Abstract
Ambient light intensity is signaled directly to hypothalamic areas that regulate energy metabolism. Observational studies have shown associations between ambient light intensity and plasma glucose and lipid levels, but human data on the acute metabolic effects of light are scarce. Since light is the main signal indicating the onset of the diurnal phase of physical activity and food intake in humans, we hypothesized that bright light would affect glucose and lipid metabolism. Therefore, we determined the acute effects of bright light on plasma glucose and lipid concentrations in 2 randomized crossover trials: (1) in 8 healthy lean men and (2) in 8 obese men with type 2 diabetes. From 0730 h, subjects were exposed to either bright light (4000 lux) or dim light (10 lux) for 5 h. After 1 h of light exposure, subjects consumed a 600-kcal mixed meal. Primary endpoints were fasting and postprandial plasma glucose levels. In healthy men, bright light did not affect fasting or postprandial plasma glucose levels. However, bright light increased fasting and postprandial plasma triglycerides. In men with type 2 diabetes, bright light increased fasting and postprandial glucose levels. In men with type 2 diabetes, bright light did not affect fasting triglyceride levels but increased postprandial triglyceride levels. We show that ambient light intensity acutely affects human plasma glucose and triglyceride levels. Our findings warrant further research into the consequences of the metabolic effects of light for the diagnosis and prevention of hyperglycemia and dyslipidemia.
Keywords: artificial light, glucose metabolism, lipid metabolism, type 2 diabetes
In modern societies, people are increasingly exposed to artificial light (Kyba et al., 2015). Intrinsically photosensitive retinal ganglion cells detect ambient light intensity and relay this information directly to hypothalamic areas, including the central brain clock in the suprachiasmatic nucleus (SCN) (Benarroch, 2011). The SCN has a role in appetite control, presumably via its connections to the arcuate nucleus and the lateral hypothalamus. In addition, the SCN controls glucose metabolism via autonomic and hormonal pathways (Stenvers et al., 2012). Therefore, ambient light may affect food intake and glucose metabolism (Versteeg et al., 2016).
In mice, chronic exposure to dim light at night (Fonken et al., 2010) or continuous light (Coomans et al., 2013) induces obesity and hyperglycemia, and exposure to prolonged day-length induces obesity (Kooijman et al., 2015). In rats, a single light pulse acutely increases expression of the gene encoding the gluconeogenic protein phosphoenolpyruvate carboxykinase (PEPCK) in the liver, via activation of the autonomic nervous system (Cailotto et al., 2009).
Observational human studies suggest adverse metabolic effects of ambient light at night. Exposure to light at night is associated with obesity (Obayashi et al., 2013; McFadden et al., 2014), increased fasting triglycerides (Obayashi et al., 2013), and diabetes (Obayashi et al., 2014; Gan et al., 2015). Furthermore, shift workers are at increased risk to develop obesity and type 2 diabetes (Gan et al., 2015).
In comparison with the observations on chronic light at night (i.e., light at the inappropriate circadian phase), there are fewer human studies on the metabolic associations of light during the day (i.e., light at the appropriate circadian phase). One study describes a positive association between daytime light exposure and body mass index (BMI) (Reid et al., 2014). Two studies show that several weeks of morning light therapy may cause a slight reduction in fat mass (despite no effect on body weight) in obese subjects (Dunai et al., 2007; Danilenko et al., 2013). A case report described a patient with seasonal affective disorder (SAD) and insulin dependent diabetes who showed a strong reduction of insulin requirements after 3 weeks of morning light therapy (Allen et al., 1992), and another case report described a similar patient who showed increased insulin sensitivity after 10 morning sessions of 30 min of 10,000 lux light therapy (Nieuwenhuis et al., 2009).
Despite these chronic studies, human data on the acute metabolic effects of light are scarce. One recent study investigated the effects of blue-enriched light in the morning or the evening and showed increased insulin resistance due to blue-enriched bright (260 lux) light exposure compared with dim (<20 lux) light in the morning and the evening (Cheung et al., 2016). Since daylight is the main signal indicating the onset of the active circadian phase (i.e., the phase of physical activity and food intake) in humans, we hypothesized that bright light with the intensity of daylight would affect glucose and lipid metabolism. Therefore, we determined in healthy men the acute effects of bright light (4000 lux, the intensity of outside light on a cloudy day; Dharani et al., 2012) on fasting and postprandial plasma glucose and lipid levels compared with the effects of dim light (10 lux, the intensity of candlelight; Dharani et al., 2012). Subsequently, since patients with type 2 diabetes by definition have increased fasting and postprandial glucose levels compared with healthy subjects (American Diabetes Association, 2015a), we investigated the effects of bright light on fasting and postprandial plasma glucose and lipid levels in men with type 2 diabetes.
Materials and Methods
Subjects and Setting
We performed 2 clinical trials. In the healthy subject trial, we included 8 healthy lean men (inclusion criteria: age 18-50 years, BMI 18-25 kg/m2, fasting plasma glucose <5.6 mmol/L, habitual wake-up time 0600-0900 h). In the type 2 diabetes patient trial, we included 8 obese men with type 2 diabetes (inclusion criteria: age 18-80 years, BMI >25 kg/m2, fasting plasma glucose >6.9 mmol/L or HbA1c >6.5% (48 mmol/mol), habitual wake-up time 0600-0900 h). The trials were not designed to make a direct comparison between the 2 subject groups. We included only men, since the reported effects of the menstrual cycle on insulin sensitivity (Valdes and Elkind-Hirsch, 1991; Yeung et al., 2010) predict larger interindividual variation in women, and therefore a larger sample size would be needed to obtain sufficient power for a similar study in women.
Exclusion criteria for both trials were the use of any medication (other than metformin for the men with type 2 diabetes) interfering with glucose metabolism or neuronal functioning, any gastrointestinal or metabolic disease affecting digestion or metabolism (mild gastroesophageal reflux disease was allowed), neuropsychiatric illness, epilepsy, uncontrolled hypertension, ophthalmological abnormalities including diabetic retinopathy, and (because of the composition of the experimental meals) lactose intolerance or soy allergy. Subjects were recruited via local advertisements.
The healthy subject trial was registered at the Netherlands Trial Registry (NTR) as NTR3881. The type 2 diabetes patient trial was registered as NTR4645. Both trials were approved by the Institutional Review Board of the Academic Medical Center and were conducted according to the Declaration of Helsinki of October 2008.
Study Design
Both trials were performed using the same study protocol, with additional measurements in the type 2 diabetes patients. The design was a 2-week randomized crossover intervention trial. At baseline, subjects underwent a physical examination and provided a fasting blood sample. Subsequently, participants were admitted to the clinical research unit twice, with a 1-week interval between admissions. Subjects were randomized to start with either bright light or dim light using a randomization list generated with nQuery Advisor 7.0 (Statistical Solutions Ltd, Cork, Ireland).
Prior to each admission, subjects were asked to maintain their normal sleep-wake schedule for 5 days, to refrain from excessive exercise for 1 day, and to use at most 3 alcoholic beverages per day for 3 days. To verify sleep-wake behavior, subjects completed a 5-day sleep-wake diary. Patients with type 2 diabetes discontinued metformin use 3 days prior to each admission.
Subjects entered the clinical research unit after a 1-h fast at 2000 h (Fig. 1). At 2130 h, subjects received a standard 800-kcal mixed meal consisting of 300 mL tomato soup and 179 g baguette with margarine and cheese (Albert Heijn, the Netherlands). Subjects remained in normal room light (200 lux) until 2330 h and slept in darkness until 0730 h. In the dim light condition, subjects were subsequently exposed to 10 lux emitted by one HF3319 EnergyLight (Philips, Eindhoven, the Netherlands) placed in the room corner. In the bright light condition, subjects were instead exposed to 4000 lux bright light emitted by two HF3319 EnergyLights (Philips) placed in front of the subject. Ten lux is comparable to the light intensity of candlelight, and 4000 lux is comparable to outside light intensity on a cloudy day (Dharani et al., 2012). All light intensities were verified at the participant’s eye level with an LM-120 Lightmeter (Amprobe, Everett, WA). A cannula was inserted in a peripheral arm vein at 0800 h. At 0830 h, participants consumed a 600-kcal liquid mixed meal within 5 min, consisting of 400 mL Ensure Plus Vanilla (carbohydrates 54% of energy, fat 29% of energy, protein 17% of energy; Abbott Nutrition, Columbus, OH). Blood samples were obtained before the meal at 0815 and 0825 h and postprandially at 0835, 0840, 0845, 0850, 0900, 0915, 0930, 0940, 0950, 1000, 1030, 1100, 1130, 1200, 1230, and 1330 h. The frequency of blood sampling was increased at postprandial times with predicted rapid changes in glucose or insulin levels (based on Dalla Man et al., 2004; Dalla Man et al., 2007) to facilitate minimal model analyses. Heart rate was continuously monitored with a Holter ECG device (GE Healthcare, Chalfont St Giles, UK) connected to the chest. During admission, subjects remained in a semirecumbent position and were not allowed to use electronic devices such as smart-phones or laptops, in order to prevent unwanted light exposure.
Figure 1.
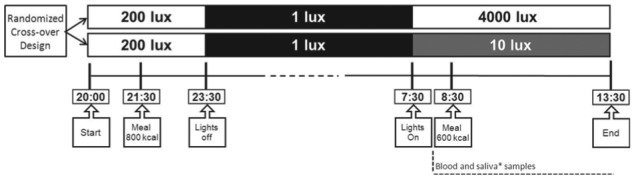
Study design. The healthy subject trial and the type 2 diabetes patient trial were both designed as randomized crossover intervention trials. Subjects were admitted to the clinical research unit twice, with a 1-week interval. Subjects entered the clinical research unit after a 1-h fast at 2000 h. At 2130 h, subjects received a standard 800-kcal mixed meal, and they were allowed to sleep in darkness from 2330 until 0730 h. In the dim light condition, subjects were subsequently exposed to 10 lux emitted by one HF3319 EnergyLight (Philips) placed in the room corner. In the bright light condition, subjects were exposed to 4000 lux bright light emitted by two HF3319 EnergyLights placed in front of the subject. A cannula was inserted in a peripheral arm vein at 0800 h, and frequent blood samples were obtained beginning at 0815 h. At 0830 h, participants consumed a 600-kcal liquid mixed meal. In the type 2 diabetes patient trial, additional measurements were performed: saliva samples (asterisk) were obtained at regular intervals, and hunger, prospective food consumption, fullness, and satiety were assessed with a questionnaire.
The experimental design of the type 2 diabetes patient trial was identical to that of the healthy subject trial with the addition of the following measurements. Saliva samples were obtained with a Salivette (Sarstedt, Nümbrecht, Germany) at regular intervals. Hunger, prospective food consumption, fullness, and satiety were assessed using a validated questionnaire (Flint et al., 2000).
Endpoints and Sample Size Determination
For both trials, the primary endpoints were fasting and postprandial plasma glucose levels. Secondary endpoints were fasting and postprandial plasma levels of insulin, free fatty acids, and triglycerides, and fasting and postprandial heart rate variability. For fasting plasma glucose values, both studies were powered to detect a difference of 0.5 mmol/L with a power of 80%, a significance level of 0.05, and an estimated standard deviation of differences of 0.5 mmol/L. For postprandial glucose values, both studies were powered to detect a difference of 100 mmol·min/L in the incremental area under the curve (iAUC) or area under the curve (AUC) of postprandial glucose excursions, with a power of 80%, a p value of 0.05, and a standard deviation of 110 mmol·min/L. Power analysis was performed using nQuery Advisor 7.0.
Measurements
Blood samples were centrifuged for 10 min at 3000 rpm at 4 °C, and plasma was separated from the cells. Glucose was determined immediately using the glucose oxidation method with the Biosen glucose analyzer (EKF Diagnostics, Barleben, Germany). Plasma aliquots were temporarily stored at −20 °C. HbA1c, plasma insulin, and plasma triglyceride levels were measured as described previously (Stenvers et al., 2014); C-peptide was determined with a 125I radioimmunoassay (Linco Research, Inc., St. Charles, MO); and plasma free fatty acid (FFA) levels were determined with an enzymatic calorimetric method (NEFA-HR(2) test kit, Wako Chemical, Neuss, Germany). In patients with type 2 diabetes, 2 additional measurements were performed: Plasma glucagon was determined with a 125I radioimmunoassay (Millipore, Billerica, MA), and saliva cortisol was determined by online solid phase extraction LC/MS/MS (Waters Corporation, Milford, MA).
Heart Rate Variability
We analyzed Holter ECG recordings with automated QRS detection followed by manual correction of noise and missed beats using a Matlab (v 2013b, The MathWorks Inc., Natick, MA) based application, as described previously (Potse et al., 2002). Subsequently, we performed heart rate variability (HRV) analysis on 1-h bins using the Kubios HRV software (version 2.2, University of Eastern Finland) (Tarvainen et al., 2014). As an indication of sympathetic-parasympathetic balance, we calculated the low-frequency/high-frequency (LF:HF) ratio with the frequency domain method.
Beta Cell Function and Insulin Sensitivity
To assess pancreatic beta cell function and peripheral insulin sensitivity, we used plasma glucose, insulin, and C-peptide levels to fit model parameters in the oral C-peptide minimal model and the oral glucose minimal model. The C-peptide minimal model is a 2-compartment model that predicts C-peptide concentrations in response to a static glucose-dependent term and a dynamic glucose-dependent term. The C-peptide minimal model outputs are the static time constant kg that represents the amount of secreted C-peptide per minute for a certain plasma glucose level above the static glucose threshold h and the dynamic constant kd that represents the amount of secreted C-peptide induced by the change in plasma glucose (Eaton et al., 1980; Van Cauter et al., 1992; Cobelli et al., 2014). The oral glucose minimal model is based on the intravenous glucose minimal model (Bergman et al., 1979) and consists of 2 ordinary coupled differential equations, predicting plasma glucose concentration based on the observed insulin concentration. The insulin sensitivity index SI is derived from the model parameters (Dalla Man et al., 2002). Fitting was performed with the Matlab GlobalSearch algorithm. Details on the models and parameter fitting are presented in the online supplemental materials.
Statistical Analysis
Normally distributed variables are presented as mean ± standard deviation (for baseline measurements) or mean ± standard error of the mean (for outcome data), and nonnormally distributed variables are presented as median (25th percentile to 75th percentile).
Fasting plasma values were defined as the average of 0815 h and 0825 h values. For postprandial plasma values, AUC and iAUC values were calculated using GraphPad Prism for Windows (version 5.01; GraphPad Software, Inc, La Jolla, CA). Since FFA levels are suppressed after a meal, for FFA the area above the curve was calculated. The effects of light on fasting plasma levels, postprandial AUC, iAUC, area above the curve, and appetite scores were determined with a 2-sided paired-samples Student t test for normally distributed variables and with a related-samples Wilcoxon signed rank test for nonnormally distributed variables.
HRV data were analyzed in a single analysis with all time points after lights-on using a generalized linear mixed model with Time (nominal), Light, and their Interaction as fixed effects. If a significant Interaction or Light effect was present, post hoc tests were performed with a related-samples Wilcoxon signed rank test. A random intercept and slope were fitted where appropriate, and robust covariances were used to test the fixed effects. If no significant Interaction was detected, the model was repeated with only the fixed factors Time and Light.
All statistical analyses were performed with IBM SPSS Statistics (version 21; SPSS, Chicago, IL) using p = 0.05 as the significance level.
Results
Healthy Men
Participants
For the healthy subject trial, 14 subjects were screened and 8 subjects were included (2 subjects did not meet inclusion criteria, 4 subjects declined participation). All included subjects completed the study. Subject characteristics are shown in Table 1. The trial was performed between March and December 2013.
Table 1.
Characteristics of healthy men (n = 8).
| Characteristic | |
|---|---|
| Age, years | 23 (21-24) |
| BMI, kg/m2 | 22 (22-23) |
| HbA1c, % | 5.2 (5.1-5.5) |
| HbA1c, mmol/mol | 34 (33-37) |
| Fasting glucose, mmol/L | 5.0 (4.8-5.0) |
| Fasting insulin, pmol/L | 40 (12-62) |
| Sleep parameters from diary | |
| Time of sleep onset | 2348 h ± 13 min |
| Time of sleep end | 0802 h ± 6 min |
Normally distributed data are expressed as mean ± SEM; nonnormally distributed data are expressed as median (25th-75th percentile).
Glucose, insulin, and C-peptide
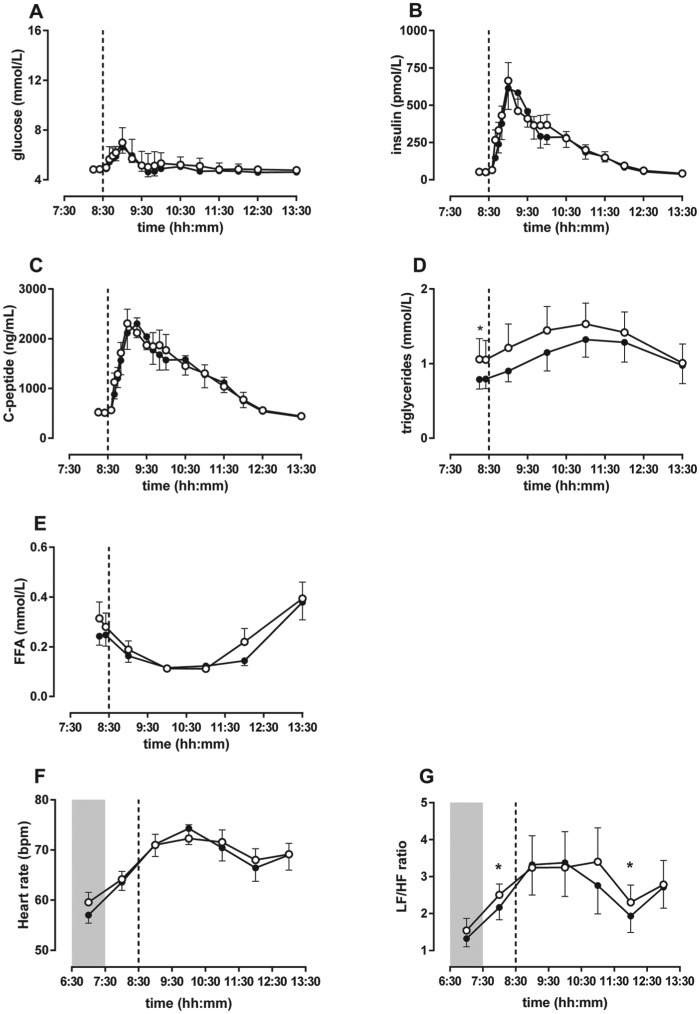
In the healthy men, fasting and postprandial glucose levels were not different between bright and dim light (Fig. 2A). The glucose iAUC was borderline increased after exposure to bright light compared with dim light (Table 2). Fasting and postprandial insulin and C-peptide levels were not different between bright and dim light (Fig. 2B and 2C, Table 2). Beta cell function and insulin sensitivity, as assessed with the C-peptide minimal model and oral glucose minimal model respectively, were not different between bright and dim light (see online supplemental materials).
Figure 2.
In healthy men, bright light did not affect plasma glucose levels but increased fasting and postprandial triglyceride levels. (A) Fasting and postprandial glucose levels were not different between bright light and dim light. (B) Insulin levels were not different between bright and dim light. (C) C-peptide levels were not different between bright and dim light. (D) Fasting and postprandial triglyceride levels were elevated due to exposure to bright light compared with dim light. (E) Free fatty acid levels did not differ between bright light and dim light. (F) Heart rate did not differ between bright light and dim light in healthy men. (G) LF:HF ratio was slightly increased in bright light compared with dim light. Data are shown as mean ± SEM of 8 subjects per group. Asterisks indicate p < 0.05. Open circles are bright light and closed circles are dim light. The dashed line represents mealtime, and the shaded area indicates lights-off.
Table 2.
Metabolic effects of light in healthy men.
| Dim Light | Bright Light | p | ||
|---|---|---|---|---|
| Glucose | Fasting, mmol/L | 4.8 ± 0.1 | 4.8 ± 0.1 | 0.720 |
| AUC, mmol·min/L | 1555 ± 47 | 1590 ± 46 | 0.245 | |
| iAUC, mmol·min/L | 39 ± 40 | 82 ± 37 | 0.081 | |
| Insulin | Fasting, pmol/L | 54 (38-64) | 57 (47-62) | 0.528 |
| AUC, nmol·min/L | 55 (46-89) | 67 (55-76) | 0.401 | |
| iAUC, nmol·min/L | 41 (28-71) | 50 (37-58) | 0.484 | |
| C-peptide | Fasting, ng/L | 515 (428-609) | 505 (463-555) | 0.674 |
| AUC, µg·min/L | 332 (274-399) | 342 (286-404) | 1.000 | |
| iAUC, µg·min/L | 172 (130-216) | 190 (136-230) | 0.674 | |
| Triglyceride | Fasting, mmol/L | 0.71 (0.55-0.92) | 0.88 (0.63-1.07) | 0.050 |
| AUC, mmol·min/L | 307 (220-423) | 354 (301-404) | 0.050 | |
| iAUC, mmol·min/L | 65 (44-163) | 90 (42-128) | 0.674 | |
| FFA | Fasting, mmol/L | 0.23 (0.15-0.29) | 0.28 (0.15-0.43) | 0.400 |
| Area above the curve, mmol·min/L | 54 (37-112) | 65 (43-114) | 0.484 |
AUC = area under the curve; FFA = free fatty acid; iAUC = incremental area under the curve. Normally distributed data are expressed as mean ± SEM; nonnormally distributed data are expressed as median (25th-75th percentile).
Triglycerides and FFA
In the healthy men, fasting triglyceride levels and the postprandial triglyceride AUC were higher in bright light compared with dim light. The postprandial triglyceride iAUC did not differ between bright and dim light (Fig. 2D). Fasting and postprandial FFA levels were not affected by bright light compared with dim light (Fig. 2E).
Heart rate variability
Continuous Holter ECG recordings were obtained in both conditions for 7 of 8 subjects; recordings for 1 subject were missing due to a technical problem. Heart rate showed a different change over time between bright and dim light (Interaction, p = 0.001; Light, p = 0.897), but there was no significant difference between groups at any individual time point (Fig. 2F). The LF:HF ratio also showed a different change over time between bright and dim light (Interaction, p = 0.007; Light, p = 0.335). Analysis of individual time points showed that the LF:HF ratio was increased in bright light compared with dim light in the first hour (p = 0.018) and fifth hour (p = 0.028) after lights-on (Fig. 2G).
Men with Type 2 Diabetes
Participants
For the type 2 diabetes patient trial, 26 subjects were screened and 8 subjects were included (17 subjects did not meet inclusion criteria, 1 subject declined participation). All included subjects completed the study. The type 2 diabetes patient trial was performed between January and July 2014. Subject characteristics are shown in Table 3.
Table 3.
Characteristics of men with type 2 diabetes (n = 8).
| Characteristic | |
|---|---|
| Age, years | 60 (54-63) |
| BMI, kg/m2 | 30 (28-35) |
| HbA1c, % | 6.8 (6.7-8.0) |
| HbA1c, mmol/mol | 51 (50-65) |
| Fasting glucose, mmol/L | 8.4 (6.2-10) |
| Fasting insulin, pmol/L | 119 (111-184) |
| Medication, n (%) | |
| Metformina | 8 (100) |
| Lipid-lowering drugs | 4 (50) |
| Antihypertensives | 3 (37.5) |
| Proton pump inhibitor | 1 (12.5) |
| Sleep parameters from diary | |
| Time of sleep onset | 0005 h ± 7 min |
| Time of sleep end | 0740 h ± 10 min |
Normally distributed data are expressed as mean ± SEM; nonnormally distributed data are expressed as median (25th-75th percentile).
Metformin was discontinued 3 days prior to each admission.
Glucose, insulin, C-peptide, glucagon, and cortisol
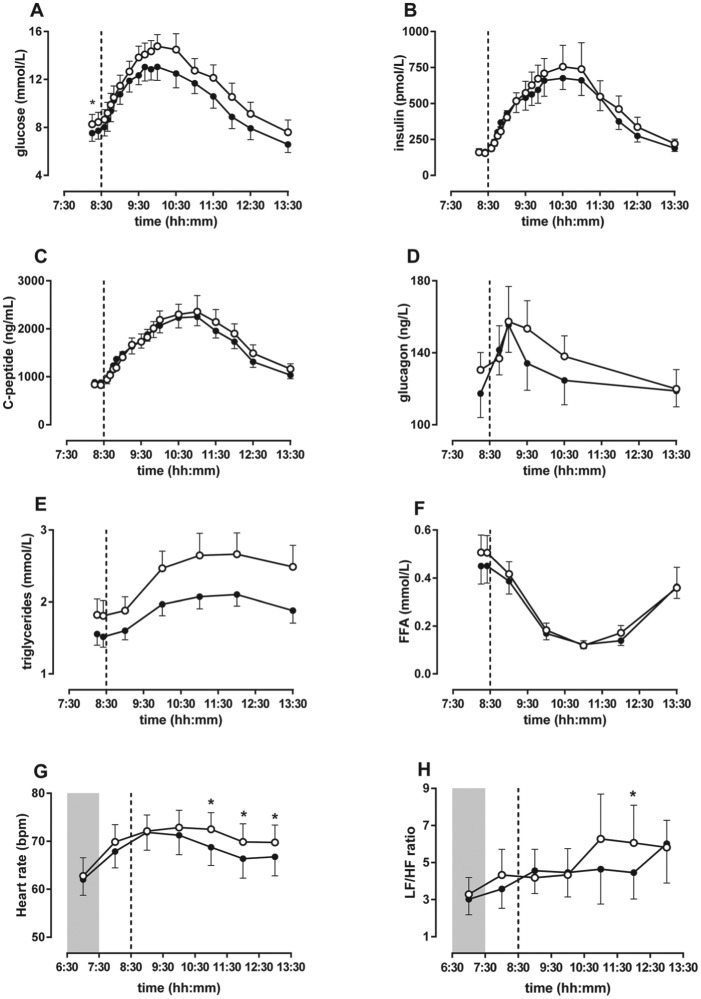
In the men with type 2 diabetes, fasting plasma glucose levels were elevated in bright light compared with dim light. The glucose AUC was increased after exposure to bright light compared with dim light, and the iAUC showed a similar trend (Fig. 3A and Table 4). Fasting and postprandial levels of insulin (Fig. 3B) and C-peptide (Fig. 3C) were not significantly different between bright and dim light. The C-peptide minimal model showed that the static time constant kg was lower in bright light compared with dim light (bright 1437 × 10-9 kg/min, dim 1564 × 10-9 kg/min, p = 0.04), indicating reduced static insulin production in bright light. The dynamic time constant kd was not different between bright light and dim light. Insulin sensitivity as assessed with the oral glucose minimal model was not different between bright light and dim light (see online supplemental materials for details). Fasting and postprandial plasma glucagon levels (Fig. 3D) and fasting and postprandial saliva cortisol levels (Table 4) were not different between bright and dim light.
Figure 3.
In men with type 2 diabetes, bright light increased fasting and postprandial glucose levels and postprandial triglyceride levels. (A) Fasting and postprandial glucose levels were elevated due to exposure to bright light compared with dim light. (E) Postprandial triglyceride levels were also elevated due to bright light. However, fasting and postprandial (B) insulin, (C) C-peptide, (D) glucagon, and (F) free fatty acid levels did not differ between bright and dim light. (G) Heart rate was increased in bright light compared with dim light. (H) LF:HF ratio was slightly increased in bright light compared with dim light. Data are shown as mean ± SEM of 8 subjects per group. Asterisks indicate p < 0.05. Open circles are bright light and closed circles are dim light. The dashed line represents mealtime, and the shaded area indicates lights-off.
Table 4.
Metabolic effects of light in men with type 2 diabetes.
| Dim Light | Bright Light | p | ||
|---|---|---|---|---|
| Glucose | Fasting, mmol/L | 7.6 ± 0.7 | 8.4 ± 0.8 | 0.013 |
| AUC, mmol·min/L | 3194 ± 279 | 3567 ± 299 | 0.002 | |
| iAUC, mmol·min/L | 791 ± 80 | 932 ± 80 | 0.075 | |
| Insulin | Fasting, pmol/L | 160 (125-193) | 146 (107-211) | 0.400 |
| AUC, nmol·min/L | 124 (105-150) | 124 (106-177) | 0.161 | |
| iAUC, nmol·min/L | 80 (61-101) | 88 (66-120) | 0.123 | |
| C-peptide | Fasting, ng/L | 905 (765-993) | 800 (683-1005) | 0.208 |
| AUC, µg·min/L | 477 (448-624) | 522 (464-604) | 0.401 | |
| iAUC, µg·min/L | 272 (168-304) | 258 (222-357) | 0.208 | |
| Glucagon | Fasting, ng/L | 128 (85-150) | 124 (109-152) | 0.123 |
| AUC, µg·min/L | 38.5 (32.0-49.7) | 40.6 (34.1-50.0) | 0.161 | |
| iAUC, µg·min/L | 3.5 (0.3-4.6) | 0.6 (-3.0-4.1) | 0.484 | |
| Triglyceride | Fasting, mmol/L | 1.40 (1.28-1.62) | 1.66 (1.27-2.46) | 0.161 |
| AUC, mmol·min/L | 580 (504-661) | 797 (509-926) | 0.012 | |
| iAUC, mmol·min/L | 97 (75-158) | 124 (104-262) | 0.012 | |
| FFA | Fasting, mmol/L | 0.44 (0.26-0.66) | 0.54 (0.35-0.69) | 0.233 |
| Area above the curve, mmol·min/L | 80 (42-113) | 87 (58-114) | 0.575 | |
| Cortisol | Fasting, nmol/L | 6.80 ± 0.91 | 7.32 ± 1.51 | 0.691 |
| AUC, nmol·min/L | 1414 ± 169 | 1541 ± 185 | 0.313 | |
| iAUC, nmol·min/L | 804 ± 169 | 931 ± 185 | 0.313 |
AUC = area under the curve; FFA = free fatty acid; iAUC = incremental area under the curve. Normally distributed data are expressed as mean ± SEM, nonnormally distributed data are expressed as median (25th percentile-75th percentile).
Triglycerides and FFA
In the men with type 2 diabetes, fasting plasma triglyceride levels were not different between bright and dim light, but the postprandial levels were increased in bright light compared with dim light (Fig. 3E and Table 4). Fasting and postprandial FFA levels were not affected by light (Fig. 3F and Table 4).
Appetite scores
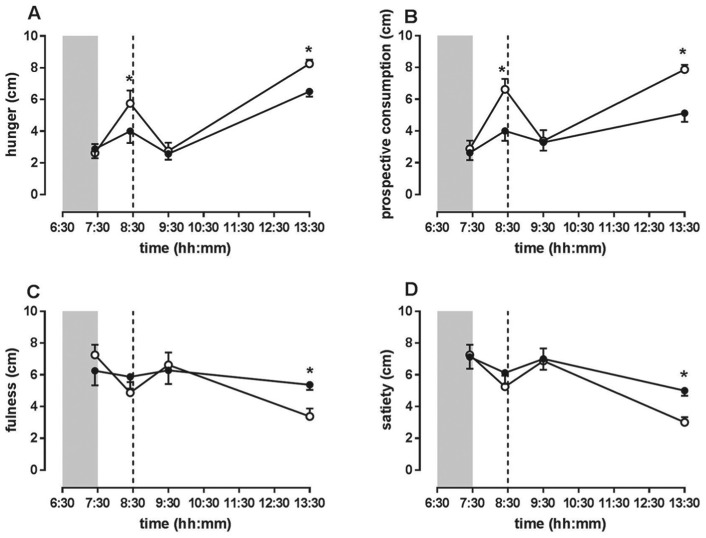
Before lights-on, the hunger, prospective food consumption, fullness, and satiety scores did not differ between bright light and dim light. After lights-on, fasting hunger and prospective food consumption scores were significantly higher in bright light compared with dim light. Five hours postprandially, hunger and prospective food consumption scores were significantly elevated in bright light while fullness and satiety scores were significantly decreased in bright light compared with dim light (Fig. 4).
Figure 4.
In men with type 2 diabetes, bright light increased appetite scores. During fasting and 5 h postprandially, (A) hunger and (B) prospective food consumption scores were elevated in bright light compared with dim light. (C) Fullness and (D) satiety scores were significantly decreased in bright light compared with dim light 5 h postprandially. Data are shown as mean ± SEM. Asterisks indicate p < 0.05. Open circles are bright light and closed circles are dim light. The dashed line represents mealtime, and the shaded area indicates lights-off.
Heart rate variability
Heart rate was increased in bright light compared with dim light (Interaction, p < 0.0001; Light, p < 0.0001), especially over the last 3 h (Fig. 3G). The LF:HF ratio showed a trend toward increase in bright light compared with dim light (Interaction, p < 0.0001; Light, p = 0.056), with a significant difference in the fifth hour after lights-on (p = 0.017) (Fig. 3H).
Discussion
In the present study, bright light did not affect plasma glucose levels in healthy men but increased fasting and postprandial plasma triglyceride levels. In men with type 2 diabetes, bright light increased fasting and postprandial glucose levels, postprandial triglyceride levels, and appetite scores.
Bright light increased fasting glucose levels in men with type 2 diabetes, which is due to either increased endogenous glucose production or decreased tissue glucose uptake during the first hour of bright light. Animal data suggest that bright light may increase hepatic glucose production, because in rats bright light caused increased expression of liver PEPCK, a rate-limiting gluconeogenetic enzyme (Cailotto et al., 2009). This effect of light on PEPCK was mediated by the autonomic nervous system (Cailotto et al., 2009), which is in line with other studies indicating that increased sympathetic signaling increases hepatic glucose production (Perseghin et al., 1997; Yi et al., 2009). Since bright light increased heart rate and tended to increase LF:HF ratio in men with type 2 diabetes in our study, we hypothesize that also in men with type 2 diabetes the light signal is transmitted from the brain to the liver via the autonomic nervous system. In addition to considering the potential role of the autonomic nervous system, we considered the glucocorticoid hormone cortisol as a candidate to transfer the light signal from the brain to the liver, because bright light increases glucocorticoid release via the SCN in rodents (Ishida et al., 2005). Human studies investigating the effects of bright morning light on cortisol yielded conflicting results; some studies showed increased cortisol levels after morning bright light (Scheer and Buijs, 1999; Leproult et al., 2001), whereas other studies showed no change in cortisol (Leproult et al., 1997) or showed decreased cortisol levels due to bright light (Jung et al., 2010). Since we did not find an effect of bright light on saliva cortisol levels in the men with type 2 diabetes, cortisol is probably not involved in the effects of bright light on fasting plasma glucose in the men with type 2 diabetes.
Bright light also increased postprandial glucose levels in men with type 2 diabetes, which is at least partly explained by reduced beta cell glucose sensitivity in bright light, as indicated by the static component of the C-peptide minimal model. Since increased sympathetic signaling toward the pancreas is thought to decrease insulin secretion (Rodriguez-Diaz and Caicedo, 2014), increased sympathetic activity during bright light could explain the decreased postprandial insulin secretion in men with type 2 diabetes. We observed no changes in whole body insulin sensitivity, as assessed with the oral glucose minimal model, that could explain the difference in postprandial plasma glucose between bright light and dim light. Furthermore, the pancreatic hormone glucagon was not affected by bright light and therefore not responsible for the increased glucose levels in bright light. Since we did not use a meal tracer, we cannot rule out that altered intestinal carbohydrate absorption (Sone et al., 2003) is partly responsible for the increased postprandial glucose levels in bright light in our study.
A recent study on the effects of morning exposure to blue-enriched room light (260 lux) compared with dim light (<20 lux) described no changes in plasma glucose but showed increased postprandial insulin levels due to blue-enriched room light (Cheung et al., 2016). The discrepancy between this published effect on insulin and our healthy subject data showing no effect of morning bright light on postprandial insulin levels may be due to differences in light intensity and wavelength, since we recently observed intensity- and wavelength-dependent effects of light on glucose tolerance in rodents (Opperhuizen et al., in press). Our second study provides the first evidence that bright light can actually affect plasma glucose levels in men with type 2 diabetes.
We performed 2 separate trials in different populations, and the trials were not designed to make a direct comparison between healthy men and obese men with type 2 diabetes. The study populations differ in age, body weight, and plasma glucose levels. Differences in outcome parameters between the study populations may be attributed to one or more of these factors.
It is however interesting to observe that the effects of bright light on triglycerides are similar between studies. Fasting triglyceride levels were significantly increased due to bright light in healthy men and showed a similar trend in men with type 2 diabetes. Elevated fasting triglyceride levels are due to either increased hepatic VLDL secretion or decreased tissue triglyceride uptake during the first hour of bright light. Since increased sympathetic signaling is known to increase hepatic VLDL secretion (Geerling et al., 2014) and we observed increased sympathetic activity due to bright light, increased VLDL secretion due to increased hepatic sympathetic signaling is a possible explanation for the observed increased fasting triglyceride levels. For postprandial triglyceride levels, increased intestinal absorption may be an additional contributing factor, given that the SCN controls intestinal lipid absorption (Hussain and Pan, 2015) and bright light affects human gastrointestinal motility (Sone et al., 2003). Our data suggest that the increased plasma triglyceride levels may persist for longer after the meal in the men with type 2 diabetes compared with the healthy men, which may be related to a prolonged intestinal transit time in patients with type 2 diabetes (Phillips et al., 2015).
The observed effects of bright light on appetite in men with type 2 diabetes contrast to a recent study showing no effect of exposure to morning blue-enriched light on appetite (Cheung et al., 2016); this again may be related to differences in light intensity, wavelength, or subject gender between the studies. The underlying pathway through which light affects appetite remains speculative. Appetite is controlled by a complex interplay of hormones, neurotransmitters, and neuropeptides (Morton et al., 2006). The hypothalamic neuropeptide orexin is a candidate, since orexin neurons are activated by light, and orexin increases appetite as well as sympathetic tone (Girault et al., 2012). The physiological role of increased appetite, plasma glucose levels, and triglyceride levels due to bright light exposure in the morning may be to mobilize energy in order to prepare the body for physical activity.
A limitation of our experimental setup is the absence of a fasting plasma sample before lights-on. The cannula was inserted after lights-on for 2 reasons. First, in order to prevent sleep disturbance due to the presence of a cannula, the cannula was not inserted in the evening. Second, in order to prevent a difficult insertion procedure in the dark or in dim red light, with resulting subject stress, the cannula was not inserted before lights-on in the morning. However, we would like to emphasize that despite the absence of a baseline measurement before lights-on, the observed changes in glucose and triglyceride metabolism are due to light, since all subjects were studied twice in a random order, and all conditions except light exposure remained constant between bright light and dim light.
The potential clinical implications of our findings are twofold. First, fasting glucose is a main clinical criterion for the diagnosis of type 2 diabetes (American Diabetes Association, 2015a) and fasting triglyceride levels can be used to assess cardiovascular risk (Nordestgaard and Varbo, 2014). We observed a difference between 10 lux and 4000 lux light exposure in fasting triglyceride in healthy men and in fasting glucose in men with type 2 diabetes. Therefore, our data warrant further investigation of the effects of intermediate light intensities on fasting plasma glucose and triglyceride levels and the practical implications for diagnostic blood sampling. Second, in the treatment of patients with type 2 diabetes, strategies to reduce HbA1c are effective to reduce microvascular and cardiovascular complications (American Diabetes Association, 2013). Fasting and postprandial glucose levels together determine HbA1c levels (American Diabetes Association, 2015b) and were acutely elevated due to bright light exposure in our study. In addition, in a noncontrolled environment, increased appetite due to bright light exposure will likely cause increased food intake. Thus, our results suggest that optimization of ambient light exposure is a potential strategy to reduce glycemia in patients with type 2 diabetes. Furthermore, if the observed effects of light exposure during the day (the appropriate phase) can be translated to the night (the inappropriate phase), our data suggest that ambient light may have a causal contribution to the observed correlations between nocturnal light exposure and metabolic disorders (Obayashi et al., 2013; McFadden et al., 2014; Obayashi et al., 2014; Gan et al., 2015). However, the metabolic effects of bright light at other times across the 24-h cycle, the metabolic effects of other light intensities and wavelengths, and the long-term effects of modified ambient light exposure on the prevention and treatment of hyperglycemia and dyslipidemia need to be evaluated in future clinical trials. It is important to note that long-term effects may differ from acute effects, as suggested by 2 case reports showing an improvement of insulin sensitivity with morning light treatment in patients with seasonal affective disorder and insulin dependent diabetes (Allen et al., 1992; Nieuwenhuis et al., 2009).
In conclusion, we showed that bright morning light increases plasma triglyceride levels in healthy men and increases plasma triglyceride and glucose levels in obese men with type 2 diabetes. Our data support the concept that ambient light can modulate human plasma glucose and triglyceride levels.
Supplementary Material
Acknowledgments
This study was financially supported by STW OnTime (Project No. 12189), and D.J.S. was supported by a ZonMW Agiko stipendium (Project No. 92003592).
Supplementary material is available on the journal’s website at http://journals.sagepub.com/home/jbr/supplemental.
Footnotes
Author Contributions: R.I.V., D.J.S., E.F., A.K., M.J.S., S.E.F., and P.H.B. designed the clinical trials. R.I.V. and D.J.S. performed the clinical trials and wrote the manuscript. R.I.V., D.J.S., D.V., A.L. and M.T. analyzed data. G.Z. and A.K.S. performed the minimal models. All authors reviewed the manuscript. P.H.B. and S.E.F. are the guarantors of this work and, as such, had full access to all the data in the study, and take responsibility for the integrity of the data and the accuracy of the data analysis.
Conflict of Interest Statement: The author(s) have no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- American Diabetes Association (2013) Standards of medical care in diabetes—2013. Diabetes Care 36 Suppl 1:S11-S66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Diabetes Association (2015. A) Classification and diagnosis of diabetes. Diabetes Care 38 Suppl: S8-S16. [DOI] [PubMed] [Google Scholar]
- American Diabetes Association (2015. B) Glycemic targets. Diabetes Care 38 Suppl: S33-40. [DOI] [PubMed] [Google Scholar]
- Allen NH, Kerr D, Smythe PJ, Martin N, Osola K, Thompson C. (1992) Insulin sensitivity after phototherapy for seasonal affective disorder. Lancet 339:1065-1066. [PubMed] [Google Scholar]
- Benarroch EE. (2011) The melanopsin system: phototransduction, projections, functions, and clinical implications. Neurology 76:1422-1427. [DOI] [PubMed] [Google Scholar]
- Bergman RN, Ider YZ, Bowden CR, Cobelli C. (1979) Quantitative estimation of insulin sensitivity. Am J Physiol 236:E667-677. [DOI] [PubMed] [Google Scholar]
- Cailotto C, Lei J, van der Vliet J, van Heijningen C, van Eden CG, Kalsbeek A, Pevet P, Buijs RM. (2009) Effects of nocturnal light on (clock) gene expression in peripheral organs: a role for the autonomic innervation of the liver. PloS One 4:e5650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung IN, Zee PC, Shalman D, Malkani RG, Kang J, Reid KJ. (2016) Morning and evening blue-enriched light exposure alters metabolic function in normal weight adults. PloS One 11:e0155601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobelli C, Dalla Man C, Toffolo G, Basu R, Vella A, Rizza R. (2014) The oral minimal model method. Diabetes 63:1203-1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coomans CP, van den Berg SA, Houben T, van Klinken JB, van den Berg R, Pronk AC, Havekes LM, Romijn JA, van Dijk KW, Biermasz NR, Meijer JH. (2013) Detrimental effects of constant light exposure and high-fat diet on circadian energy metabolism and insulin sensitivity. FASEB J 27:1721-1732. [DOI] [PubMed] [Google Scholar]
- Dalla Man C, Caumo A, Basu R, Rizza R, Toffolo G, Cobelli C. (2004) Minimal model estimation of glucose absorption and insulin sensitivity from oral test: validation with a tracer method. 287:E637-643. [DOI] [PubMed] [Google Scholar]
- Dalla Man C, Caumo A, Cobelli C. (2002) The oral glucose minimal model: estimation of insulin sensitivity from a meal test. IEEE Trans Biomed Eng 49:419-429. [DOI] [PubMed] [Google Scholar]
- Dalla Man C, Rizza RA, Cobelli C. (2007) Meal simulation model of the glucose-insulin system. IEEE Trans Biomed Eng 54:1740-1749. [DOI] [PubMed] [Google Scholar]
- Danilenko KV, Mustafina SV, Pechenkina EA. (2013) Bright light for weight loss: results of a controlled crossover trial. Obes Facts 6:28-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharani R, Lee CF, Theng ZX, Drury VB, Ngo C, Sandar M, Wong TY, Finkelstein EA, Saw SM. (2012) Comparison of measurements of time outdoors and light levels as risk factors for myopia in young Singapore children. Eye 26:911-918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunai A, Novak M, Chung SA, Kayumov L, Keszei A, Levitan R, Shapiro CM. (2007) Moderate exercise and bright light treatment in overweight and obese individuals. Obesity 15:1749-1757. [DOI] [PubMed] [Google Scholar]
- Eaton RP, Allen RC, Schade DS, Erickson KM, Standefer J. (1980) Prehepatic insulin production in man: kinetic analysis using peripheral connecting peptide behavior. J Clin Endocrinol Metab 51:520-528. [DOI] [PubMed] [Google Scholar]
- Flint A, Raben A, Blundell JE, Astrup A. (2000) Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int J Obes Relat Metab Disord 24:38-48. [DOI] [PubMed] [Google Scholar]
- Fonken LK, Workman JL, Walton JC, Weil ZM, Morris JS, Haim A, Nelson RJ. (2010) Light at night increases body mass by shifting the time of food intake. Proc Natl Acad Sci U S A 107:18664-18669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan Y, Yang C, Tong X, Sun H, Cong Y, Yin X, Li L, Cao S, Dong X, Gong Y, et al. (2015) Shift work and diabetes mellitus: a meta-analysis of observational studies. Occup Environ Med 72:72-78. [DOI] [PubMed] [Google Scholar]
- Geerling JJ, Boon MR, Kooijman S, Parlevliet ET, Havekes LM, Romijn JA, Meurs IM, Rensen PC. (2014) Sympathetic nervous system control of triglyceride metabolism: novel concepts derived from recent studies. J Lipid Res 55:180-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girault EM, Yi CX, Fliers E, Kalsbeek A. (2012) Orexins, feeding, and energy balance. Prog Brain Res 198:47-64. [DOI] [PubMed] [Google Scholar]
- Hussain MM, Pan X. (2015) Circadian regulators of intestinal lipid absorption. J Lipid Res 56:761-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida A, Mutoh T, Ueyama T, Bando H, Masubuchi S, Nakahara D, Tsujimoto G, Okamura H. (2005) Light activates the adrenal gland: timing of gene expression and glucocorticoid release. Cell Metab 2:297-307. [DOI] [PubMed] [Google Scholar]
- Jung CM, Khalsa SB, Scheer FA, Cajochen C, Lockley SW, Czeisler CA, Wright KP., Jr. (2010) Acute effects of bright light exposure on cortisol levels. J Biol Rhythms 25:208-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooijman S, van den Berg R, Ramkisoensing A, Boon MR, Kuipers EN, Loef M, Zonneveld TC, Lucassen EA, Sips HC, Chatzispyrou IA, et al. (2015) Prolonged daily light exposure increases body fat mass through attenuation of brown adipose tissue activity. Proc Natl Acad Sci U S A 112:6748-6753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyba CC, Tong KP, Bennie J, Birriel I, Birriel JJ, Cool A, Danielsen A, Davies TW, Outer PN, Edwards W, et al. (2015) Worldwide variations in artificial skyglow. Sci Rep 5:e8409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leproult R, Colecchia EF, L’Hermite-Balériaux M, Van Cauter E. (2001) Transition from dim to bright light in the morning induces an immediate elevation of cortisol levels. J Clin Endocrinol Metab 86:151-157. [DOI] [PubMed] [Google Scholar]
- Leproult R, Van Reeth O, Byrne MM, Sturis J, Van Cauter E. (1997) Sleepiness, performance, and neuroendocrine function during sleep deprivation: effects of exposure to bright light or exercise. J Biol Rhythms 12:245-258. [DOI] [PubMed] [Google Scholar]
- McFadden E, Jones ME, Schoemaker MJ, Ashworth A, Swerdlow AJ. (2014) The relationship between obesity and exposure to light at night: cross-sectional analyses of over 100,000 women in the Breakthrough Generations Study. Am J Epidemiol 180:245-250. [DOI] [PubMed] [Google Scholar]
- Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. (2006) Central nervous system control of food intake and body weight. Nature 443:289-295. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis RF, Spooren PF, Tilanus JJ. (2009) [Less need for insulin, a surprising effect of phototherapy in insulin-dependent diabetes mellitus]. Tijdschr Psychiatr 51:693-697. [PubMed] [Google Scholar]
- Nordestgaard BG, Varbo A. (2014) Triglycerides and cardiovascular disease. Lancet 384:626-635. [DOI] [PubMed] [Google Scholar]
- Obayashi K, Saeki K, Iwamoto J, Ikada Y, Kurumatani N. (2014) Independent associations of exposure to evening light and nocturnal urinary melatonin excretion with diabetes in the elderly. Chronobiol Int 31:394-400. [DOI] [PubMed] [Google Scholar]
- Obayashi K, Saeki K, Iwamoto J, Okamoto N, Tomioka K, Nezu S, Ikada Y, Kurumatani N. (2013) Exposure to light at night, nocturnal urinary melatonin excretion, and obesity/dyslipidemia in the elderly: a cross-sectional analysis of the HEIJO-KYO Study. J Clin Endocrinol Metab 98: 337-344. [DOI] [PubMed] [Google Scholar]
- Opperhuizen AL, Stenvers DJ, Jansen RD, Foppen E, Fliers E, Kalsbeek A. (in press). Light at night acutely impairs glucose tolerance in a time- intensity- and wavelength-dependent manner in rats. Diabetologia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perseghin G, Regalia E, Battezzati A, Vergani S, Pulvirenti A, Terruzzi I, Baratti D, Bozzetti F, Mazzaferro V, Luzi L. (1997) Regulation of glucose homeostasis in humans with denervated livers. J Clin Invest 100:931-941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips LK, Deane AM, Jones KL, Rayner CK, Horowitz M. (2015) Gastric emptying and glycaemia in health and diabetes mellitus. Nat Rev Endocrinol 11:112-128. [DOI] [PubMed] [Google Scholar]
- Potse M, Linnenbank AC, Grimbergen CA. (2002) Software design for analysis of multichannel intracardial and body surface electrocardiograms. Comput Methods Programs Biomed 69:225-236. [DOI] [PubMed] [Google Scholar]
- Reid KJ, Santostasi G, Baron KG, Wilson J, Kang J, Zee PC. (2014) Timing and intensity of light correlate with body weight in adults. PloS One 9:e92251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Diaz R, Caicedo A. (2014) Neural control of the endocrine pancreas. Best Pract Res Clin Endocrinol Metab 28:745-756. [DOI] [PubMed] [Google Scholar]
- Scheer FA, Buijs RM. (1999) Light affects morning salivary cortisol in humans. J Clin Endocrinol Metab 84:3395-3398. [DOI] [PubMed] [Google Scholar]
- Sone Y, Hyun KJ, Nishimura S, Lee YA, Tokura H. (2003) Effects of dim or bright-light exposure during the daytime on human gastrointestinal activity. Chronobiol Int 20:123-133. [DOI] [PubMed] [Google Scholar]
- Stenvers DJ, Jonkers CF, Fliers E, Bisschop PH, Kalsbeek A. (2012) Nutrition and the circadian timing system. Prog Brain Res 199:359-376. [DOI] [PubMed] [Google Scholar]
- Stenvers DJ, Schouten LJ, Jurgens J, Endert E, Kalsbeek A, Fliers E, Bisschop PH. (2014) Breakfast replacement with a low-glycaemic response liquid formula in patients with type 2 diabetes: a randomised clinical trial. Br J Nutr 112:504-512. [DOI] [PubMed] [Google Scholar]
- Tarvainen MP, Niskanen JP, Lipponen JA, Ranta-Aho PO, Karjalainen PA. (2014) Kubios HRV—heart rate variability analysis software. Comput Methods Programs Biomed 113:210-220. [DOI] [PubMed] [Google Scholar]
- Valdes CT, Elkind-Hirsch KE. (1991) Intravenous glucose tolerance test-derived insulin sensitivity changes during the menstrual cycle. J Clin Endocrinol Metab 72:642-646. [DOI] [PubMed] [Google Scholar]
- Van Cauter E, Mestrez F, Sturis J, Polonsky KS. (1992) Estimation of insulin secretion rates from C-peptide levels. Comparison of individual and standard kinetic parameters for C-peptide clearance. Diabetes 41:368-377. [DOI] [PubMed] [Google Scholar]
- Versteeg RI, Stenvers DJ, Kalsbeek A, Bisschop PH, Serlie MJ, la Fleur SE. (2016) Nutrition in the spotlight: metabolic effects of environmental light. Proc Nutr Soc 75:451-163. [DOI] [PubMed] [Google Scholar]
- Yeung EH, Zhang C, Mumford SL, Ye A, Trevisan M, Chen L, Browne RW, Wactawski-Wende J, Schisterman EF. (2010) Longitudinal study of insulin resistance and sex hormones over the menstrual cycle: the BioCycle Study. J Clin Endocrinol Metab 95:5435-5442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi CX, Serlie MJ, Ackermans MT, Foppen E, Buijs RM, Sauerwein HP, Fliers E, Kalsbeek A. (2009) A major role for perifornical orexin neurons in the control of glucose metabolism in rats. Diabetes 58:1998-2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.