Various hydration regimens and supplementation strategies are used to prevent cisplatin‐induced kidney injury; however, evidence‐based recommendations on specific hydration regimens are limited. This systematic review of the literature evaluates clinical studies that have examined hydration and supplementation strategies to prevent cisplatin‐induced nephrotoxicity.
Keywords: Cisplatin, Nephrotoxicity, Hydration, Mannitol, Magnesium
Abstract
Introduction.
Cisplatin, a platinum‐based antineoplastic agent, is the cornerstone for the treatment of many malignancies. Nephrotoxicity is the primary dose‐limiting toxicity, and various hydration regimens and supplementation strategies are used to prevent cisplatin‐induced kidney injury. However, evidence‐based recommendations on specific hydration regimens are limited. A systematic review was performed to evaluate clinical studies that have examined hydration and supplementation strategies to prevent cisplatin‐induced nephrotoxicity.
Materials and Methods.
PubMed and Excerpta Medica databases were searched from 1966 through October 2015 for clinical trials and other studies focused on hydration regimens to prevent nephrotoxicity in cancer patients treated with cisplatin. The University of Oxford Centre for Evidence‐Based Medicine criteria were used to grade level of evidence.
Results.
Among the 1,407 identified studies, 24 were included in this systematic review. All studies differed on type, volume, and duration of hydration. Among the 24 studies, 5 evaluated short‐duration hydration, 4 evaluated low‐volume hydration, 4 investigated magnesium supplementation, and 7 reviewed forced diuresis with hydration. Short‐duration and lower‐volume hydration regimens are effective in preventing cisplatin‐induced nephrotoxicity. Magnesium supplementation may have a role as a nephroprotectant, and forced diuresis may be appropriate in some patients receiving cisplatin.
Conclusion.
Hydration is essential for all patients to prevent cisplatin‐induced nephrotoxicity. Specifically, short‐duration, low‐volume, outpatient hydration with magnesium supplementation and mannitol forced diuresis (in select patients) represent best practice principles for the safe use of cisplatin. The Oncologist 2017;22:609–619
Implications for Practice.
The findings contained within this systematic review show that (a) hydration is essential for all patients to prevent cisplatin‐induced nephrotoxicity, (b) short‐duration, low‐volume, outpatient hydration regimens appear to be safe and feasible, even in patients receiving intermediate‐ to high‐dose cisplatin, (c) magnesium supplementation (8–16 milliequivalents) may limit cisplatin‐induced nephrotoxicity, and (d) mannitol may be considered for high‐dose cisplatin and/or patients with preexisting hypertension. These findings have broad implications for clinical practice and represent best practice principles for the prevention of cisplatin‐induced nephrotoxicity.
Introduction
Cisplatin (cis‐diamminedichloroplatinum [II]; CDDP; Platinol) is an antineoplastic that serves as the backbone of myriad treatment regimens across a broad spectrum of malignancies, and has led to improved survival as well as cure [1], [2]. Cisplatin is a platinum‐based alkylating compound that reacts with DNA to form interstrand cross‐links and intrastrand bifunctional N‐7 DNA adducts at d(GpG) and d(ApG) [3].
Cisplatin is renally excreted and can accumulate in the renal proximal tubules, leading to nephrotoxicity. Its use is generally limited to patients with a creatinine clearance (CrCl) >60 mL/min; however, cisplatin‐induced nephrotoxicity is common and may limit dosing and/or dose intensity [4]. In one study, moderate to severe nephrotoxicity was noted in 25%–33% of patients receiving a single intravenous (IV) 50–75 mg/m2 dose [5]. A majority of patients (50%–75%) who received cisplatin (15–20 mg/m2 IV daily) over five consecutive days also experienced moderate to severe nephrotoxicity [6]. There was a higher incidence of severe and irreversible nephrotoxicity in patients who received high cisplatin doses (>100 mg/m2) [7].
In clinical practice, the overall prevalence of cisplatin‐induced nephrotoxicity approaches one third of treated patients, and typically presents approximately 10 days after treatment [8], [9]. Hydration significantly reduces cisplatin half‐life, urinary cisplatin concentrations, and proximal tubule transit time [10], [11], [12]. Cisplatin can also cause high rates of electrolyte wasting (e.g., hypomagnesemia) [13], [14], but magnesium supplementation may reduce renal tubular damage [15], [16].
In clinical practice, the overall prevalence of cisplatin‐induced nephrotoxicity approaches one third of treated patients, and typically presents approximately 10 days after treatment. Hydration significantly reduces cisplatin half‐life, urinary cisplatin concentrations, and proximal tubule transit time.
While it is standard practice to use hydration with cisplatin, various hydration regimens are utilized with limited data. Based on the lack of guidance regarding cisplatin hydration, multiple hydration protocols exist among different health systems, and even within a single institution [17]. We performed a systematic review of the literature to evaluate clinical studies that have examined hydration and supplementation strategies to prevent cisplatin‐induced nephrotoxicity.
Materials and Methods
Data Source/Study Selection
The selection and systematic review of appropriate studies was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses statement [18]. Studies were eligible if they directly examined hydration regimens, hydration with supplementation, and/or hydration with forced diuresis. Animal studies, review articles, and studies where the primary outcome was not prevention of cisplatin‐induced nephrotoxicity were excluded. Only studies published in full manuscript form, in English, and in peer‐reviewed journals were screened for eligibility.
Search Strategy and Data Extraction
Independent reviews of citations between 1966 and 2015 were performed in April 2015 and repeated in October 2015. A MEDLINE/PubMed search, using Medical Subject Heading search terms, was conducted followed by a search of the Excerpta Medica database (EMBASE). Key search terms reviewed and agreed upon by all authors for MEDLINE/PubMed and EMBASE included the following: “cisplatin/cisdiamminedichloroplatinum/CDDP,” “nephrotoxic/nephrotoxicity/renal or kidney toxicity,” “renal/kidney failure,” “renal/kidney function,” “creatinine clearance/glomerular filtration rate and adverse effects/toxicity,” “fluid therapy/fluid management,” “hydrate/hydration/prehydrate/prehydration/posthydrate/posthydration/rehydrate/rehydration,” “saline/sodium chloride,” and “diuresis/diuretic/mannitol/furosemide.”
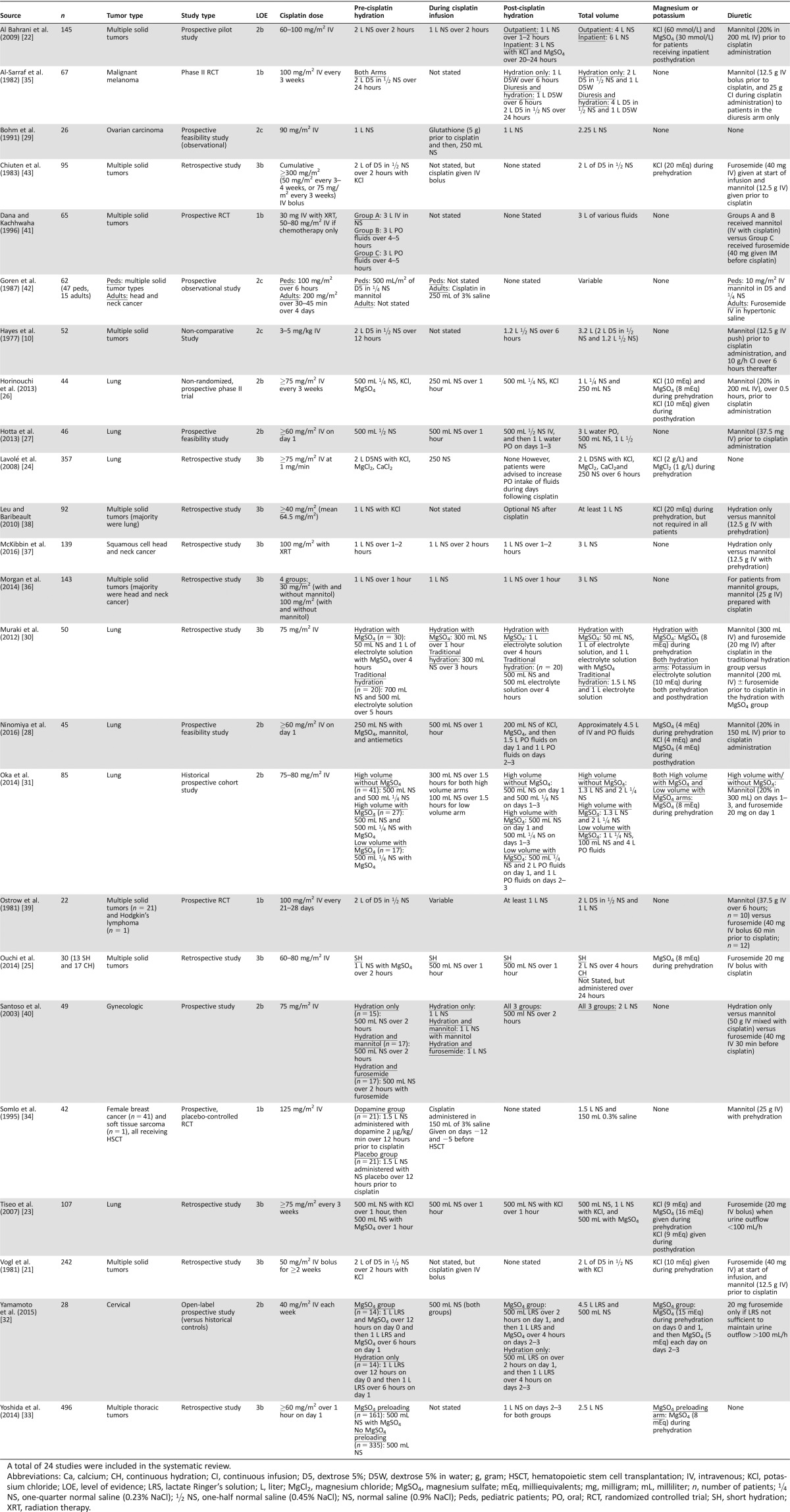
Data extraction of abstracts containing search terms was conducted, and duplications were removed. Two independent reviews of abstract titles followed by entire abstracts were conducted, and authors agreed on selection of full text articles for review. Full text articles were retrieved, and authors independently confirmed eligibility. Eleven cisplatin hydration articles, familiar to the authors, were selected a priori to serve as positive controls for the review's search term strategy. Information collected from each study included author name, publication year, study type, number of patients, malignancy type, level of evidence, hydration regimen details, magnesium or potassium supplementation information, and forced diuresis information (Table 1).
Table 1. Cisplatin hydration studies evaluating nephrotoxicity identified for the systematic review.
A total of 24 studies were included in the systematic review.
Abbreviations: Ca, calcium; CH, continuous hydration; CI, continuous infusion; D5, dextrose 5%; D5W, dextrose 5% in water; g, gram; HSCT, hematopoietic stem cell transplantation; IV, intravenous; KCl, potassium chloride; LOE, level of evidence; LRS, lactate Ringer's solution; L, liter; MgCl2, magnesium chloride; MgSO4, magnesium sulfate; mEq, milliequivalents; mg, milligram; mL, milliliter; n, number of patients; ¼ NS, one‐quarter normal saline (0.23% NaCl); ½ NS, one‐half normal saline (0.45% NaCl); NS, normal saline (0.9% NaCl); Peds, pediatric patients; PO, oral; RCT, randomized controlled trial; SH, short hydration; XRT, radiation therapy.
The definition of nephrotoxicity varied across the selected studies. A summary of the different nephrotoxicity definitions is provided (Table 2). Level of evidence criteria were developed by the University of Oxford Centre for Evidence‐Based Medicine [19].
Table 2. Summary of definitions of nephrotoxicity used in the included studies.
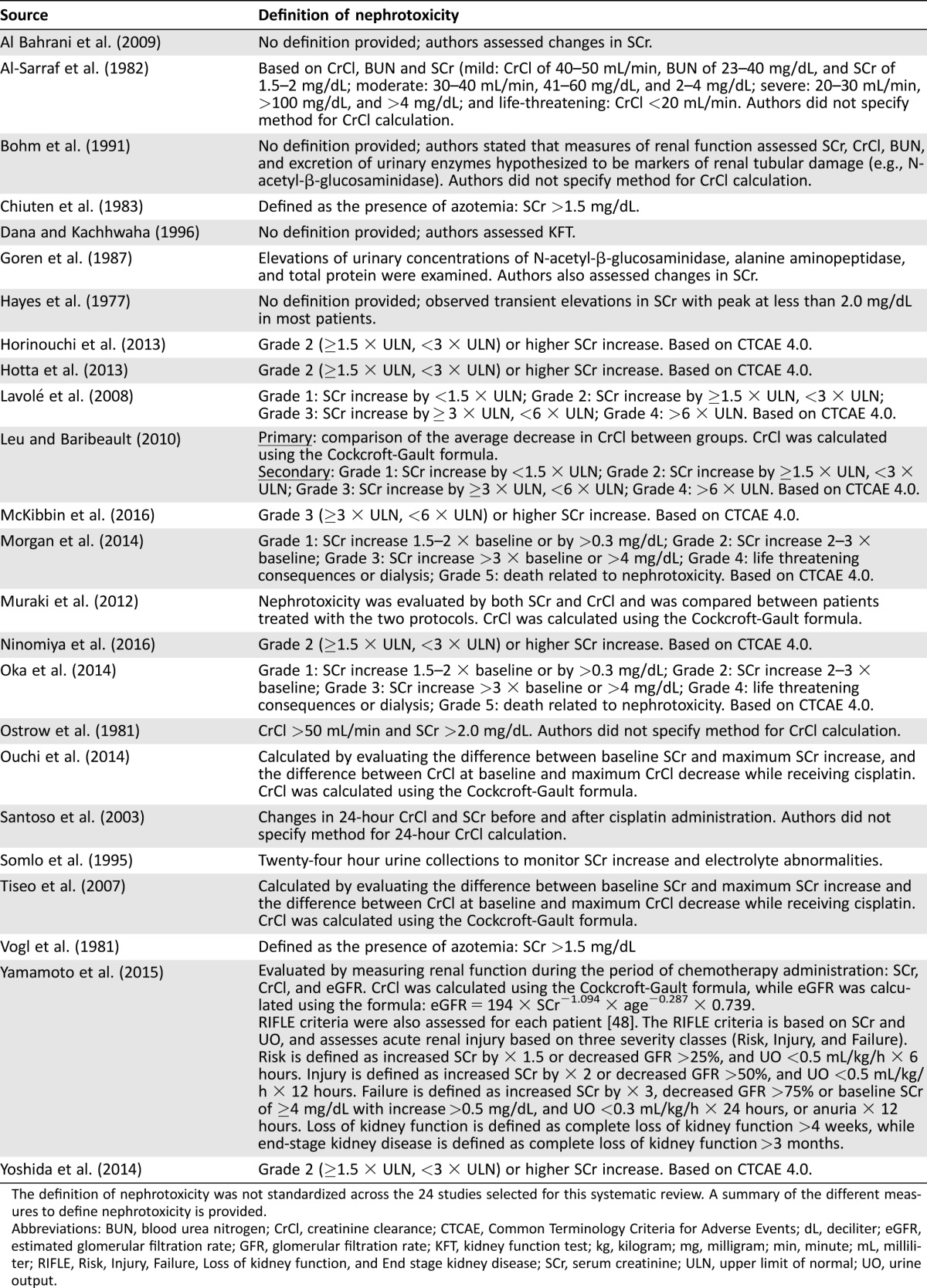
The definition of nephrotoxicity was not standardized across the 24 studies selected for this systematic review. A summary of the different measures to define nephrotoxicity is provided.
Abbreviations: BUN, blood urea nitrogen; CrCl, creatinine clearance; CTCAE, Common Terminology Criteria for Adverse Events; dL, deciliter; eGFR, estimated glomerular filtration rate; GFR, glomerular filtration rate; KFT, kidney function test; kg, kilogram; mg, milligram; min, minute; mL, milliliter; RIFLE, Risk, Injury, Failure, Loss of kidney function, and End stage kidney disease; SCr, serum creatinine; ULN, upper limit of normal; UO, urine output.
Results
Search Results
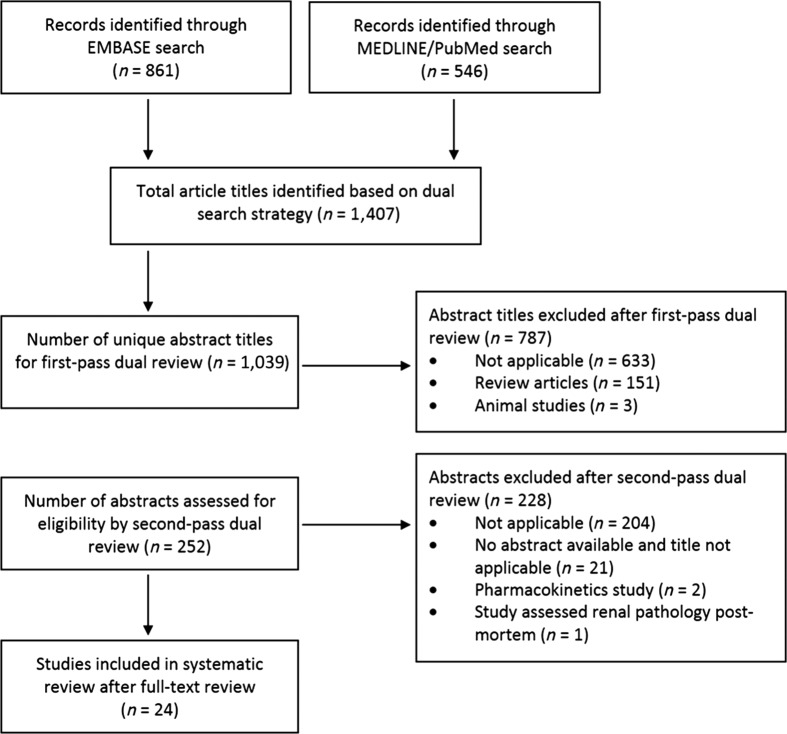
The search identified 1,407 articles (861 EMBASE, 546 MEDLINE/PubMed) with 371 overlapping. Of the remaining 1,036 article abstract titles, 787 were excluded. All 11 articles selected a priori as positive controls were among the 1,036 article abstracts identified. The remaining 249 articles underwent a full review to determine eligibility for inclusion. Twenty‐four studies met eligibility criteria and were included (Fig. 1).
Figure 1.
Schematic describing the selection process for studies included in the systematic review.
Characteristics of Reviewed Studies
There was wide variation across the 24 studies related to study design, tumor type, cisplatin dose, hydration administered (prehydration, hydration during cisplatin, and posthydration), supplements, hydration vehicle, and the type of forced diuresis used (Table 1). Lung, head and neck, and gynecologic malignancies comprised 10 of 24 (42%) studies. Twenty‐one studies (88%) and seven studies (29%) evaluated hydration in the setting of cisplatin doses ≥50 mg/m2 and ≥100 mg/m2, respectively. Total hydration volume ranged from 1–6 L. Only four studies (17%) did not implement forced diuresis, but in each of those studies, supplementation with magnesium or potassium was included.
There was wide variation in how studies defined nephrotoxicity. Nine studies (38%) used the Common Terminology Criteria for Adverse Events (CTCAE) version 4.0 [20], while five (21%) used a calculated CrCl change from baseline after cisplatin administration (Table 2).
Fourteen studies (58%) prospectively evaluated hydration regimens and their effects on cisplatin nephrotoxicity; however, only four (17%) were identified as high‐quality prospective studies (e.g., phase II clinical trial or randomized, placebo‐controlled trial [RCT]). Types of studies included RCTs with evidence level 1b (n = 4; 17%), individual cohort studies with evidence level 2b (n = 9; 38%), observational studies with evidence level 2c (n = 2; 8%), and individual case‐control studies with evidence level 3b (n = 9; 38%; Table 1).
Short‐Duration Hydration
Five studies investigated the safety and efficacy of short‐duration hydration regimens in the outpatient setting. Vogl et al. performed a retrospective study to assess short‐duration hydration safety (2 L with control of diuresis over 2 hours) with cisplatin 50 mg/m2 (n = 242). Azotemia and serum creatinine (SCr) >2.0 mg/dL occurred in <5% of patients leading the authors to conclude that short‐duration hydration and forced diuresis can be administered safely in the outpatient setting [21]. Al Bahrani et al. performed a retrospective study comparing short‐duration outpatient hydration (n = 88; 4 L with forced diuresis over 5–6 hours) versus inpatient hydration (n = 57), with no significant differences in nephrotoxicity between the two arms for patients receiving either cisplatin 60–80 mg/m2 (p = .63), or >80 mg/m2 (p = .96) [22]. Tiseo et al. performed a retrospective study of short‐duration hydration (2 L with control of diuresis over 3 hours) in lung cancer patients receiving cisplatin ≥75 mg/m2 (n = 107) and monitored for SCr and CrCl changes. Cisplatin was discontinued in only 4.6% of the patients due to renal toxicity. Among the remaining patients, associations between SCr increase and number of chemotherapy cycles (p = .36), and between SCr increase and cumulative cisplatin doses (p = .39) were not significant. Similarly, associations between CrCl decline and number of chemotherapy cycles (p = .64), and between CrCl decline and cumulative cisplatin doses (p = .65) were also not significant. They concluded that short‐duration, outpatient hydration is safe for patients receiving intermediate‐ to high‐dose cisplatin [23]. Lavolé et al. also conducted a retrospective study of short‐duration, outpatient hydration (2.25 L over 6 hours) in lung cancer patients receiving cisplatin ≥75 mg/m2 (n = 357). Twenty‐one patients (6%) experienced a grade ≥1 nephrotoxic event, and only one of those patients experienced a grade ≥2 event (<1%), such that the authors concluded that short‐duration hydration is feasible without increased nephrotoxicity [24]. Ouchi et al. performed a retrospective study in solid tumor patients receiving cisplatin ≥60 mg/m2 to evaluate the safety and efficacy of short‐duration, outpatient hydration (2 L with forced hydration over 4 hours; n = 13) versus inpatient, continuous hydration (CH; n = 17). Grade 2 or greater nephrotoxicity was only observed in two patients (both in the CH group). No significant differences in maximum SCr increase (p = .43) or maximum CrCl decline (p = .28) were observed between the two groups [25].
Low‐Volume Hydration
Four studies investigated the safety and efficacy of low‐volume hydration regimens in the outpatient setting. Horinouchi et al. conducted a prospective phase II trial in lung cancer patients (n = 44) receiving cisplatin ≥75 mg/m2 to assess the safety and efficacy of low‐volume hydration (1.25 L total) with forced diuresis and potassium/magnesium supplementation. Of these patients, 97.8% completed treatment without grade ≥2 nephrotoxicity [26]. Hotta et al. performed a prospective feasibility trial of low‐volume hydration (1.5 L total during outpatient infusion and 3 L oral posthydration) in chemo‐naïve lung cancer patients (n = 46) receiving cisplatin ≥60 mg/m2. No patients experienced grade ≥2 nephrotoxicity during the first cycle [27]. Ninomiya et al. conducted a prospective, low‐volume hydration feasibility trial (950 mL total during outpatient infusion and 3.5 L oral posthydration) in chemo‐naïve lung cancer patients (n = 45) receiving cisplatin ≥60 mg/m2. During the first cycle, only one patient experienced grade ≥2 nephrotoxicity (2%), and five patients (11%) experienced a grade ≥1 event [28]. Bohm et al. also showed that low‐volume hydration (2.25 L total) provided nephroprotection in ovarian cancer patients (n = 26) receiving cisplatin 90 mg/m2. No patients experienced nephrotoxicity (SCr >1.5 mg/dL) and only two patients experienced hypomagnesemia (<1.5 mg/dL) while on study [29].
Supplementation in Hydration Regimens
While many of the studies included potassium as part of their standard hydration regimen (Table 1), four studies specifically evaluated the role of magnesium supplementation to prevent cisplatin‐induced nephrotoxicity. Two studies also evaluated dopamine and glutathione. Muraki et al. performed a retrospective study in lung cancer patients receiving cisplatin 75 mg/m2. Patients received either hydration with forced diuresis (n = 30) or hydration with forced diuresis and magnesium (n = 20). After one cycle, patients who received magnesium experienced significantly increased CrCl (p = .0004) and decreased SCr (p = .0148) [30]. Oka et al. performed a historical prospective cohort study in lung cancer patients receiving cisplatin 75–80 mg/m2. Post‐cisplatin SCr significantly increased and CrCl decreased from baseline levels in patients who did not receive magnesium (both p < .001). However, among patients who received high‐volume magnesium, no differences between pre‐treatment and post‐treatment SCr and CrCl levels were observed (p = .118 and p = .254, respectively) [31]. Yamamoto et al. performed an open‐label, prospective study in patients with cervical cancer (n = 28) receiving cisplatin 40 mg/m2 prior to radiotherapy. SCr significantly increased and estimated glomerular filtration rate (eGFR) significantly decreased when compared with baseline (p < .05 for both) in patients who did not receive magnesium (n = 14), but not for those who received magnesium (n = 14; p = .35, and p = .27, respectively) [32]. Yoshida et al. performed a single‐center retrospective study of 496 patients with thoracic malignancies (n = 496) receiving cisplatin ≥60 mg/m2. Grade ≥2 SCr increase was significantly lower during the first cycle and all cycles (p < .001 for both) in patients who received magnesium in their prehydration (n = 161) when compared with patients who did not (n = 335). A multivariate analysis revealed that magnesium supplementation significantly reduced the risk of nephrotoxicity by 3.8‐fold during the first cycle (p < .001) and by 4.3‐fold over all cycles (p < .001) [33].
Two additional prospective studies evaluated the use of dopamine and glutathione for prevention of cisplatin‐induced nephrotoxicity. Bohm et al. evaluated glutathione supplementation in a prospective feasibility study in patients with ovarian cancer (n = 26) receiving 90 mg/m2 of cisplatin and found that no patients experienced nephrotoxicity [29]. Somlo et al. performed a prospective RCT to evaluate the nephroprotective effects of dopamine in solid tumor patients (n = 42) receiving cisplatin 125 mg/m2 prior to hematopoietic stem cell transplantation. The authors observed that patients who received dopamine (n = 21) experienced increased SCr (p =.04) when compared with patients who received placebo (n = 21) [34].
Forced Diuresis
Eighteen studies implemented forced diuresis, with seven directly examining the nephroprotective effects of mannitol or furosemide in cisplatin hydration regimens. Hayes et al. showed that prehydration and posthydration with mannitol resulted in a reduced risk of nephrotoxicity [10]. Al‐Sarraf et al. performed a prospective phase II RCT in patients with malignant melanoma receiving cisplatin 100 mg/m2. Patients were randomized to receive hydration (n = 33) or hydration with mannitol (n = 34). After the first cycle, patients who received mannitol experienced lower rates of nephrotoxicity when compared with hydration alone (15% versus 30%; no p value reported); however, in subsequent cycles, the nephroprotective effect of mannitol was not maintained (32% versus 39% experienced nephrotoxicity; no p value reported) [35]. Morgan et al. performed a retrospective study in solid tumor patients receiving either 30 or 100 mg/m2 of cisplatin (n = 143). Among patients who did not receive mannitol (n = 85), there was a 2.6‐fold increased risk of nephrotoxicity (p = .048) when compared with patients who received mannitol (n = 58). In patients who received the higher dose of cisplatin but no mannitol, there was an 11.5‐fold increased risk of nephrotoxicity (p < .0001) and a 3.2‐fold increased risk in patients with hypertension (p = .017). The authors concluded that mannitol should be included in hydration regimens for cisplatin ≥100 mg/m2 and/or for patients with preexisting hypertension [36]. McKibbin et al. performed a retrospective study of squamous cell head and neck patients receiving 100 mg/m2 of cisplatin with concurrent radiation (n = 139). Patients who received mannitol were at 84% lower risk for grade 3 SCr increase (p = .01). However, these same patients were twice as likely to experience grade 3 hyponatremia (p = .026) [37]. Conversely, Leu and Baribeault performed a retrospective study in patients with multiple tumor types (n = 92) receiving ≥40 mg/m2 of cisplatin. Patients received either mannitol with prehydration (n = 46) or hydration alone (n = 46). No differences in average CrCl decrease (p = .09), incidence of nephrotoxicity, or hypomagnesemia and hypokalemia rates were observed between the two arms (no p values reported) [38].
Ostrow et al. conducted an RCT in patients with multiple tumor types receiving 100 mg/m2 of cisplatin, and either mannitol (n = 10) or furosemide (n = 12). Nephrotoxicity occurred in 19% of patients who received furosemide versus 28% of patients who received mannitol, and no significant differences in decreased CrCl (p > .25) or increased SCr (p > .45) was observed between the two arms [39]. Santoso et al. conducted a prospective, randomized study in patients with gynecologic malignancies receiving 75 mg/m2 of cisplatin, with mannitol (n = 17), furosemide (n = 17), or hydration alone (n = 15). After one cycle, no CrCl differences were detected between patients who received furosemide versus hydration alone (p = .66). The authors noted that CrCl decreased in patients who received mannitol compared with those that received furosemide or hydration alone (p = .02 for both). While the authors concluded that hydration with furosemide supplementation may provide better nephroprotection than mannitol, they did not provide evidence that furosemide was more nephroprotective than hydration alone [40].
Oral Hydration or Hypertonic Saline to Prevent Nephrotoxicity
Dana et al. performed a prospective RCT in solid tumor patients receiving 50–80 mg/m2 of cisplatin (n = 65) to assess the efficacy of oral versus IV prehydration. The authors concluded that adequate oral prehydration with diuresis is not inferior to IV hydration in preventing cisplatin‐induced nephrotoxicity [41]. Goren et al. conducted an observational study of head and neck cancer patients receiving (n = 62). They observed no significant differences in urinary markers of nephrotoxicity (N‐acetyl‐β‐glucosaminidase, alanine aminopeptidase, and total protein) between cisplatin (50 mg/m2 daily over 4 days) delivered in hypertonic saline and cisplatin (one 100 mg/m2 dose) delivered in 5% glucose and 0.22% saline (no p value reported) [42].
Hydration and Cumulative Cisplatin Exposure
Chiuten et al. performed a retrospective study in solid tumor patients who received cumulative cisplatin ≥300 mg/m2 (n = 95) to examine nephrotoxicity in the setting of “adequate” hydration (2 L of fluids with forced diuresis and potassium). Only four patients (4.2%) developed transient renal dysfunction, and in no instance was SCr >1.8 mg/dL. In all cases, azotemia was transient and SCr normalized to ≤1.5 mg/dL within 2–8 weeks. The authors surmised that if cisplatin‐induced nephrotoxicity is to occur, it is more likely to happen early in treatment. And, they concluded there is no cumulative dose effect on nephrotoxicity when cisplatin is administered at moderate doses, and with adequate hydration [43].
Discussion
Cisplatin revolutionized the treatment of many malignancies, leading to cures in several cancers, such as advanced germ cell tumors. This current systematic review examined studies that have directly evaluated the role of cisplatin hydration and supplementation strategies to prevent nephrotoxicity.
Several clinical factors predict nephrotoxicity, and are used to select for cisplatin treatment including dose and schedule. Adequate renal function is essential, with most trials excluding patients with SCr ≥1.5 mg/dL and/or CrCl <50 mL/min; however, there are major inconsistencies in the methods used to assess renal function. Additionally, clinical factors, such as comorbid disease states (e.g., diabetes mellitus), age, and concomitant nephrotoxic medications (e.g., aminoglycoside antibiotics), can increase risk of cisplatin‐induced renal injury and must be considered when assessing for the risk of nephrotoxicity.
While cisplatin‐induced nephrotoxicity can be dose‐ and dose intensity‐limiting, studies have shown adequate hydration limits the incidence and extent of renal injury. Early studies established the importance of CH during cisplatin administration in the inpatient setting. However, safe and effective cisplatin administration with short‐duration and/or low‐volume hydration is feasible in the outpatient setting [23], [24], [25], [26], [27], [28].
Hypomagnesemia can upregulate OCT‐2, leading to increased cisplatin transport to the kidneys and resulting in nephrotoxicity [44]. Several of the reviewed studies suggest that magnesium supplementation (8–16 milliequivalents [mEq]) may limit cisplatin‐induced nephrotoxicity [30], [31], [32], [33]. Moreover, it is conceivable that the inclusion of magnesium contributed to the safety and feasibility of short‐duration hydration regimens in patients who received intermediate‐ to high‐dose cisplatin (≥75 mg/m2) [23], [26]. Hypokalemia can occur secondary to the excessive urinary losses of magnesium that result in hypomagnesemia, and serum concentrations of potassium and magnesium may underestimate total electrolyte wasting [45]. Nine (38%) studies included potassium supplementation (up to 20 mEq) as part of their nephrotoxicity prevention strategy, but none of these studies directly examined the role of potassium in the prevention of cisplatin‐induced nephrotoxicity. Therefore, we are not able to provide definitive recommendations regarding the optimal amount, and timing of potassium replacement. Glutathione supplementation may also protect against cisplatin‐induced nephrotoxicity, but dopamine provides no benefit in cisplatin hydration regimens [29], [34].
While several studies support the use of short‐duration, low‐volume hydration regimens and magnesium supplementation in the outpatient setting, the use of hypertonic saline, oral hydration, and alternative supplements (e.g., glutathione) have far less convincing evidence. Goren et al. showed that urinary markers of nephrotoxicity, including urinary protein levels, did not significantly differ between patients who received cisplatin administered in a vehicle composed of hypertonic solution versus normal saline [42]. Despite promising data from phase II studies in which a hypertonic saline delivery vehicle was used [46], [47], there have been no prospective RCTs to assess hypertonic saline in this setting.
Mannitol reduces the concentration of cisplatin in the kidneys, which is the presumed mechanism underlying a potential nephroprotective effect [10], [35]. Some studies have shown a benefit of hydration with mannitol forced diuresis [35], [36], [37], while others have not [38]. There is concern that mannitol may over‐diurese some patients, resulting in dehydration. Moreover, data from studies showing the benefit of mannitol suggest that only patients receiving high‐dose cisplatin (e.g., 100 mg/m2) benefit from forced diuresis [35], [36]. There is insufficient evidence to support using furosemide for forced diuresis [39], [40].
There is concern that mannitol may over‐diurese some patients, resulting in dehydration. Moreover, data from studies showing the benefit of mannitol suggest that only patients receiving high‐dose cisplatin (e.g., 100 mg/m2) receive benefit from forced diuresis.
There are several limitations to this systematic review. First, there is inherent subjectivity in the design of any systematic review, including the creation of the list of search terms, subsequent data extraction, and the final selection of articles. We sought to limit subjectivity or bias at each step by involving all authors when devising the search terms, and at a minimum dual review of the studies for all steps. Second, the differing tumor types, cisplatin doses, study types, hydration regimens, and definitions of nephrotoxicity across studies limit our ability to make concrete recommendations regarding optimal cisplatin hydration regimens. Third, the referenced studies span several decades. Because many practice advances have been introduced over this time, and because clinical trials often do not mirror what actually occurs in routine clinical practice, results from some studies may not be generalizable. Finally, cisplatin hydration publications prior to 2008 often advocated for inpatient administration for cisplatin and/or the use of large‐volume IV hydration prior to the approval of 5‐HT3 and NK‐1 inhibitors. The use of NK‐1 inhibitors as part of a three‐drug regimen that includes a 5‐HT3 inhibitor and corticosteroid has become the standard of care for emesis control when patients receive high‐dose cisplatin. Adequate emesis control prevents intravascular depletion of fluids and electrolytes, and therefore decreases the potential for dehydration leading to acute renal injury, a likely major reason for the decline in cisplatin‐induced nephrotoxicity.
Conclusion
In conclusion, this review systematically summarizes studies that have focused on strategies to prevent cisplatin‐induced nephrotoxicity. In spite of over four decades of research on the subject of cisplatin hydration, concrete recommendations regarding the exact hydration type, volume/duration, supplementation, and use of forced diuresis remain unavailable. While it may not be realistic to perform more robust, prospective, cisplatin hydration RCTs for numerous reasons (e.g., cost), this systematic review supports short‐duration, low‐volume, outpatient hydration regimens, even in patients receiving intermediate‐ to high‐dose cisplatin. In addition, magnesium supplementation (or glutathione, but not dopamine) and mannitol forced diuresis (but not furosemide) may be considered. Oral posthydration and/or the use of a hypertonic saline vehicle may be effective in preventing nephrotoxicity; however, data are limited, and additional validation is required. Collectively, these findings represent best practice principles for the prevention of cisplatin‐induced nephrotoxicity (Table 3).
Table 3. Summary of hydration and supplementation recommendations to prevent nephrotoxicity from cisplatin.
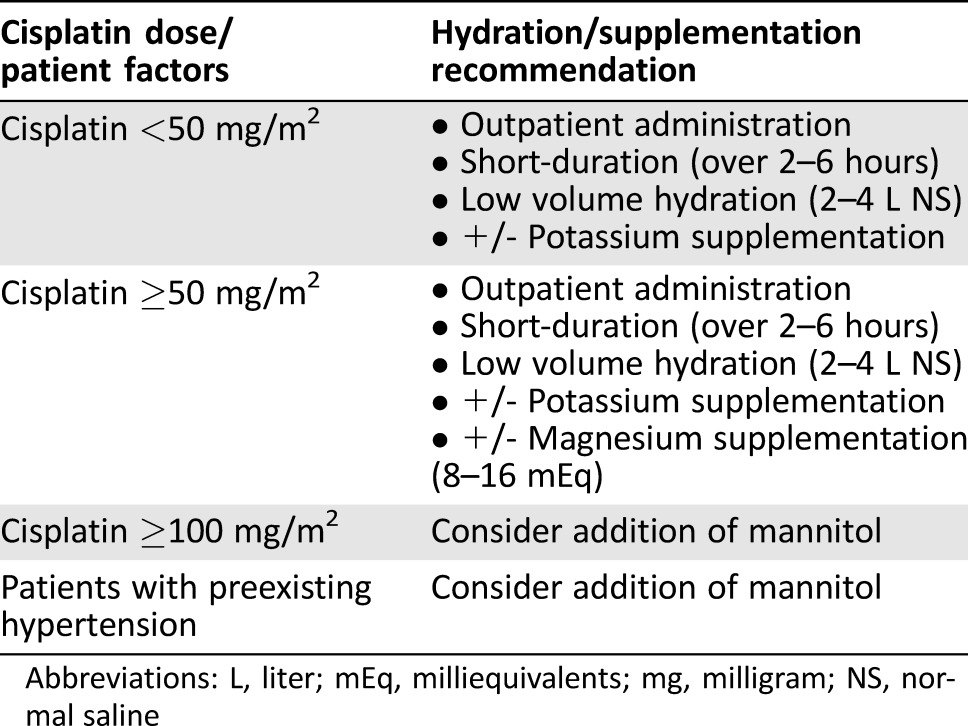
Abbreviations: L, liter; mEq, milliequivalents; mg, milligram; NS, normal saline
Acknowledgments
During the development and writing of this systematic review, Dr. Crona received support from the National Institute of General Medicine Sciences (NIGMS, T32GM086330). The authors would like to acknowledge Dr. Lucas Wind for his assistance during the development phase of this systematic review. The authors would also like to thank Dr. Lana Crona for reviewing and editing the manuscript.
Author Contributions
Conception/Design: Daniel J. Crona, Aimee Faso, Matthew I. Milowsky
Provision of study material or patients: Daniel J. Crona
Collection and/or assembly of data: Daniel J. Crona, Aimee Faso, Kathleen A. McGraw, Matthew D. Galsky, Matthew I. Milowsky
Data analysis and interpretation: Daniel J. Crona, Aimee Faso, Tomohiro F. Nishijima, Kathleen A. McGraw, Matthew D. Galsky, Matthew I. Milowsky
Manuscript writing: Daniel J. Crona, Aimee Faso, Tomohiro F. Nishijima, Kathleen A. McGraw, Matthew D. Galsky, Matthew I. Milowsky
Final approval of manuscript: Daniel J. Crona, Aimee Faso, Tomohiro F. Nishijima, Kathleen A. McGraw, Matthew D. Galsky, Matthew I. Milowsky
Disclosures
Matthew D. Galsky: Merck, Genentech (C/A), Bristol‐Myers Squibb, Genentech, Merck (RF); Matthew I. Milowsky: Mirati Thera, Pfizer, Cerulean Pharma, Merck, Seattle Gen, Acerta Pharma, BioClin Thera, Roche, Genentech (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1. Loehrer PJ, Einhorn LH. Drugs five years later. Cisplatin. Ann Intern Med 1984;100:704–713. [DOI] [PubMed] [Google Scholar]
- 2. Pabla N, Dong Z. Cisplatin nephrotoxicity: Mechanisms and renoprotective strategies. Kidney Int 2008;73:994–1007. [DOI] [PubMed] [Google Scholar]
- 3. Go RS, Adjei AA. Review of the comparative pharmacology and clinical activity of cisplatin and carboplatin. J Clin Oncol 1999;17:409–422. [DOI] [PubMed] [Google Scholar]
- 4. Caglar K, Kinalp C, Arpaci F et al. Cumulative prior dose of cisplatin as a cause of the nephrotoxicity of high‐dose chemotherapy followed by autologous stem‐cell transplantation. Nephrol Dial Transplant 2002;17:1931–1935. [DOI] [PubMed] [Google Scholar]
- 5. Madias NE, Harrington JT. Platinum nephrotoxicity. Am J Med 1978;65:307–314. [DOI] [PubMed] [Google Scholar]
- 6. Lippman AJ, Helson C, Helson L et al. Clinical trials of cis‐diamminedichloroplatinum (NSC‐119875). Cancer Chemother Rep 1973;57:191–200. [PubMed] [Google Scholar]
- 7. Higby DJ, Wallace HJ Jr., Holland JF. Cis‐diamminedichloroplatinum (NSC‐119875): A phase I study. Cancer Chemother Rep 1973;57:459–463. [PubMed] [Google Scholar]
- 8. Cornelison TL, Reed E. Nephrotoxicity and hydration management for cisplatin, carboplatin, and ormaplatin. Gynecol Oncol 1993;50:147–158. [DOI] [PubMed] [Google Scholar]
- 9. Gonzales‐Vitale JC, Hayes DM, Cvitkovic E et al. The renal pathology in clinical trials of cis‐platinum (II) diamminedichloride. Cancer 1977;39:1362–1371. [DOI] [PubMed] [Google Scholar]
- 10. Hayes DM, Cvitkovic E, Golbey RB et al. High dose cis‐platinum diammine dichloride: Amelioration of renal toxicity by mannitol diuresis. Cancer 1977;39:1372–1381. [DOI] [PubMed] [Google Scholar]
- 11. Daugaard G, Abildgaard U. Cisplatin nephrotoxicity. A review. Cancer Chemother Pharmacol 1989;25:1–9. [DOI] [PubMed] [Google Scholar]
- 12. Frick GA, Ballentine R, Driever CW et al. Renal excretion kinetics of high‐dose cis‐dichlorodiammineplatinum(II) administered with hydration and mannitol diuresis. Cancer Treat Rep 1979;63:13–16. [PubMed] [Google Scholar]
- 13. Lajer H, Kristensen M, Hansen HH et al. Magnesium and potassium homeostasis during cisplatin treatment. Cancer Chemother Pharmacol 2005;55:231–236. [DOI] [PubMed] [Google Scholar]
- 14. Lajer H, Daugaard G. Cisplatin and hypomagnesemia. Cancer Treat Rev 1999;25:47–58. [DOI] [PubMed] [Google Scholar]
- 15. Bodnar L, Wcislo G, Gasowska‐Bodnar A et al. Renal protection with magnesium subcarbonate and magnesium sulphate in patients with epithelial ovarian cancer after cisplatin and paclitaxel chemotherapy: A randomised phase II study. Eur J Cancer 2008;44:2608–2614. [DOI] [PubMed] [Google Scholar]
- 16. Willox JC, McAllister EJ, Sangster G et al. Effects of magnesium supplementation in testicular cancer patients receiving cis‐platin: A randomised trial. Br J Cancer 1986;54:19–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Greystoke AP, Jodrell DI, Cheung M et al. How many cisplatin administration protocols does your department use? Eur J Cancer Care (Engl) 2010;19:80–90. [DOI] [PubMed] [Google Scholar]
- 18. Moher D, Liberati A, Tetzlaff J et al. Preferred reporting items for systematic reviews and meta‐analyses: The PRISMA statement. J Clin Epidemiol 2009;62:1006–1012. [DOI] [PubMed] [Google Scholar]
- 19.The University of Oxford. OCEBM Levels of Evidence Working Group. “The Oxford Levels of Evidence 2”. Oxford Centre for Evidence‐Based Medicine. Available at http://www.cebm.net/wp-content/uploads/2014/06/CEBM-Levels-of-Evidence-2.1.pdf. Accessed March 9, 2017.
- 20.National Cancer Institute . Common Terminology Criteria for Adverse Events v4.0. NCI, NIH, DHHS. May 29, 2009. NIH publication # 09‐7473. Available at http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdf. Accessed March 9, 2017.
- 21. Vogl SE, Zaravinos T, Kaplan BH et al. Safe and effective two‐hour outpatient regimen of hydration and diuresis for the administration of cis‐diamminedichloroplatinum (II). Eur J Cancer 1981;17:345–350. [DOI] [PubMed] [Google Scholar]
- 22. Al Bahrani BJ, Moylan EJ, Forouzesh B et al. A short outpatient hydration schedule for cisplatin administration. Gulf J Oncolog 2009:30–36. [PubMed] [Google Scholar]
- 23. Tiseo M, Martelli O, Mancuso A et al. Short hydration regimen and nephrotoxicity of intermediate to high‐dose cisplatin‐based chemotherapy for outpatient treatment in lung cancer and mesothelioma. Tumori 2007;93:138–144. [DOI] [PubMed] [Google Scholar]
- 24. Lavolé A, Danel S, Baudrin L et al. Routine administration of a single dose of cisplatin ≥ 75 mg/m2 after short hydration in an outpatient lung‐cancer clinic. Bull Cancer 2012;99:E43–E48. [DOI] [PubMed] [Google Scholar]
- 25. Ouchi A, Asano M, Aono K et al. Comparison of short and continuous hydration regimen in chemotherapy containing intermediate‐ to high‐dose Cisplatin. J Oncol 2014;2014:767652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Horinouchi H, Kubota K, Itani H et al. Short hydration in chemotherapy containing cisplatin (≥75 mg/m2) for patients with lung cancer: A prospective study. Jpn J Clin Oncol 2013;43:1105–1109. [DOI] [PubMed] [Google Scholar]
- 27. Hotta K, Takigawa N, Hisamoto‐Sato A et al. Reappraisal of short‐term low‐volume hydration in cisplatin‐based chemotherapy: Results of a prospective feasibility study in advanced lung cancer in the Okayama Lung Cancer Study Group Trial 1002. Jpn J Clin Oncol 2013;43:1115–1123. [DOI] [PubMed] [Google Scholar]
- 28. Ninomiya K, Hotta K, Hisamoto‐Sato A et al. Short‐term low‐volume hydration in cisplatin‐based chemotherapy for patients with lung cancer: The second prospective feasibility study in the Okayama Lung Cancer Study Group Trial 1201. Int J Clin Oncol 2016;21:81–87. [DOI] [PubMed] [Google Scholar]
- 29. Bohm S, Battista Spatti G, Di Re F et al. A feasibility study of cisplatin administration with low‐volume hydration and glutathione protection in the treatment of ovarian carcinoma. Anticancer Res 1991;11:1613–1616. [PubMed] [Google Scholar]
- 30. Muraki K, Koyama R, Honma Y et al. Hydration with magnesium and mannitol without furosemide prevents the nephrotoxicity induced by cisplatin and pemetrexed in patients with advanced non‐small cell lung cancer. J Thorac Dis 2012;4:562–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Oka T, Kimura T, Suzumura T et al. Magnesium supplementation and high volume hydration reduce the renal toxicity caused by cisplatin‐based chemotherapy in patients with lung cancer: A toxicity study. BMC Pharmacol Toxicol 2014;15:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yamamoto Y, Watanabe K, Tsukiyama I et al. Nephroprotective effects of hydration with magnesium in patients with cervical cancer receiving cisplatin. Anticancer Res 2015;35:2199–2204. [PubMed] [Google Scholar]
- 33. Yoshida T, Niho S, Toda M et al. Protective effect of magnesium preloading on cisplatin‐induced nephrotoxicity: A retrospective study. Jpn J Clin Oncol 2014;44:346–354. [DOI] [PubMed] [Google Scholar]
- 34. Somlo G, Doroshow JH, Lev‐Ran A et al. Effect of low‐dose prophylactic dopamine on high‐dose cisplatin‐induced electrolyte wasting, ototoxicity, and epidermal growth factor excretion: A randomized, placebo‐controlled, double‐blind trial. J Clin Oncol 1995;13:1231–1237. [DOI] [PubMed] [Google Scholar]
- 35. Al‐Sarraf M, Fletcher W, Oishi N et al. Cisplatin hydration with and without mannitol diuresis in refractory disseminated malignant melanoma: A southwest oncology group study. Cancer Treat Rep 1982;66:31–35. [PubMed] [Google Scholar]
- 36. Morgan KP, Snavely AC, Wind LS et al. Rates of renal toxicity in cancer patients receiving cisplatin with and without mannitol. Ann Pharmacother 2014;48:863–869. [DOI] [PubMed] [Google Scholar]
- 37. McKibbin T, Cheng LL, Kim S et al. Mannitol to prevent cisplatin‐induced nephrotoxicity in patients with squamous cell cancer of the head and neck (SCCHN) receiving concurrent therapy. Support Care Cancer 2016;24:1789–1793. [DOI] [PubMed] [Google Scholar]
- 38. Leu L, Baribeault D. A comparison of the rates of cisplatin (cDDP)–induced nephrotoxicity associated with sodium loading or sodium loading with forced diuresis as a preventative measure. J Oncol Pharm Pract 2010;16:167–171. [DOI] [PubMed] [Google Scholar]
- 39. Ostrow S, Egorin MJ, Hahn D et al. High‐dose cisplatin therapy using mannitol versus furosemide diuresis: comparative pharmacokinetics and toxicity. Cancer Treat Rep 1981;65:73–78. [PubMed] [Google Scholar]
- 40. Santoso JT, Lucci JA 3rd, Coleman RL et al. Saline, mannitol, and furosemide hydration in acute cisplatin nephrotoxicity: A randomized trial. Cancer Chemother Pharmacol 2003;52:13–18. [DOI] [PubMed] [Google Scholar]
- 41. Dana R, Kachhwaha VS. Comparison of oral and intravenous hydration and diuretic, choice for protecting cisplatin induced nephrotoxicity. Indian J Cancer 1996;33:168–170. [PubMed] [Google Scholar]
- 42. Goren MP, Forastiere AA, Wright RK et al. Carboplatin (CBDCA), iproplatin (CHIP), and high dose cisplatin in hypertonic saline evaluated for tubular nephrotoxicity. Cancer Chemother Pharmacol 1987;19:57–60. [DOI] [PubMed] [Google Scholar]
- 43. Chiuten D, Vogl S, Kaplan B et al. Is there cumulative or delayed toxicity from cis‐platinum? Cancer 1983;52:211–214. [DOI] [PubMed] [Google Scholar]
- 44. Yokoo K, Murakami R, Matsuzaki T et al. Enhanced renal accumulation of cisplatin via renal organic cation transporter deteriorates acute kidney injury in hypomagnesemic rats. Clin Exp Nephrol 2009;13:578–584. [DOI] [PubMed] [Google Scholar]
- 45. Lajer H, Bundgaard H, Secher NH et al. Severe intracellular magnesium and potassium depletion in patients after treatment with cisplatin. Br J Cancer 2003;89:1633–1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Planting AS, van der Burg ME, de Boer‐Dennert M et al. Phase I/II study of a short course of weekly cisplatin in patients with advanced solid tumours. Br J Cancer 1993;68:789–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Planting AS, de Mulder PH, de Graeff A et al. Phase II study of weekly high‐dose cisplatin for six cycles in patients with locally advanced squamous cell carcinoma of the head and neck. Eur J Cancer 1997;33:61–65. [DOI] [PubMed] [Google Scholar]
- 48. Bellomo R, Ronco C, Kellum JA et al. Acute renal failure ‐ Definition, outcome measures, animal models, fluid therapy and information technology needs: The Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 2004;8:R204–R212. [DOI] [PMC free article] [PubMed] [Google Scholar]