sHR) with 95% confidence intervals (95% CI). In order to obtain a new risk score based on the estimated multivariable model, one point was assigned to the factor with the lowest estimated coefficient and the remaining points were determined accordingly (as proposed in [19]). Discrimination of the derived risk score was assessed using the time‐dependent receiver operating characteristic (ROC) curve and time‐dependent area under the ROC curve (AUC) [20]. All statistical analyses were performed using R version 3.2.0 (R Foundation for Statistical Computing, Vienna, Austria; https://www.R-project.org/). Competing risk analysis was performed using package cmprsk (R package version 2.2‐7. https://CRAN.R-project.org/package=cmprsk).
Results
Characteristics of the Study Population and Outcomes
The baseline characteristics of our population (n = 843) are summarized in Table 2. With the exception of patients who underwent surgery, in whom low‐molecular weight heparin was routinely continued up to 4 weeks after discharge, no patient received heparin prophylaxis or platelet‐active drugs at the enrollment time and throughout the study period.
Table 2. Characteristics of the patient population.
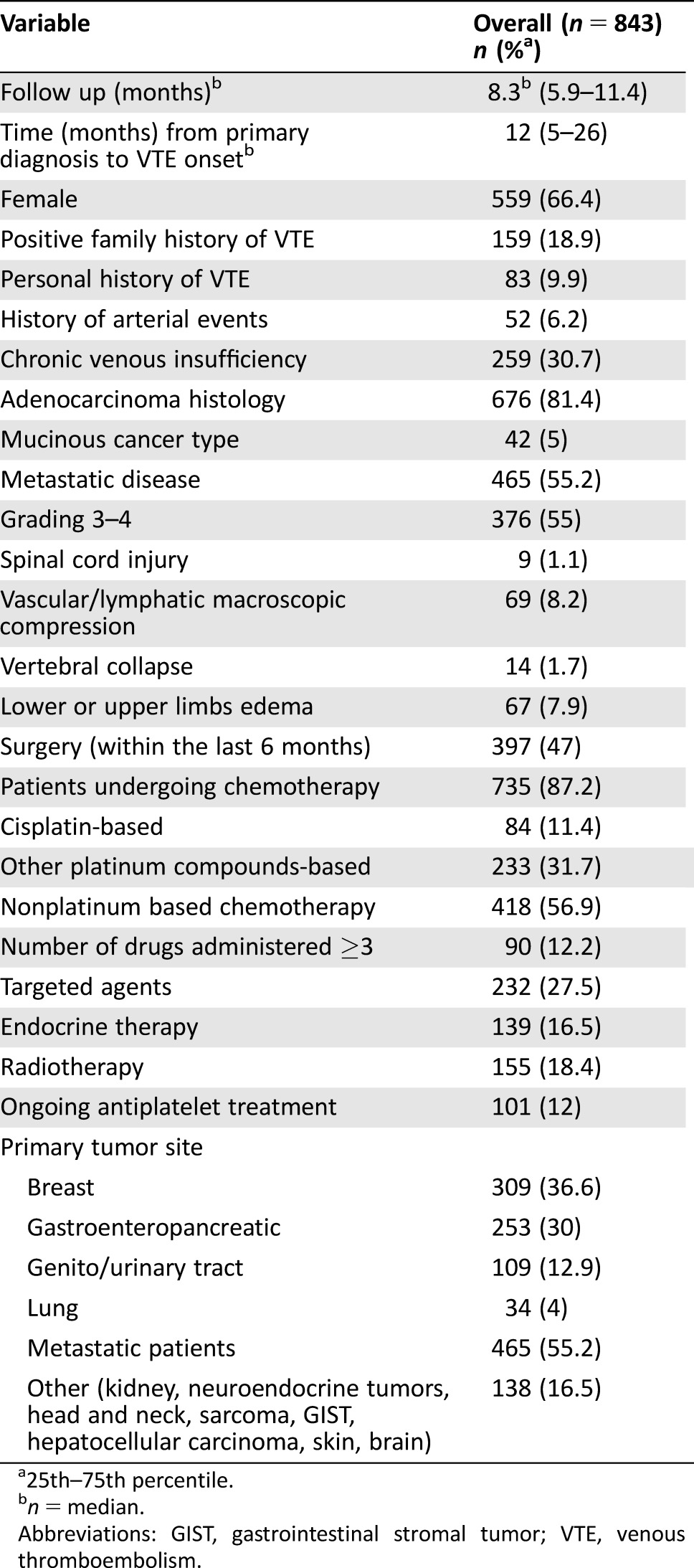
25th–75th percentile.
n = median.
Abbreviations: GIST, gastrointestinal stromal tumor; VTE, venous thromboembolism.
The median observation period of the entire population was 8.3 months (interquartile range 5.9–11.4). Overall 73 (8.6%) VTEs were diagnosed. All thrombotic events were non‐fatal: 45% (n = 33/73) occurred exclusively at lower limbs, 18% (n = 13/73) at head and neck veins, 15% (n = 11/73) at major abdominal veins, and 11% (n = 8/73) at multiple site. Pulmonary embolism occurred in 11% (n = 8/73). Among the VTE events screened with ultrasound, only 0.07% (5/73 patients) were asymptomatic and occurred at the following sites: four at lower limbs and one at jugular vein. Among all upper limb events, only one was related to the presence of a central venous catheter. Silent PE or abdominal thrombosis were commonly diagnosed with CT scan, which was part of the routine assessment. The total percentage of asymptomatic events is 31% (23/73).
All VTE events are summarized in Table 3.
Table 3. Distribution of venous thromboembolisms.
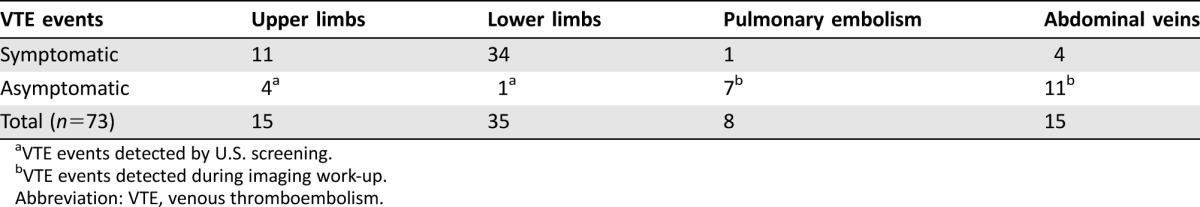
VTE events detected by U.S. screening.
VTE events detected during imaging work‐up.
Abbreviation: VTE, venous thromboembolism.
Risk Assessment and Rate of Thrombotic Events According to Khorana Score.
According to the available clinical characteristics at baseline, the Khorana score was calculated on 96.4% of the study population (n = 813) patients, as shown in Table 4. The cumulative incidence of VTE at 12 months in the three Khorana risk groups was 8.8% (n = 32), 9.2% (n = 30), and 21.7% (n = 11), respectively.
Table 4. Performance of Khorana score in the ONKOTEV trial.
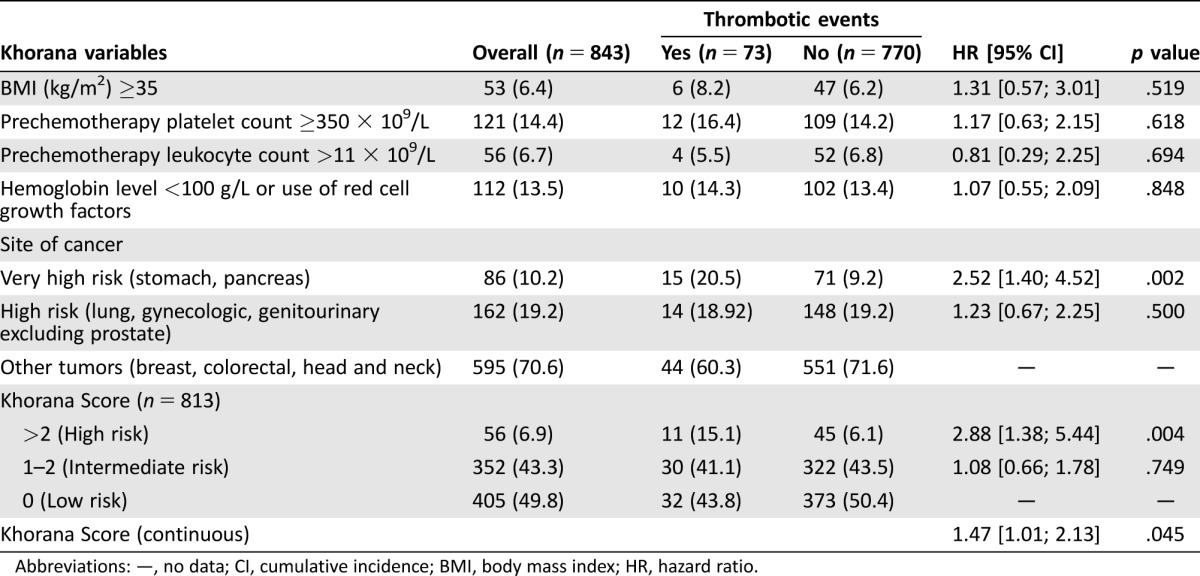
Abbreviations: —, no data; CI, cumulative incidence; BMI, body mass index; HR, hazard ratio.
The sHR for developing a VTE was 2.74 (95% CI: 1.38 to 5.44, p = .004) and 1.08 (95% CI: 0.66 to 1.78, p = .749) for Khorana high and intermediate risk, respectively, compared with the Khorana low‐risk category. Assuming the Khorana score as a continuous predictor, every unit increase in the score led to a 47% increase in the risk of developing VTE (sHR 1.47, 95% CI 1.01 to 2.13, p = .045). When the six variables included in the Khorana score were separately analyzed, only the very high‐risk primary site of cancer (stomach and pancreas) significantly predicted VTE (s‐HR 2.52, 95% CI: 1.40 to 4.52, p = .002) in the present population.
Multiparametric Risk Assessment.
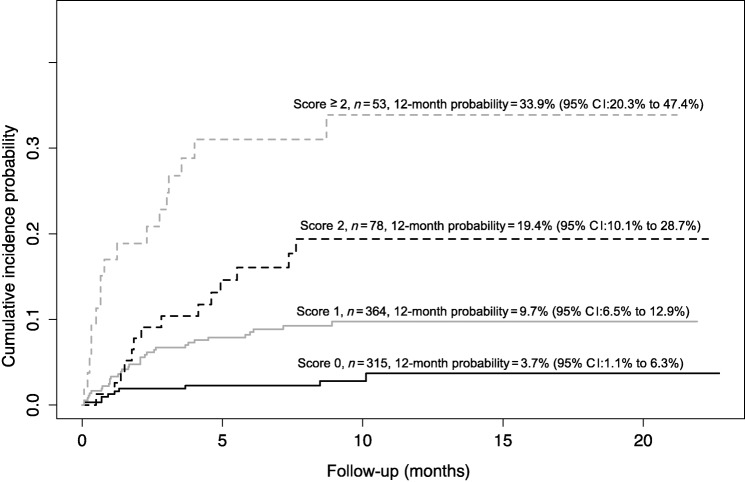
The risk factors that significantly increased the risk of VTE at univariate analysis are summarized in Table 5. In the multivariate analysis, after bootstrap validation, the factors that independently showed a significant association with the outcome are shown in Table 6. In order to set up a multi‐item score, we assigned one point to each of these four variables, as shown in Table 7. The cumulative incidence function for developing a VTE in the four categories of the score is shown in Figure 1. The cumulative incidence probability at 12 months in patients with ONKOTEV score 0, 1, 2, and >2 is, respectively, 3.69% (95% CI: 1.07% to 6.31%), 9.74% (95% CI: 6.53% to 12.94%), 19.39% (95% CI:10.1% to 28.68%), and 33.87% (95% CI:20.32% to 47.41%). The sHR of developing a VTE in the ONKOTEV “score = 1”, “score = 2” and “score > 2” risk categories were 3.29 (95% CI: 1.57 to 6.89, p = .002), 6.54 (95% CI: 2.84 to 15.03, p < .001), and 13.74 (95% CI 6.08 to 31.07, p < .001) respectively, considering ONKOTEV “score = 0” as reference category. Time dependent AUCs were significantly higher for the new score with respect to the Khorana score at 3 months (71.9% vs. 57.9%, p = .001), 6 months (75.4% vs. 58.6%, p < .001), and 12 months (69.8% vs. 58.3%, p = .014).
Table 5. Predictive factors for venous thromboembolism at univariate analysis.
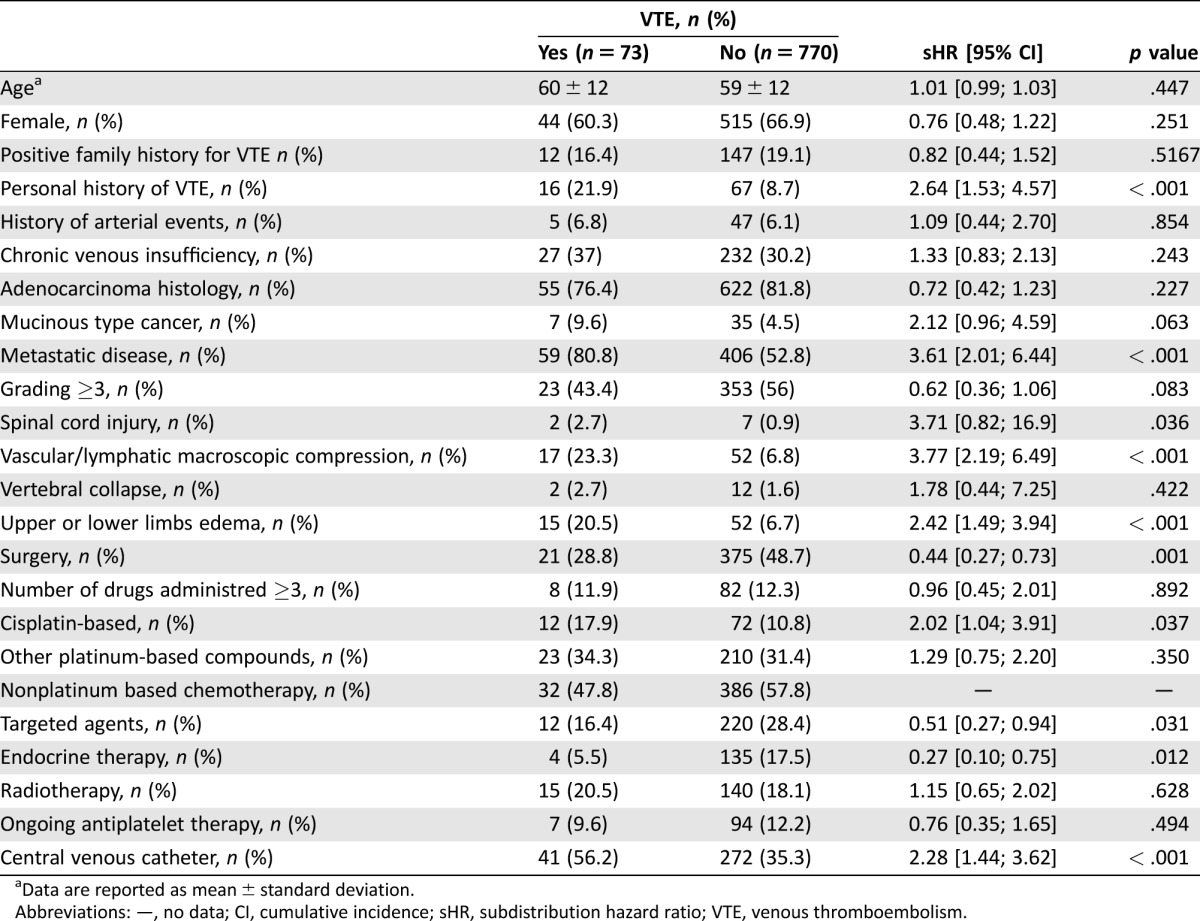
Data are reported as mean ± standard deviation.
Abbreviations: —, no data; CI, cumulative incidence; sHR, subdistribution hazard ratio; VTE, venous thromboembolism.
Table 6. Predictive risk model.
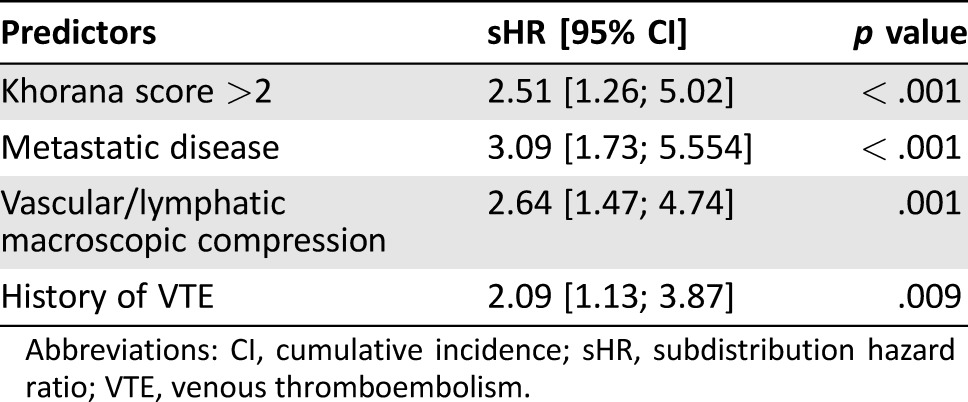
Abbreviations: CI, cumulative incidence; sHR, subdistribution hazard ratio; VTE, venous thromboembolism.
Table 7. The ONKOTEV score.
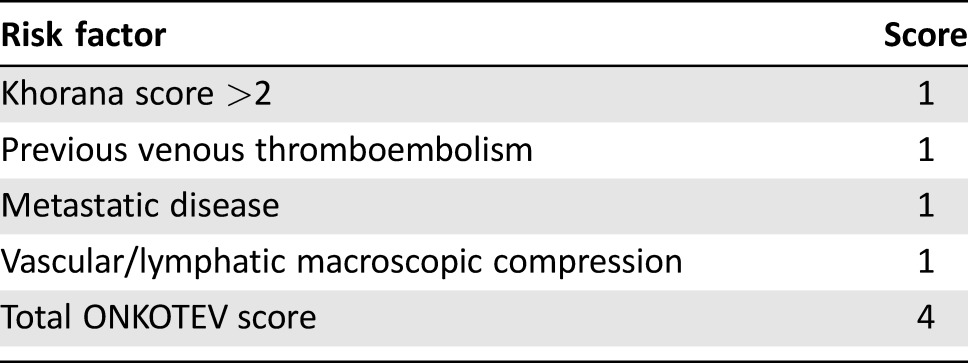
Figure 1.
Cumulative incidence function for the risk of developing a venous thromboembolism according to the ONKOTEV score.
Abbreviation: CI, confidence interval.
Discussion
The stratification of VTE risk in cancer outpatients is an emerging area of investigation. Current guidelines recommend the use of primary thromboprophylaxis only in patients with multiple myeloma receiving thalidomide or lenalidomide, especially when combined with high‐dose dexamethasone. As for the other cancer settings, the international panels emphasize the need for stratifying the VTE risk by easy‐to‐use tools that have emerged in the last few years [21], [22], [23], [24]. The mostly widespread, the Khorana risk‐scoring method, is based on five variables and is able to stratify cancer patients into three risk categories (low risk for a 0‐point score, intermediate risk for 1‐ or 2‐point score, and high risk if the point score >2) [11].
Although achieving a good risk stratification of cancer outpatients, the Khorana score has, in our opinion, some weaknesses: (a) in spite of the relevant patient cohort (2,701 individuals) evaluated, the VTE incidence is rather low (2.2%), maybe due to the relatively short median observation period (73 days); (b) the validation of the score was retrospectively carried out; (c) the proportion of hospitalized/ambulatory cancer patients is not reported; (d) additional tumor‐related VTE risk factors (e.g., the impact of poly‐chemotherapy, endocrine therapy, and/or target therapies) were not analyzed; and (e) anatomic conditions predisposing to VTE as the presence of central venous catheter or the encasement of vascular structures by the tumor have not been considered.
Two years later, the predictive effectiveness of the Khorana score has been confirmed and improved by the Vienna Cancer Group study, with the addition of two laboratory parameters: D‐dimer and P‐selectin [25], chosen according to previous findings from the same authors [13], [26], [27]. In the study, the population was more heterogeneous, including patients with different cancer types and patients who had not received chemotherapy within the previous 3 months but undergone radiotherapy or surgery within the last 2 weeks. As for primary cancer sites, high‐grade gliomas and lymphomas were included in the analysis, allocating brain tumors to the “very high‐risk” sites group and multiple myeloma to the “high‐risk” sites group. In addition, the median follow‐up (2 years) was significantly longer than Khorana's work. However, the evaluation of highly specific biomarkers, like P‐selectin, significantly reduces its widespread clinical use and increases the overall costs for the VTE risk assessment.
In our experience, 843 patients with active cancers were prospectively assessed, collecting clinical and laboratory data. We pioneered an extensive screening with upper and lower limbs ultrasound to all patients to have the most precise incidence of VTE in cancer outpatients; we also explored the efficacy of the existing Khorana risk model in our population.
Several tumor or treatment‐related risk factors, selected according to previous evidences, were investigated in our 843 patients [28], [29], [30], [31], [32], [33], [34]. The number and type of chemotherapeutic agents administered in monotherapy or in combination, the impact of novel target agents (especially those with antiangiogenic activity), the macroscopic involvement of vascular structures for tumor compression or infiltration, the stage of disease (localized or metastatic), and the role of other antitumoral strategies (radiotherapy, endocrine therapy, surgery) appeared to be relevant as to an integrated evaluation of the individual risk. This makes the patients of the ONKOTEV trial rather comparable to everyday patient populations. A previous personal history of VTE is related to any VTE that occurred more than 6 months before enrollment. Patients who were still receiving anticoagulation therapy for VTE—even for one that had occurred more than 6 months before study evaluation—were not included in the protocol. One concern may be raised about the distribution of primary sites in the ONKOTEV population, which is certainly not fully balanced and is not exactly reflecting the epidemiological frequency of tumor diseases. However, the prevalence of primary sites reflects precisely what we see every day in outpatient treatment units, where, for instance, metastatic lung cancer is less frequently treated than metastatic colorectal cancer. The goal of the ONKOTEV trial is to observe a mixed population of patients typically treated in an outpatient setting. Another concern could be related to lymphovascular macroscopic compression assessment; however, standardized imaging, like CT scan or MRI, have accurately detected and discriminated compression by other forms of vascular involvement (like encasement or direct infiltration).
In our population, we registered only one CVC‐related VTE among all upper limb events; this is probably imputable to the improvement in types, implantation techniques, and management of port‐a‐caths in recent years. In this direction, we may assume that traditional separation between cancer‐related and CVC‐related thrombosis may have its drawbacks.
As for PEs rates, we registered an incidence of 11%. Even though we can reasonably consider this finding similar to the other major evidences, we may speculate that peripheral screening has contributed to prevent a further increase in PE incidence, by early management of a deep vein thrombosis with anticoagulation therapy. We additionally analyzed the effect of both cisplatin‐based regimen and other platinum compounds. Surprisingly, the VTE risk associated with cisplatin was found to be statistically significant only in the univariate, but not in the multivariate analysis. If we speculate on reasons, we should take into consideration that—apart from its own increased VTE risk—cisplatin infusion is often associated with other chemotherapic agents in more complex 3‐ to 5‐day scheduled regimens, which are often given in an inpatient setting (e.g., cisplatin‐etoposide for small cell lung cancer or PEB regimen for testicular cancer). As inpatients were excluded from the ONKOTEV trial, those receiving cisplatin‐based chemotherapy in an outpatient setting may not suffer the additional VTE risks related to the hospitalization.
At the multivariate analysis, it was found that a Khorana score >2 independently predicted the outcome as much as the presence of one of the following three clinical covariates: metastatic disease status, vascular/lymphatic compression, or previous history of VTE (Table 5); therefore, the derived multi‐item score includes these four variables.
Upper and lower limb edema, spinal cord injury, surgery, central venous catheter, or endocrine therapy are not included in the final score, because after bootstrap validation, the factors that independently showed a significant association with the outcome are only those included in the final score.
Compared with a 3.7% marginal probability in patients with an ONKOTEV score of 0, those with an ONKOTEV score > 2 exhibited a very high marginal probability of developing VTE (33.87) at 12 months (Table 7). Thus, in our cohort, the derived model was characterized by a higher predictive power compared with the Khorana score. This is also remarked by the further evidence that, in our ambulatory population, 49.8% of patients (n = 405/843) had a Khorana score of 0. Among them, 7.9% (n = 32/405) developed a VTE and, as a consequence, 32/73 (43.8%) of VTE cases were not identified by Khorana score in the present setting. In addition, the marginal probability of VTEs at 12 months was very similar in the Khorana low‐ and intermediate‐risk groups (8.8% and 9.2%, respectively), suggesting a limited power of the Khorana score in stratifying patients with mild to moderate risk of venous thromboembolism. Moreover, by analyzing the individual variables included in the Khorana score, only the “very high‐risk primary site” variable (pancreas, stomach) significantly predicted VTE events in the present ambulatory population (p = .02). Surgery—a traditional risk factor for VTE—in our population most likely contributed little to the VTE events detected. As part of the routine clinical management, low molecular weight heparin is prophylactically used after discharge from surgery for up to 4 weeks. In contrast, with regard to endocrine therapy, we can speculate that the recent spread of aromatase inhibitors, both in adjuvant and metastatic breast cancers, may have reduced the high VTE incidence with tamoxifen reported in the past.
One criticism of the ONKOTEV study could be the fact that not all of the observed events for calculation of the ONKOTEV score were symptomatic or clinically relevant at the timepoint of detection, for example, by ultrasound screening or by thoraco‐abdominal CT or MRI in the context of staging or re‐staging of the tumoral disease. However, even though they may not be not clinically relevant at the timepoint of screening, deep vein/major abdominal vein thromboses are in some cases followed by PE, which is a major cause of cancer‐related morbidity and mortality. On the other hand, the inclusion of such events improved the capability of the ONKOTEV score to detect the real incidence of VTEs.
Future research should also consider implementation of biomarkers, especially soluble plasma factors. Tissue factor‐bearing microparticle, for example, is increased in plasma and in tumor tissue, playing an interesting role in the angiogenic process, hemostasis, and tumor progression. We have tried to separately investigate the role of D‐dimer and P‐selectin according to the Ay et al. score [13], but we were able to measure D‐dimer and P‐selectin in only 150 patients. Because the limited data cannot be implemented into our clinical risk score, we are planning to separately report these findings.
Conclusion
We here show that by adding three commonly employed easy‐to‐integrate clinical parameters (i.e., metastatic disease, malignancy‐related macroscopic vascular or lymphatic compression, and a history of VTE), the prediction of VTE by the Khorana score may be improved in ambulatory cancer patients. As for the large majority of previous reports in the field, we detected asymptomatic VTE by performing an extensive Doppler ultrasound examination and a careful evaluation of CT scans.
Although the bootstrap approach that we used may have reduced the risk of overfitting, an independent validation cohort is the mandatory next step for the present derived risk score. This is the goal of the ONKOTEV‐2 study, as recently endorsed and approved by the European Organization for Research and Treatment of Cancer Young Investigators Program.
Aknowledgments
We acknowledge Prof. Dirk Arnold and Dr. Ramon Salazar (European Organization for Research and Treatment of Cancer [EORTC] members for Young Investigator Program) for proofreading the manuscript, Dr. Murielle Mauer (biostatistician in the EORTC group) for statistical revision, and Dr. Mario Rosanova and Dr. Stefano De Falco for data collecting and database management. C.A.C. is currently affiliated with the Instituto Europeo di Oncologia, Milan, Italy, and the Department of Molecular and Translational Medicine, University of Brescia, Brescia, Italy.
Footnotes
For Further Reading: Patrizia Ferroni, Fiorella Guadagni, Anastasia Laudisi et al. Estimated Glomerular Filtration Rate Is an Easy Predictor of Venous Thromboembolism in Cancer Patients Undergoing Platinum‐Based Chemotherapy. The Oncologist 2014;19:562–567.
Implications for Practice: All major society guidelines currently recommend no thromboprophylaxis for chemotherapy‐treated cancer outpatients. Nonetheless, there is a common need to identify risk assessment models that may be predictive of cancer‐associated venous thromboembolism in at‐risk patients who might benefit from appropriate prevention measures. In this respect, the Khorana score correctly assigns patients to the high‐risk category; however, clinical decision making remains challenging in approximately 50% of patients, who fall in the intermediate risk class. Assessment of pretreatment estimated glomerular filtration rate could represent a simple and cost‐effective predictor of venous thromboembolic events, at no additional cost to health care systems.
Disclosures
The authors indicated no financial relationships.
References
- 1. Stein PD, Beemath A, Meyers FA et al. Pulmonary embolism as a cause of death in patients who died with cancer. Am J Med 2006;119:163–165. [DOI] [PubMed] [Google Scholar]
- 2. Ashrani AA, Silverstein MD, Rooke TW et al. Impact of venous thromboembolism, venous stasis syndrome, venous outflow obstruction and venous valvular incompetence on quality of life and activities of daily living: A nested case‐control study. Vasc Med 2010;15:387–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Prandoni P, Lensing AW, Piccioli A et al. Recurrent venous thromboembolism and bleeding complications during anticoagulant treatment in patients with cancer and venous thrombosis. Blood 2002;100:3484–3488. [DOI] [PubMed] [Google Scholar]
- 4. Lee AY, Levine MN. Venous thromboembolism and cancer: Risks and outcomes. Circulation 2003;107(23 suppl 1):I17–I21. [DOI] [PubMed] [Google Scholar]
- 5. Khorana A, Dalal M, Lin J et al. Incidence and predictors of venous thromboembolism (VTE) among ambulatory high‐risk cancer patients undergoing chemotherapy in the united states. Cancer 2013;119:648–655. [DOI] [PubMed] [Google Scholar]
- 6. Agnelli G, Gussoni G, Bianchini C et al. Nadroparin for the prevention of thromboembolic events in outpatients with metastatic or locally advanced solid cancer receiving chemotherapy: A randomised, placebo‐controlled, double‐blind study. Lancet Oncol 2009;10:943–949. [DOI] [PubMed] [Google Scholar]
- 7. Barni S, Labianca R, Agnelli G et al. Chemotherapy‐associated thromboembolic risk in cancer outpatients and effect of nadroparin thromboprophylaxis: Result of a retrospective analysis of the PROTECHT study. J Transl Med 2011;9:179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Oettle H, Petzer U, Stieler J. Oxaliplatin/Folinic acid/5‐fluoroouracil [24h] (OFF) plus best supportive care versus best supportive care alone (BSC) in second‐line therapy of gemcitabine‐refractory advanced pancreatic cancer (CONKO‐003). Proc Am Soc Clin Oncol: 2005;23:4031. [Google Scholar]
- 9. Maravejas A, Waters J, Roy R et al. Gemcitabine versus gemcitabine plus dalteparin thromboprophylaxis in pancreatic cancer. Eur J Cancer 2012;48:1283–1292. [DOI] [PubMed] [Google Scholar]
- 10. Kuderer NM, Khorana AA, Lyman GH, et al. A meta‐analysis and systematic review of the efficacy and safety of anticoagulants as cancer treatment: Impact on survival and bleeding complications. Cancer 2007;110:1149–1161. [DOI] [PubMed] [Google Scholar]
- 11. Khorana AA, Kuderer NM, Culakova E et al. Development and validation of a predictive model for chemotherapy‐associated thrombosis. Blood 2008;111:4902–4907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mandala M, Clerici M, Corradino I et al. Incidence, risk factors and clinical implications of venous thromboembolism in cancer patients treated within the context of phase I studies: The ‘SENDO experience'. Ann Onco 2012;23:1416–1421. [DOI] [PubMed] [Google Scholar]
- 13. Ay C, Simanck R, Vormittag R et al. High plasma levels of soluble P‐selectin are predictive of venous thromboembolism in cancer patients: Results from the Vienna Cancer and Thrombosis Study (CATS). Blood 2008;112:2703–2708. [DOI] [PubMed] [Google Scholar]
- 14. Moore RA1, Adel N, Riedel E et al. High incidence of thromboembolic events in patients treated with cisplatin‐based chemotherapy: A large retrospective analysis. J Clin Oncol 2011;29:3466–3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Concato J, Peduzzi P, Holfold TR et al. Importance of events per independent variable in proportional hazards analysis. I. Background, goals, and general strategy. J Clin Epidemiol 1995;48:1495–1501 [DOI] [PubMed] [Google Scholar]
- 16. Peduzzi P, Concato J, Feinstein AR et al. Importance of events per independent variable in proportional hazards regression analysis. II. Accuracy and precision of regression estimates. J Clin Epidemiol 1995;48:1503–1510. [DOI] [PubMed] [Google Scholar]
- 17. Sauerbrei W, Schumacher M. A bootstrap resampling procedure for model building: Application to the Cox regression model. Stat Med 1992;11:2093–2109. [DOI] [PubMed] [Google Scholar]
- 18. Kuk D, Varadhan R. Model selection in competing risks regression. Stat Med 2012; 32:3077–3088. [DOI] [PubMed] [Google Scholar]
- 19. Sullivan LM, Massaro JM, D'Agostino RB Sr. Presentation of multivariate data for clinical use: The Framingham Study risk score functions. Stat Med 2004;23:1631–1660. [DOI] [PubMed] [Google Scholar]
- 20. Blanche P, Dartigues J F, Jacqmin‐Gadda H. Estimating and comparing time‐dependent areas under receiver operating characteristic curves for censored event times with competing risks. Stat Med 2013;32:5381–5397. [DOI] [PubMed] [Google Scholar]
- 21. Guyatt GH, Akl EA, Crowther M et al. Executive summary: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence‐based clinical practice guidelines. Chest 2012;141:7S–47S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mandala M, Falanga A, Roila F. Management of venous thromboembolism (VTE) in cancer patients: ESMO clinical practice guidelines. Ann Oncol 2011;22:vi85–vi92. [DOI] [PubMed] [Google Scholar]
- 23. Streiff MB, Bockenstedt PL, Cataland SR et al. On behalf of the National Comprehensive Cancer Network venous thromboembolic disease. J Natl Compr Canc Netw 2013;11:1402–1429. [DOI] [PubMed] [Google Scholar]
- 24. Easaw JC, Shea‐Budgell MA, Wu CM et al. Canadian consensus recommendations on the management of venous thromboembolism in patients with cancer. Part 1: prophylaxis. Curr Oncol 2015;22:133–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ay C, Dunkler D, Simanek R et al. Prediction of venous thromboembolism in cancer patients. Blood 2010;116:5377–5382. [DOI] [PubMed] [Google Scholar]
- 26. Pabinger I, Ay C. Biomarkers and venous thromboembolism. Arter Thromb and Vasc Biol 2009;29:332–326. [DOI] [PubMed] [Google Scholar]
- 27. Arcopinto M, Cella CA, Wesolowski R et al. Primary prevention of cancer‐related thrombosis: special focus on ambulatory patients. Int J Cardiol 2014;173:583–584. [DOI] [PubMed] [Google Scholar]
- 28. Haddad TC1, Greeno EW. Chemotherapy‐induced thrombosis. Thromb Res 2006;118:555–68. [DOI] [PubMed] [Google Scholar]
- 29. Moore RA, Adel N, Riedel E et al. High incidence of thromboembolic events in patients treated with Cisplatin‐based chemotherapy: A large retrospective analysis. J Clin Oncol 2011;29:3466–3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kabbinavar FF, Hurwitz HI, Yi J et al. Addition of bevacizumab to fluorouracil‐based first‐line treatment of metastatic colorectal cancer: Pooled analysis of cohorts of older patients from two randomized clinical trials. J Clin Oncol 2009;27:199–205. [DOI] [PubMed] [Google Scholar]
- 31. Byrne M, Reynolds JV, O'Donnell JS et al. Long‐term activation of the pro‐coagulant response after neoadjuvant chemoradiation and major cancer surgery. Br J Cancer. 2010;102:73–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bohlius J, Wilson J, Seidenfeld J et al. Recombinant human erythropoietins and cancer patients: Updated meta‐analysis of 57 studies including 9353 patients. J Natl Cancer Inst 2006;98:708–714. [DOI] [PubMed] [Google Scholar]
- 33. Akl EA, Vasireddi SR, Gunukula S et al. Anticoagulation for patients with cancer and central venous catheters. Cochrane Database Syst Rev: 2011;2:CD006468. [DOI] [PubMed] [Google Scholar]
- 34. Simanek R, Vormittag R, Ay C et al. High platelet count associated with venous thromboembolism in cancer patients: Results from the Vienna Cancer and Thrombosis Study (CATS). J Thromb Haemost 2010;8:114–120. [DOI] [PubMed] [Google Scholar]