Table 4. Summary of three drugs (two targeted and one cytotoxic) in combinationa.
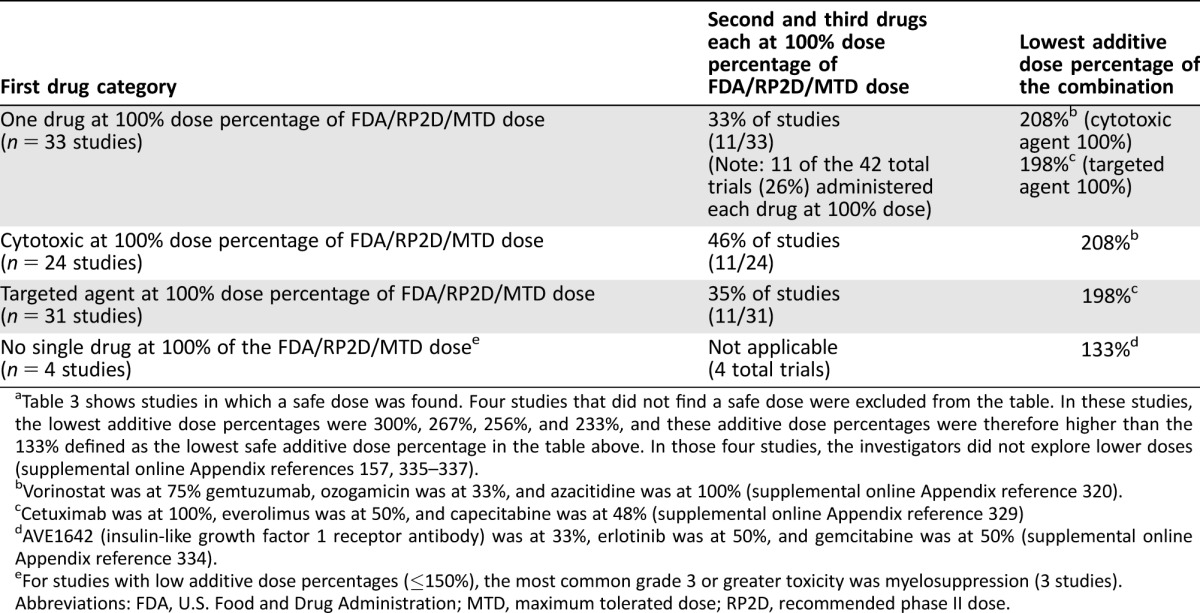
Table 3 shows studies in which a safe dose was found. Four studies that did not find a safe dose were excluded from the table. In these studies, the lowest additive dose percentages were 300%, 267%, 256%, and 233%, and these additive dose percentages were therefore higher than the 133% defined as the lowest safe additive dose percentage in the table above. In those four studies, the investigators did not explore lower doses (supplemental online Appendix references 157, 335–337).
Vorinostat was at 75% gemtuzumab, ozogamicin was at 33%, and azacitidine was at 100% (supplemental online Appendix reference 320).
Cetuximab was at 100%, everolimus was at 50%, and capecitabine was at 48% (supplemental online Appendix reference 329)
AVE1642 (insulin‐like growth factor 1 receptor antibody) was at 33%, erlotinib was at 50%, and gemcitabine was at 50% (supplemental online Appendix reference 334).
For studies with low additive dose percentages (≤150%), the most common grade 3 or greater toxicity was myelosuppression (3 studies).
Abbreviations: FDA, U.S. Food and Drug Administration; MTD, maximum tolerated dose; RP2D, recommended phase II dose.
