Abstract
The purpose of our study was to assess quality of life (QoL) among Georgian HIV-infected individuals and to examine factors associated with QoL. Our cross-sectional study sample consisted of 201 HIV-infected adult outpatients recruited at the National AIDS Center in Tbilisi, Georgia. WHOQOL-HIV-BREF was used to measure QoL. Data about other variables of interest were obtained from medical records. Modified Poisson regression with robust variance estimates was performed to create a predictive model of factors that influenced QoL. The study results showed the following factors as predictors of good general QoL: antiretroviral (ARV) treatment (prevalence ratio (PR)=2.87 (95% CI: 1.45, 5.67)); higher education level (PR = 1.51 (95% CI: 1.05, 2.17)); CD4 cells ≥200 cells/mm3 (PR = 1.83 (95% CI: 1.13, 2.94)); and age ≥40 years (PR = 1.60 (95% CI: 1.09, 2.36)). However, all factors examined were associated with at least one QoL domain. Our study suggests that HIV-infected individuals younger than 40 years and those with lower education level are more likely to have poorer QoL, while those receiving ARV treatment tend to have better QoL. This highlights the importance of educational interventions and ARV treatment in HIV patients. Future research should seek to implement additional evidence-based actions to improve QoL in this population.
Keywords: HIV, AIDS, QoL, WHOQOL-HIV
Introduction
HIV/AIDS is an emerging problem in the country of Georgia. Although Georgia does not belong to the countries with high HIV prevalence (0.07%), HIV incidence in Georgia has been increasing since the mid-1990s and there is a high risk of an expanding epidemic due to growing HIV incidence in neighboring countries and risk factors for HIV/AIDS such as high prevalence of sexually transmitted infections, injection drug use, and low levels of knowledge about HIV in the Georgian population.1 About 4700 HIV/AIDS cases have been officially registered in Georgia as of 31 December 2014, with an estimated total number of people infected with HIV around 6400 (Spectrum/PP).1 The main routes of transmission are believed to be heterosexual contact (41.6 %) and injection drug use (49.1%).1
Georgia has made significant progress in providing treatment and care services for people living with HIV (PLWHIV): the model of service delivery ensures high patient engagement in care after HIV diagnosis providing basis for universal access to free antiretroviral (ARV) therapy for all those in need (at the time of study ARV was prescribed at CD4 threshold of ≤350 cells/mm3).2 A total of 2750 PLWHIV are retained in care countrywide, as of the end of 2014.1 Advances in HIV care have dramatically improved AIDS outcomes and prolonged life expectancy of PLWHIV,3 and the focus of care of HIV patients has shifted to improving their quality of life (QoL).4 Thus, evaluating QoL and identifying factors that influence it may lead to changes in planning and improvement in care of HIV/AIDS patients.5
QoL has not previously been examined in Georgian PLWHIV. However, a number of studies assessed QoL among HIV patients in different countries. The factors influencing QoL differ in different populations. In the Estonian PLWHIV population, being employed and early stage of the disease were associated with better QoL,6 while in the Croatian HIV-infected population, younger age, higher level of education, and being in a relationship were predictors of better QoL.7 In a Brazilian study,8 patients who were employed had better QoL. In the study conducted in Burkina-Faso, female gender, not having support for medical care, and younger age were shown to be risk factors for poor QoL in PLWHIV9; existing data about association of CD4 cell count and ARV treatment status with QoL also differ by study. In most studies, CD4 cell count was not significantly associated with QoL, but in the study conducted in India,10 these markers influenced some QoL domains. ARV treatment also affected QoL in some studies: the study by Razera et al. in Brazil revealed that patents using ARV drugs had poorer QoL while the cohort study among HIV-infected individuals living in Guangxi11 showed a positive association between ARV treatment and better QoL. Considering the differing results from previous research, it is important to find out which factors influence QoL in the Georgian HIV-infected population.
The purpose of our study is to assess QoL among Georgian HIV-infected individuals and to examine associations of socio-demographic and disease-related factors with general and domain-specific QoL.
Methods
We conducted a cross-sectional study of HIV-infected adults who were 18 years of age or older, seen as outpatients, and who were able to speak and read Georgian. Our sample consisted of 201 patients attending Infectious Diseases, AIDS and Clinical Immunology Research Center (IDACIRC/ National AIDS Center) in Tbilisi, Georgia, which is Georgia’s referral institution serving as a national coordinator for HIV diagnosis, treatment, and care, providing specific treatment and care for the majority of Georgian HIV patients. We excluded from the study pregnant women, individuals who were hospitalized, and decisionally impaired/psychiatric patients.
Participants were recruited by physicians at IDACIRC. The recruitment period was about three months and almost every HIV patient on his/her regular visit was offered participation in the study. WHOQOL-HIV BREF questionnaire was used to measure QoL in the participants. WHOQOL-HIV was developed and validated by the World Health Organization specifically for PLWHIV; it evaluates QoL based on six domains (physical, psychological, level of independence, social relationships, environment, and spiritual/beliefs) and includes questions specific to HIV/AIDS. WHOQOL-HIV BREF is a short version containing 31 items.12 Each item is rated on a 5 point Likert scale with 1 indicating a negative perception and 5 indicating a positive perception. Thus, final scores are scaled in a positive direction where higher scores indicate better QoL.
Five HIV-infected persons who were asked to participate refused. A total of 212 HIV patients agreed to fill out the questionnaire, but 11 of them were excluded from the analysis because of incomplete forms. The high participation rate might be related to some degree of confidence (the patients were recruited at the medical facility) and the simplicity of the procedure (completing a questionnaire taking about 20 minutes, while confidentiality is ensured). Thus, we analyzed the data from 201 participants. The scores for the six domains and general QoL were calculated according to the Manual for Scoring and Coding WHOQOL-HIV BREF.13 The domain scores range between 4 and 20, with 4 signifying the worst result and 20 signifying the best result. General QoL score ranges between 1 and 5, with 1 corresponding to very poor QoL and 5 corresponding to very good QoL. We categorized the participants by general QoL into two groups: those with poor Qol and those with good QoL, using as the cut-point the median score of 3.
The following data were gathered from the electronic database of IDACIRC: gender (male/female), age in years, education level (education level is defined “high” if a person finished University and is defined “low” if a person did not finish University), time since the diagnosis in years, route of transmission (heterosexual contact/homosexual contact/IDU/blood transfusion/unknown), information on ARV treatment (receiving/ not receiving ARV drugs), CD4 cell count (in cell/mm3) and co-infection with hepatitis C (diagnosed HCV infection).
The descriptive analysis was performed using mean ± standard deviation for all continuous variables and frequency/percentage for categorical data for the population overall and by general QoL status.
Bivariate analysis was performed between both domain-specific and general QoL and each of the factors of interest using two sample t-tests or Chi square tests. The variables which were associated with QoL at a significance level of 0.05 were included in the multivariate analysis.
We performed modified Poisson regression with robust variance estimates to create a predictive model and to identify which factors influenced QoL. In the multivariate model QoL was the dependent variable: general QoL and the six domains were included in separate models as dichotomous variables, using their medians as cutoff points. Modified Poisson regression with robust variance has been shown to provide more accurate estimates than logistic regression for cross-sectional studies with binary outcomes.14
We performed a goodness-of-fit test of the final predictive model and generated a Receiver Operative Characteristic (ROC) curve to assess the predictive ability and fit of our model.
For the statistical analysis, we used SAS 9.2 software (SAS Institute Inc., Cary, NC, USA).
The study was approved by the Institutional Review Board of the IDACIRC (approval #13-002) and the Institutional Review Board of the University at Albany (approval #13166-01). Written informed consent was obtained from each study participant.
Results
Sample characteristics
Our study sample (n = 201) had a higher percentage of male participants (72.1%) and persons with a low level of education (e.g. persons who did not finish University) (61%). The mean age of participants was 40.3 years and 79% were on ARV treatment. The majority of HIV patients had poor general QoL (63.7%). Detailed information about the sample characteristics and the domain scores is presented in Table 1.
Table 1.
Socio-demographic, HIV disease-related, and QoL-related characteristics for a sample of 201 HIV patients in Georgia.
| Age in years | |
| Mean (±SD) | 40.3 (±10) |
| Sex, n (%) | |
| Male | 145 (72.1) |
| Female | 56 (27.9) |
| Education,a n (%) | |
| High | 76 (39.0) |
| Low | 119 (61.0) |
| Route of transmission, n (%) | |
| IDU | 69 (34.3) |
| Heterosexual contact | 116 (57.7) |
| Blood transfusion | 3 (1.5) |
| Homosexual/MSM | 10 (5.0) |
| Unknown | 3 (1.5) |
| Time since diagnosis in years | |
| Mean (±SD) | 2.9 (±3.5) |
| CD4 in cells/mm3 | |
| Mean (±SD) | 358.5 (±200) |
| ARV treatment, n (%) | |
| Yes | 159 (79.0) |
| No | 42 (21.0) |
| Co-infection with hepatitis C, n (%) | |
| Yes | 52 (26.0) |
| No | 149 (74.0) |
| Feeling ill (self-report), n (%) | |
| Yes | 81 (40.3) |
| No | 120 (59.7) |
| Self-reported health, n (%) | |
| Very bad | 14 (7.0) |
| Bad | 25 (12.4) |
| Not good and not bad | 108 (53.7) |
| Good | 54 (26.9) |
| Very good | 0 (0) |
| General QoL,b n (%) | |
| Poor quality of life | 128 (63.7) |
| Good quality of life | 73 (36.3) |
| Scores for general QoL and 6 domains (Mean ±SD) | |
| General QoL (Overall QoL & general health perception) | 3.0 ± 0.7 |
| Physical domain | 13.3 ± 3.6 |
| Psychological domain | 12.9 ± 2.2 |
| Level of independence domain | 12.2 ± 3.3 |
| Social relationship domain | 14.8 ± 2.7 |
| Environment domain | 12.8 ± 2.3 |
| Spirituality domain | 14.5 ± 3.2 |
Variable Education has missing data for six participants; education level is considered high if a person finished University and it is considered low if a person did not finish University and has only secondary education.
General QoL is defined as “Poor” if the score of general QoL and overall health perception is less than or equal to 3 points and it is defined as “Good” if the score is more than 3 points. General QoL score is derived from the questionnaire as the mean of question 1 (“How would you rate your quality of life?”) and question 2 (How satisfied are you with your health?”) based on the Manual for Scoring and Coding WHOQOL-HIV-BREF by WHO. It ranges from 1 to 5, with 1 corresponding to very poor QoL and 5 corresponding to very good QoL.
Sample characteristics by general QoL
Descriptive statistics by general QoL (good vs. poor) are presented in Table 2. On average, participants with poor QoL were younger (p = 0.01), and had a lower level of education (p = 0.02). The percentage being treated with ARV was significantly higher in those with good vs. poor QoL (p = 0.003). The average time period since diagnosis of HIV was four years for participants with good QoL and two years for those with poor QoL (p < 0.001).
Table 2.
Socio-demographic, HIV disease-related and QoL-related characteristics of study participants by general QoL (poor vs good).a
| Poor QoL | Good QoL | p-Valueb | |
|---|---|---|---|
| Men | 60.7% | 39.3% | 0.15 |
| Women | 71.4% | 28.6% | |
| Receiving ARV treatment | 58.5% | 41.5% | 0.003 |
| Not on ARV treatment | 83% | 17% | |
| Co-infection with hepatitis C | 52% | 48% | 0.04 |
| No co-infection | 68% | 32% | |
| Low education | 71% | 29% | 0.02 |
| High education | 54% | 46% | |
| Route of transmission | |||
| Heterosexual contact | 63% | 37% | 0.70 |
| IDU | 67% | 33% | |
| Other | 56% | 44% | |
| Mean age in years | 39.0 | 42.5 | 0.01 |
| Mean CD4 cell count in cells/mm3 | 355.4 | 363.8 | 0.77 |
| Time since diagnosis in years | 2 | 4 | <0.001 |
General QoL score is derived from the questionnaire as the mean of question 1 (“How would you rate your quality of life?”) and question 2 (How satisfied are you with your health?”) based on Users’ Manual for Scoring and Coding WHOQOL-HIV-BREF by WHO. It ranges from 1 to 5, with 1 corresponding to very poor QoL and 5 corresponding to very good QoL. Median of general QoL (3) was used as the cutoff point to define poor and good QoL.
p-Values are from the Chi square tests for the categorical variables (gender, education level, ARV treatment, co-infections); from Fisher’s exact test for Route of Transmission; and from two sample t-tests for the continuous variables (age, CD4 cell count, time since diagnosis), using significance level of 0.05.
Multivariable analysis results
Modified Poisson regression was performed to examine associations between participant characteristics and general QoL, as well as domain-specific QoL (Table 3). The results suggest that HIV patients who received ARV treatment were more likely to have good general QoL (prevalence ratio (PR) = 2.87 (95% CI: 1.45, 5.67)) as compared to those not using ARV drugs. Persons with high education were more likely to have good general QoL as compared with those with low education (PR = 1.51(95% CI: 1.05, 2.17)). The participants with CD4 cell count ≥ 200 cells/mm3 had higher general QoL as compared to those with lower levels of CD4 cells (PR = 1.83 (95% CI: 1.13, 2.94)). Persons 40 years of age or older were more likely to have good general QoL as compared to younger individuals (PR = 1.60 (95% CI: 1.09, 2.36)). Male and female participants did not have a statistically significant difference in general QoL. Moreover, the multivariate analysis did not reveal any association of general QoL with other characteristics such as route of transmission, date since diagnosis, or co-infection with hepatitis C. The detailed information about associations between specific QoL domains and patients’ characteristics is given in Table 3.
Table 3.
Prevalence ratios and 95% confidence intervals for the association between participants’ characteristics and high vs. low score for general and domain-specific QoL for a sample of 201 HIV Patients in Georgia (modified Poisson regression with robust variance).
| General QoL | Physical | Psychological | Independence | Social | Environment | Spirituality | |
|---|---|---|---|---|---|---|---|
| ARV treatment (treatment vs no treatment) | 2.87a (1.45, 5.67) | 2.08a (1.32, 3.27) | 1.08 (0.73, 1.72) | 1.04 (0.75, 1.43) | 1.96a (1.12, 3.45) | 1.11 (0.72, 1.62) | 1.18 (0.73, 1.92) |
| Age (40 years of age or older vs younger than 40 years of age) | 1.60a (1.09, 2.36) | 0.97 (0.69, 1.38) | 0.98 (0.73, 1.39) | 0.81 (0.58, 1.15) | 0.55a (0.38, 0.78) | 1.14 (0.85, 1.73) | 1.39 (0.95, 2.05) |
| CD4 (≥200 cells/mm3 vs < 200 cells/mm3) | 1.83a (1.13, 2.94) | 2.25a (1.57, 3.24) | 1.45 (0.95, 2.21) | 1.08 (0.76, 1.55) | 0.93 (0.64, 1.35) | 1.74a (1.04, 2.92) | 1.28a (1.15, 2.17) |
| Co-infection with hepatitis C (co-infection with hepatitis C vs no co-infection) | 1.16 (0.72, 1.77) | 0.95 (0.59, 1.42) | 0.67a (0.44, 0.94) | 1.03 (0.67, 1.57) | 0.97 (0.64, 1.49) | 0.65 (0.40, 1.07) | 0.78 (0.52, 1.18) |
| Gender (male vs female) | 1.08 (0.67, 1.75) | 0.49a (0.34, 0.69) | 1.39 (0.98, 1.99) | 0.67a (0.47, 0.93) | 1.25 (0.76, 2.06) | 0.87 (0.56, 1.34) | 2.40a (1.37, 4.19) |
| Time since diagnosis (diagnosed at least 3 years ago vs diagnosed less than 3 years) | 0.99 (0.68, 1.43) | 1.15 (0.82, 1.60) | 1.39a (1.05, 1.85) | 1.38 (0.99, 1.93) | 1.06 (0.72, 1.58) | 1.11 (0.77, 1.60) | 1.59a (1.12, 2.27) |
| Education (high vs low) | 1.51a (1.05, 2.17) | 1.35a (1.03, 1.81) | 1.18 (0.86, 1.61) | 1.44a (1.10, 1.94) | 0.99 (0.70, 1.41) | 1.61a (1.16, 2.22) | 1.41 (0.98, 2.03) |
| IDU (vs hetero) route of transmission Other types of transmission vs heterob | 0.87 (0.53, 1.40) 1.41 (0.74, 2.69) | 0.73 (0.42, 1.28) 3.14a (1.94, 5.07) | 0.64a (0.43, 0.96) 0.79 (0.42, 1.49) | 0.60 (0.35,1.02) 2.20a (1.49, 3.25) | 1.33 (0.84, 2.11) 1.97a (1.10, 3.32) | 1.23 (0.80, 1.90) 1.76a (1.13, 3.72) | 0.83 (0.53,1.27) 0.49 (0.15, 1.42) |
Statistically significant at 0.05 level of significance.
“Other” route of transmission includes homosexual contact, blood transfusion and unknown transmission route. These were combined due to the small number of participants in each listed category.
Goodness-of-fit statistics
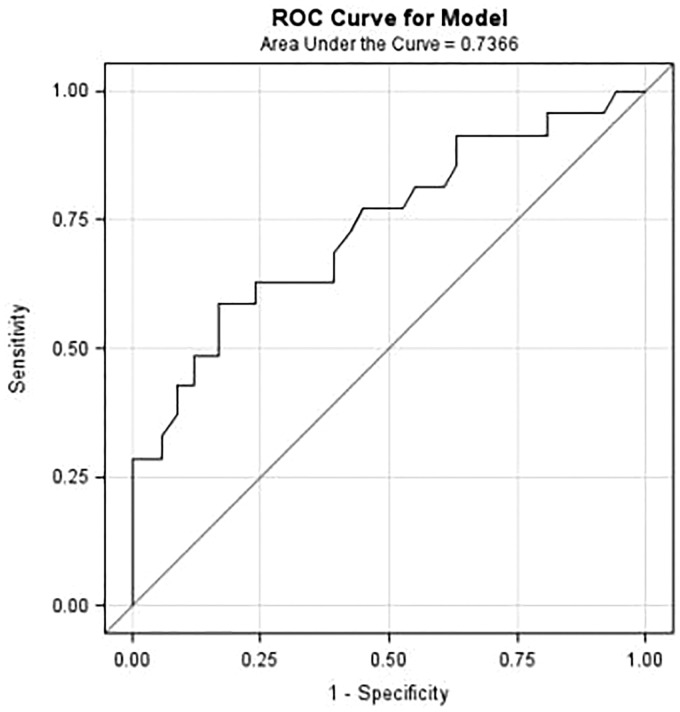
To assess the predictive accuracy of our modified Poisson’s regression model, we generated a ROC curve. The Area Under the ROC Curve (AUC) is a measure of the predictive ability of the model and typically ranges between 0.5 and 1, with 0.5 corresponding to a model that is no better than chance and 1 corresponding to 100% accuracy. In our analysis, the AUC was approximately 0.74 as shown in Figure 1.
Figure 1.
ROC curve for modified Possion regression model predicting general QoL from the study participants’ characteristics.
The internal consistency reliability of the Georgian version of WHOQOL HIV BREF instrument was also assessed. Chronbach’s alpha for six domains ranged from 0.73 to 0.83 (0.74 for Physical, 0.80 for Psychological, 0.74 for Independence, 0.82 for Social relations, 0.73 for Environment and 0.83 for Spirituality domains), and Spearman–Brown coefficient for general QoL was 0.83, indicating acceptable reliability of the questionnaire.
Discussion
The purpose of our study was to assess QoL among Georgian PLWHIV and to examine associations of socio-demographic and disease-related factors with general and domain-specific QoL. The results suggest that the majority of Georgian HIV-infected patients had poor general QoL. Among the study participants, the best QoL was observed in the dimensions related to social relationship and spirituality, and the worst QoL was observed in level of independence.
In our study, ARV treatment and higher CD4 levels were the greatest contributors to good general QoL. People living with HIV who received ARV treatment were more likely to have good general QoL, as well as better physical and social QoL as compared to those not using ARV drugs. Previous studies showed inconsistent results for the association between ARV treatment and QoL. In Estonian and Croatian HIV patients, ARV treatment did not appear to have an effect on QoL. However, in the study among HIV-infected Brazilians by Razera et al., participants receiving ARV treatment had poorer general QoL as compared to those not receiving the treatment. In Guangxi study by Ming et al., use of ARV drugs was associated with better QoL. Beneficial effects of ARV treatment on QoL might be explained by decreasing intensity of clinical symptoms of the disease. On the other hand, it is possible that more resilient patients have a higher QoL and are more likely to take medications. In any case, the results highlight the importance of enhancing ARV treatment and adherence programs.
The analysis of CD4 cell count and QoL was suggestive of an association. A CD4 cell count less than 200 cells/mm3 is classified by WHO as severe immunosuppression.15 Thus, CD4 cell count is one of the indicators of disease progression in HIV/AIDS and therefore can potentially influence a person’s self-perception of QoL. However, most previous studies did not document an association between CD4 level and QoL. In contrast, Handajani et al. found a positive association between higher CD4 level and better general QoL.16 Our study revealed that HIV-infected persons with a CD4 cell count ≥200 cells/mm3 are more likely to have better general QoL as compared to those with a CD4 cell count <200 cells/mm3, after adjusting for other predictors of QoL. This was also true for the physical, environment, and spirituality domains.
A significant association was observed between education and QoL in our study: persons with higher education (e.g. those who finished University) were more likely to have good general QoL as well as better QoL scores in the physical, environment, and independence domains as compared to those with low education. Education potentially provides opportunities for employment and social support, and thus can contribute to a sense of good QoL. However, the relationship between education and general QoL was not documented in previous studies. Instead, in some prior studies, employment had a positive effect on QoL6,8; however, data on occupation were unavailable for participants in our study and therefore we were unable to examine this association in our sample.
In our study, younger age was associated with worse general QoL, while there was no significant difference in general QoL between female and male participants. In some of the previous studies of this association, general QoL differed across both gender and age categories.7,9 Generally, older people are expected to have worse QoL due to the factors related to ageing (physical conditions, fears about the future); however, HIV-associated stress level did not differ by age.17 Further stratification of our sample by age showed a significant difference in ARV status: 85% of older patients and only 73% of younger individuals were receiving ARV treatment (p = 0.04). Considering that ARV treatment is associated with better QoL, the above difference between the two populations might have somehow influenced our results. Our study findings are consistent with a multicultural QoL study showing poorer QoL in young PLWHIV as compared to older persons; older persons reported less negative feelings, lower social exclusion, and greater access to health care. According to the authors, these differences cannot be entirely explained by age.18 Our study has several limitations. First of all, given the cross-sectional design of the study, we could not demonstrate temporality of the associations observed. However, many of the covariates assessed are fixed (education, gender, etc.) and therefore could not have been affected by QoL. Second, our sample size was moderate (201 participants) and we might have missed some true associations because of lack of statistical power. Third, we used convenience sampling: HIV-patients were recruited at a medical facility by the physicians, which may have increased the possibility of selection bias or decreased generalizability. However, convenience sampling is a common practice in HIV research, and it would be difficult to obtain a truly representative sample because of stigma related to the disease. Previous studies also used a similar design and sampling method. Other limitations include absence of information on some important variables, such as employment status (which was shown to be associated with QoL in previous studies), as well as income, current injection drug use, or other diseases except for HCV. Data on these variables were unavailable or inaccurate, and therefore they were not included in the model. We should also note the absence of ethnic minority status. The reason is low percentage of ethnic minority groups in general population in Georgia19 as well as among PLWHIV (6%) (Georgian AIDS Center Database). It is less likely that this variable could considerably affect the study results.
On the other hand, this study is the first to examine QoL among HIV-infected individuals in Georgia, making this a new direction in HIV research in Georgia as well as in the Caucasus region. Another strength of the study is that the data about socio-demographic and disease-related factors were obtained from medical records, decreasing the possibility of information bias. In addition, the previously validated self-administered instrument used in the study might decrease the potential for social desirability bias, which is more likely to be present when participants encounter face-to-face interviews.20
Our study can be considered as a standing point for future research on QoL in HIV patients in Georgia. While the study findings provide information about QoL and some influencing factors among PLWHIV in Georgia, the limitations of the current study can be considered and minimized in future studies. Social support and social interventions are factors potentially related to QoL, and confirming these associations in future research would have major public health implications.
Conclusions
Although Georgia has achieved considerable success in controlling HIV/AIDS through prevention and treatment programs, social problems related to QoL, including stigma and discrimination, still remain challenges in PLWHIV. Quality of life itself is not a pre-determined characteristic – it can be modified if public health specialists target appropriate interventions at specific groups of people. Our study showed that individuals younger than 40 years of age and those with lower education level are at higher risk of having poorer QoL and health perception, while individuals with higher CD4 levels and those receiving ARV treatment tend to have better QoL. These findings highlight the importance of educational interventions as well as the importance of adherence to ARV treatment in HIV patients to improve their general QoL. Georgian public health specialists working in the HIV field should prioritize implementation of such interventions among HIV patients. This study is the first step in researching factors influencing QoL in HIV patients in Georgia, and it highlights the need for future studies to further direct evidence-based action towards improving QoL in this population.
Acknowledgment
The research was conducted with the assistance of clinicians of IDACIRC.
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by the New York State International Training and Research Program (grant no. D43TW000233), NIH–Fogarty International Center and National Institute on Drug Abuse.
References
- 1.National Center for Disease Control and Public Health. Georgia country progress report. Global AIDS response progress report, Tbilisi, December 2014.
- 2.Chkhartishvili N, Sharavdze L, Chokoshvili O, et al. The cascade of care in the Eastern European country of Georgia. HIV Med 2015; 16: 62–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chkhartishvili N, Sharvadze L, Chokoshvili O, et al. Mortality and causes of death among HIV-infected individuals in the country of Georgia: 1989–2012. AIDS Res Hum Retrovirus 2014; 30: 560–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Drewes J, Gusy B, Rüden U. More than 20 years of research into the quality of life of people with HIV and AIDS—a descriptive review of study characteristics and methodological approaches of published empirical studies. J Int Assoc Provid AIDS Care 2013; 12: 18–22. [DOI] [PubMed] [Google Scholar]
- 5.Webb A, Norton M. Clinical assessment of symptom-focused health-related quality of life in HIV/AIDS. J Assoc Nurses AIDS Care 2004; 15: 67–78. [PubMed] [Google Scholar]
- 6.Rüütel K, Pisarev H, Loit HM, et al. Factors influencing quality of life of people living with HIV in Estonia: a cross-sectional survey. J Int AIDS Soc 2009; 12: 13–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Belak Kovacević S, Vurusić T, Duvancić K. Quality of life of HIV-infected persons in Croatia. Coll Antropol 2006; 30: 79–84. [PubMed] [Google Scholar]
- 8.Razera F, Ferreira J, Bonamigo RR. Factors associated with health-related quality-of-life in HIV-infected Brazilians. Int J STD AIDS 2008; 19: 519–523. [DOI] [PubMed] [Google Scholar]
- 9.Bakiono F, Ouédraogo L, Sanou M, et al. Quality of life in people living with HIV: a cross-sectional study in Ouagadougou, Burkina Faso. Springerplus 2014; 3: 372. [DOI] [PMC free article] [PubMed]
- 10.Chandra PS, Gandhi C, Satishchandra P, et al. Quality of life in HIV subtype C infection among asymptomatic subjects and its association with CD4 counts and viral loads-a study from South India. Qual Life Res 2006; 15: 1597–1605. [DOI] [PubMed] [Google Scholar]
- 11.Ming Z, Prybylski D, Cheng F, et al. Two-year prospective cohort study on quality of life outcomes among people living with HIV after initiation of antiretroviral therapy in Guangxi, China. J Assoc Nurses AIDS Care 2014; 25: 603–613. [DOI] [PubMed]
- 12.WHOQOL HIV group. WHOQOL-HIV for quality of life assessment among people living with HIV and AIDS: results from the field test. AIDS Care 2004; 16: 882–882. [DOI] [PubMed] [Google Scholar]
- 13.World Health Organization. Users’ manual for scoring and coding WHOQOL-HIV instruments. Department of Mental Health and Substance Dependence: Geneva, Switzerland, 2002.
- 14.Barros A, Hirakata V. Alternatives for logistic regression in cross-sectional studies: an empirical comparison of models that directly estimate the prevalence ratio. BMC Med Res Methodol 2003; 3: 21–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Health Organization. WHO case definitions of HIV for surveillance and revised clinical staging and immunological classification of HIV-related disease in adults and children. HIV/AIDS Programme, Geneva: World Health Organization, 2007. [Google Scholar]
- 16.HandajaniYS, Djoerban Z, Irawan H. Quality of life in people living with HIV/AIDS: outpatient in Kramat 128 Hospital Jakarta. Acta Med Indones 2012; 44: 310–316. [PubMed] [Google Scholar]
- 17.Webel A, Longenecker C, et al. Age, stress, and isolation in older adults living with HIV. AIDS Care 2014; 26: 523–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Skevington S. Is quality of life poorer for older adults with HIV/AIDS? International evidence using the WHOQOL-HIV. AIDS Care 2012; 24: 1219–1225. [DOI] [PubMed]
- 19.National Statistics Office of Georgia. General population census. Tbilisi, Georgia, 2014.
- 20.Okamotoa K, Ohsukab K, Shiraishib T, et al. Comparability of epidemiological information between self- and interviewer-administered questionnaires. J Clin Epidemiol 2002; 55: 505–511. [DOI] [PubMed] [Google Scholar]