Abstract
While it is now well known that social deprivation during early development permanently perturbs affective responding, accumulating evidence suggests that less severe restriction of the early social environment may also have deleterious effects. In the present report, we evaluate the affective responding of rhesus macaque (Macaca mulatta) infants raised by their mothers in restricted social environments or by their mothers in large social groups by indexing autonomic nervous system activity. Following a 25-hr evaluation of biobehavioral organization, electrocardiogram and an index of respiration were recorded for ten minutes. This allowed for an evaluation of both heart rate and respiratory sinus arrhythmia, an index of parasympathetic activity, during a challenging situation. Three to four month old infants raised in restricted social environments had significantly higher heart rates and lower respiratory sinus arrhythmia as compared to infants raised in unrestricted social environments, consistent with a more potent stress response to the procedure. These results are consistent with mounting evidence that the environment in which individuals are raised has important consequences for affective processing.
Keywords: Macaca mulatta, rhesus macaque, autonomic nervous system, respiratory sinus arrhythmia, heart rate, social environment
Decades of research with humans and nonhuman animals demonstrate that infants who develop in adverse environments experience life-long challenges in the ability to generate and regulate normal affective responses. The persisting impact of early life stress suggests there is a critical window during which affective experience and regulation may be programmed. Much of what is known about the impact of early life experience on affective development comes from studies on nonhuman primates raised without their mothers. While the role of mother is certainly critical, accumulating evidence suggests that it may not alone be sufficient for normal development. Rather, early development in the context of a social group may also be important for the development of affective processing, through, for example, the process of peer play and via relationships developed with kin. The goal of the present study was to evaluate whether variation in early environment, including the number of individuals in one’s social group, influenced a translational index of affective processing – activity of the parasympathetic nervous system – in infant rhesus macaques (Macaca mulatta).
Following from the hallmark work of Harlow (e.g., Harlow & Harlow, 1965; Ruppenthal, Arling, Harlow, Sackett, & Suomi, 1976; Suomi, Collins, Harlow, & Ruppenthal, 1976), restriction of the social environment early in development is now recognized as a stressor. Compared to infants raised with their mothers, infant macaques who are raised without their mothers evidence more frequent abnormal and self-directed behaviors and stereotypies (e.g., Champoux, Metz, & Suomi, 1991; Gottlieb, Capitanio, & McCowan, 2013; Winslow, Noble, Lyons, Sterk, & Insel, 2003) and are less socially engaged (Chamove, Rosenblum, & Harlow, 1973; Champoux, et al., 1991; Winslow, et al., 2003). They generate more frequent affective behaviors (e.g., they spasm and vocalize more frequently: Capitanio, Mason, Mendoza, Del Rosso, & Roberts, 2006; Champoux, et al., 1991; Gottlieb & Capitanio, 2013) and are more perturbed and harder to console when handled (Champoux et al., 2002). While some of these differences normalize as animals age (e.g., hair cortisol levels, Dettmer, Novak, Suomi, & Meyer, 2012; growth, pregnancy outcomes, Sackett, Ruppenthal, & Davis, 2002) others persist into adulthood (Capitanio, et al., 2006; Chamove, et al., 1973; Novak, 2003) suggesting that some features of affective reactivity are programed early in development.
While the behavioral consequences of early social isolation are clear (Capitanio, 1986), less clear is the impact of a restricted early social environment on the development of affective reactivity. A small number of studies document that infants raised with their mothers (who may or may not have an adult pair-mate), but in the absence of a social group, experience alterations in their behavioral reactivity compared to those raised with their mothers in social groups. For example, compared to 3–4 month old monkeys raised with their mothers in large social groups, when presented with a threatening stimulus (i.e., an unfamiliar human), infants raised by their mothers in restricted social environments were significantly more affectively reactive (i.e., the behavioral factor called “emotionality” (Gottlieb & Capitanio, 2013), evidenced greater anxiety-related behaviors (i.e., yawning and scratching; Karere et al., 2009) and were more physically active (Capitanio, et al., 2006; Gottlieb & Capitanio, 2013). Further, unlike 3–4 month old infants raised with their mothers in large social groups, infants raised by their mothers in restricted social environments did not show the expected dexamethasone suppression of cortisol activity (Capitanio, et al., 2006). Finally, the frequency of stereotypic behaviors increases as the early social rearing environment becomes more restricted such that animals raised in small social groups evidence greater frequencies of stereotypies than animals raised in large social groups, and animals raised with their mothers in restricted social environments or are peer-reared in the nursery show significantly greater numbers of stereotypies than those raised in social groups (Gottlieb, et al., 2013). Whether affective processing differences arise in other domains remains a question, as does the extent to which these differences are translatable across species. Importantly, restricted social environments also may differ from robust social environments in terms of other features (e.g., being indoors versus outdoors, having greater contact with humans, amount of available space) which themselves may influence behavioral variation. The goal of the present study was to investigate in infant rhesus monkeys the efficacy of a translational tool that we have proposed could be of great value for evaluating individual differences in affective processing in nonhuman primates – the measurement of the activity of the parasympathetic and sympathetic branches of the autonomic nervous system (Bliss-Moreau, Machado, & Amaral, 2013). Affect is an ongoing state characterized by some degree of valence (hedonics) and arousal that is present in all animals and forms the basis of emotions in humans (Barrett & Bliss-Moreau, 2009; Bliss-Moreau, submitted). Physiological states instantiated by the autonomic nervous system are thought be critical for the experience of affect, giving “color” to experience (Barrett & Bliss-Moreau, 2009; Craig, 2003; Critchley & Nagai, 2012). By translational tool, we mean a tool or measurement that can be deployed without modification in multiple species (allowing for cross-species translation) to measure the same phenomenon. To that end, we indexed activity of the parasympathetic branch of the autonomic nervous system by computing respiratory sinus arrhythmia (RSA) in infants raised by mothers in their social groups versus those raised by mothers alone. Activity of the parasympathetic nervous system, and in particular RSA, has long been recognized to be important for affective and social functioning (Cacioppo, Bernston, Larsen, Poehlmann, & Ito, 2000; Porges, 2007) and accumulating evidence points to stable individual differences in its activity that relate to socioaffective phenotypes (e.g. extraversion) in humans. In particular, people who experience more positive affect and are more social (i.e., those high in extraversion) have higher RSA than those who experience relatively less positive affect and are less social (i.e., those low in extraversion) (Kogan et al., 2014; Oveis et al., 2009; Wang, Lu, & Qin, 2013). While two older reports of research with nonhuman primates document changes in momentary RSA in response to either social grooming (i.e., leading to an increase in RSA; Aureli, Preston, & de Waal, 1999) or the presence of a discrete stressors (i.e., leading to a decrease in RSA; Shively et al., 2007) only recently has variance in RSA across the affective spectrum (ranging from negative to positive) been documented in rhesus macaques (Bliss-Moreau, et al., 2013) opening the possibility that activity of the ANS could be a valuable translational tool. To further explore the utility of this tool, we hypothesized that infants living with their mothers alone in indoor caging would have lower RSA than infants living with their mothers in large social groups outdoors, based on their increased affective reactivity documented in previous studies (e.g., Gottlieb & Capitanio, 2013). Because RSA is calculated from electrocardiogram data, we were also able to evaluate rearing condition influences on heart rate which reflects a blend of parasympathetic and sympathetic activity. We hypothesized that infants raised with their mothers in restricted environments would have higher heart rates than those raised in with their mothers in large social groups.
Methods
All procedures were approved by the Institutional Animal Care and Use Committee of the University of California, Davis.
Subjects
Subjects were 14 female rhesus macaque infants (Mage = 104.93 days; Rangeage = 92 to 115 days) who participated in the 25-hour-long BioBehavioral Assessment (BBA) Program at the California National Primate Research Center immediately prior to the data collection described below. (BBA data are not presented in this report.) Because we were limited in the number of animals we could assess, and because it was unknown whether there would be sex differences in these measures, we elected to power the study to evaluate rearing group differences by sampling only female infants. BBA testing procedures include behavioral observations in the home-cage, the Human Intruder testing, a video playback test, a visual memory test, and four blood draws, distributed across the 25-hr period (see details in Golub et al., 2009). A total of 203 subjects (97 females) completed the BBA program during the season in which the present study was conducted. Of those, 153 (79 female) were born to mothers living in the large outdoor social groups (0.2hA, ~60–150 animals per cage) and 22 (9 female) were born to mothers living indoors in standard primate caging with or without a social partner– the remaining 28 were born to mothers living in small outdoor social groups. Animals in the BBA program were tested in cohorts of up to 8 animals.
We selected 2 animals per available cohort (i.e., cohorts comprising animals not involved with other projects) to enroll in this study. Subjects for the present study were selected randomly from infants meeting the following criteria: 1) they were female, 2) they were not enrolled on any other projects, 3) they were raised either outdoors in the large social groups or by their mothers in a restricted social environment. In total, we tested 8 infants raised in the large social groups and 6 infants raised by their mothers in restricted social environments.
Procedure
Testing occurred immediately following the BBA testing so as not to influence standardized BBA procedures. Subjects’ chests were shaved for electrode placement in the BBA testing room by a trained animal technician. Infants were then transported in a standard infant transport box (30cm W × 30cm H × 32cm D) to the physiological laboratory. Seven human neonatal Ag-AgCl electrodes (Huggables, ConMed Corp., Utica, New York) were placed on each animal’s chest in a standard configuration (Bliss-Moreau, et al., 2013) for recording electrocardiogram and impedance cardiogram data. Subjects’ chests were wrapped in disposable adhesive bandage (VetWrap Bandaging Tape, Animal Care Products/3M, St. Paul, MN) to secure the electrodes in place and to prevent the infants from accessing the lead-wires. Subjects were placed in a modified infant transport box for testing (30cm W × 30cm H × 10cm D) – the box allowed for room to move (i.e., they were not restrained) but not enough room to become tangled in the lead-wires. The box was placed in a darkened, sound attenuated test room for data collection.
Electrocardiogram and impedance cardiogram data were recorded using MindWare Technologies (Gahana, OH) hardware and software with standard settings. Physiological data were recorded at 1000 Hz. We recorded two five-minute epochs of physiological data collection. Between the two epochs we introduced a challenge event by blowing a whistle (Rescue Howler, Survive Outdoors Longer™, Littleton, NY) and simultaneously playing a bike horn (Bugle horn, Bell Sports, Inc., Rantual, IL) for approximately 1 second. At the end of testing, infants were returned to the BBA staff to be reunited with their mothers via standard procedures.
Data Analysis Strategy
RSA was computed via standard scoring procedures using commercially available software (MindWare HRV3.0.25; MindWare Technologies Gahana OH). RSA was computed from the ECG data using the cardiac impedance signal (Zo)1 as the respiration signal according to standardized scoring procedures (Berntson et al., 1997). The ECG signal for each 15 s epoch was visually inspected. Artifacts were removed or corrected if possible, or epochs scored as missing data if artifacts could not be corrected. Additionally, proper placement of each R-spike was ensured. The data were detrended, tapered, and fast Fourier transformed prior to RSA computations. RSA was computed as the natural log integral of the high frequency power (0.24 to 1.04 Hz) that was set based on literature (Quigley & Stifter, 2006) and visual inspection of the respiration frequency for this sample. Heart rate was computed as the number of R-spikes per minute. Data were averaged across epoch within each 5 min block.
Normality of the data was assessed using Kolmogorov-Smirnov and Shapiro-Wilk tests. Heart rate was normally distributed but RSA was not. As a result, we elected to log10+1 transform RSA, which rendered its distribution normal. Raw RSA means are presented in the figures for ease of interpretation.
Results
Heart Rate
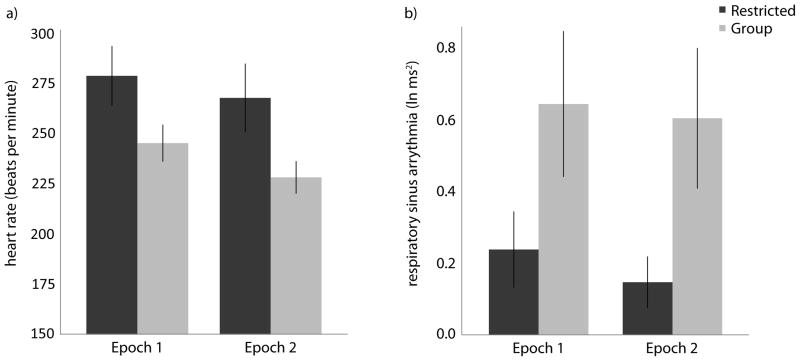
Consistent with our hypotheses, there was a significant effect of rearing condition on heart rate, F(1, 12)=4.92, p<0.05, ηp2=0.29 (Figure 1A). Monkeys reared with their mothers in social groups had significantly slower heart rates than monkeys reared with their mothers in restricted social environments. There was also a significant effect of epoch on heart rate, F(1, 12)=11.76, p<0.01, ηp2=.495. All animals had slower heart rates during the second as compared to the first epoch. The rearing condition X epoch interaction was not significant, F(1,12)=0.548, p=0.47, ηp2=0.04.
Figure 1.
Cardiac data collected from 3–4 month old infants raised by their mothers in restricted social environments (Restricted, dark bars) or by their mothers in large social groups (Group, light bars), during the first 5-minutes of testing (Epoch 1) and following the challenge event during the second 5-minutes of testing (Epoch 2). Panel A depicts heart rate data and Panel B depicts RSA data. Error bars represent SEM.
Respiratory Sinus Arrhythmia
Consistent with our hypotheses, there was a significant effect of rearing condition on RSA, F(1, 12)=4.53, p≤0.05, ηp2=0.27 (Figure 1B). Monkeys reared with their mothers in social groups had significantly higher RSA than monkeys reared with their mothers in restricted social environments. Neither the effect of epoch nor the epoch X rearing condition effects were significant, F(1,12)=0.37, p=0.56, ηp2=.029, and F(1,12)=0.12, p=0.74, ηp2=0.01, respectively.
Discussion
Consistent with our hypotheses, infants raised by their mothers in restricted social environments had higher heart rates and lower respiratory sinus arrhythmia than infants raised by their mothers in large social groups during our 10-min procedure. This suggests that infants raised by their mother in restricted social environments, compared to those raised in groups, either came into the challenging context with lower resting parasympathetic activity, responded more robustly to the challenging context, or some combination of the two. While we intended the loud noise presented after 5 minutes to be a challenging event, all animals’ heart rates actually decreased from the first to second epoch. One possible reason for this is that the data were collected following the BBA, in a context that itself was a challenging event. It is possible, even probable, that this context produced ceiling effects – that is, ANS activity reflected conditions of stress such that the impact of the noise challenge was negligible. Given that heart rate decreased from Epoch 1 to Epoch 2, but there was not a significant difference in RSA, it is possible that the heart rate deceleration was driven by changes in sympathetic rather than parasympathetic activity. Future studies will be able to evaluate this possibility by measuring cardiac impedance to compute pre-ejection period in order to index sympathetic activity.
Whether physiological activity indexed in this experiment represents state (i.e., momentary, stimulus driven) or trait (i.e., stable individual differences) variation is not entirely clear. A growing body of evidence in the human literature recognizes that physiological variation occurs on these two different scales. For example, while people may evidence lower parasympathetic activity during momentary stress inductions compared to baseline or control trials (for a review Mendes, 2009), people also vary in the magnitude of their parasympathetic activity at rest (with no apparent stimuli). For example, people high in extraversion – a personality trait characterized by high levels of positive affect and/or social engagement – have greater parasympathetic activity at rest reflecting trait variation (Kogan, et al., 2014; Oveis, et al., 2009; Wang, et al., 2013). Typically, protocols like the one we implemented are thought to index individual differences in responsivity because they do not employ specific tasks. Yet, it is critical to note that the experimental procedure itself might be considered a challenging event – infants needed to be shaved, handled to place the electrodes, and then were placed in a darkened test cage alone for the 10-minute test period. It is therefore possible that the observed variation represents differential magnitude of affect induction by the test procedure, rather than stable individual differences. Importantly, autonomic nervous system activity at rest (procedure-free) is typically the standard measure of basal individual differences. Future work that follows animals over time and contexts, and habituates them to the testing procedures to allow for the collection of true resting basal data, will be able to determine whether this is the case. Nevertheless, we believe that the induction should have been less, and not more, potent or stress-inducing for the animals reared by their mothers in restricted social environments because they were raised indoors, in housing rooms similar to the testing room (in terms of light, temperature, smell, etc.) and had greater experience with humans (closer contact with technicians cleaning, feeding, etc.). Yet it was those infants who evidenced higher heart rates and lower parasympathetic activity suggesting a more robust response to challenge.
The present findings are consistent with the growing literature that suggests that early affective processing is perturbed not only by severe experiences such as early social isolation (Capitanio, 1986; Harlow & Harlow, 1965) but also by more modest social restriction early in life (Capitanio, et al., 2006; Gottlieb & Capitanio, 2013; Gottlieb, et al., 2013). It is possible, even probable, that the greater affective reactivity observed in infants raised in restricted social environments, as compared to infants raised by their mothers in large social groups (i.e., Gottlieb & Capitanio, 2013) may be driven by variation in either their baseline autonomic nervous system activity (i.e., the homeostatic set points) or in their momentary autonomic responses to being placed in novel environments. Repeating the present experiment with a larger sample size will allow us to evaluate the relationships between variation in affective behavior during the BBA testing and variation in autonomic responding. The present sample was too small for reliable individual difference analyses.
One important limitation of the present work is that the sample size was small, although within the range of similar studies with nonhuman primates. The size of the sample was driven by the number of female subjects that were available, in concert with the timing restraints associated with the parent study. Infants participating in the BBA must be returned to their mothers very soon after the end of the 25-hour testing procedure. That meant that we had only a short period of time within each BBA session to hone our methods and evaluate infants. The small sample size precluded an evaluation of the relationship between physiological variables and the biobehavioral phenotypes identified in BBA because each rearing group was not powered for correlational or predictive analyses. We were, however, able to confirm that the general patterns of behavioral differences previously reported between rearing groups did also manifest in this group. For example, Gottlieb & Capitanio (2013) reported that infants raised by their mothers in restricted social environments, compared to those raised by their mothers in large social groups, were significantly more affectively reactive (i.e., the behavioral factor called “emotionality”) during the human intruder task – that pattern replicated in this group (Mann-Whitney U=9.0, p=0.039; Mmother-restricted=1.39, SDmother-restricted=1.85; Mmother-group=−0.21, SDmother-group=0.58). Expanding the sample size to evaluate the relationship between ANS activity and behavioral phenotypes is an important avenue for future research. We elected to test only female infants because the presence of sex differences in macaque autonomic nervous system activity is unknown and we sought to power the study to detect rearing differences. It will be critical to test males moving forward. Despite the small sample and being limited to female subjects, we believe that these data are important because of the increased focus on group-rearing macaque infants at research facilities and changing regulations on environmental conditions in which macaques live. As a result, the opportunity for similar evaluations may not be possible in the future. That being said, tracking individual differences across the variations in early social environments that do exist at primate facilities (e.g., infants raised by mothers with large versus small social networks) will be possible and should speak to the importance of enriched social environments for the early programing of affective processing. Future research need also consider how the other factors that differed between the restricted social environment and large social groups (e.g., indoor versus outdoor; temperature and light controlled or not; variation in contact with humans) influence autonomic nervous system activity in order to paint a complete picture of how variation in early environment shapes affective responding.
In conclusion, we believe that our data demonstrate the utility of using cardiovascular measures as a translational tool across species, inasmuch as our physiological results are consistent with the behavioral data (reported in the Introduction) showing that monkeys reared with their mothers but not in large social groups evidence altered affective processing from those raised with their mothers in large social groups. Specifically, we believe the present data provide additional evidence that, for other nonverbal species (or for humans that are pre-verbal), autonomic nervous system activity may be a sensitive window into understanding affective processes despite species differences in the specific behaviors executed during affective experience and regulation. Such a tool may have great value in understanding the evolution of affective processes in mammals.
Acknowledgments
The authors thank Laura Del Rosso and Laura Calonder for their assistance with this research. This research was supported by the funding from the National Institutes of Health: R24OD010962 to JPC; P51OD011107 to the California National Primate Research Center. EBM was funded by K99MH10138.
Footnotes
The impedance data were visually inspected for all subjects to ensure the integrity of low frequency variation that can be used as the respiration signal in the computation of RSA (Berntson et al, 1997). We have elected not to report indices from the impedance data in this report because the integrity of the first derivative of Zo was questionable for enough subjects that the power of the sample was compromised. Over the course of the study, we experimented with placement of the impedance electrodes such that we were able to maximize signal integrity of dZ/dt. Future studies will utilize this adapted protocol.
References
- Aureli F, Preston SD, de Waal FB. Heart rate responses to social interactions in free-moving rhesus macaques (Macaca mulatta): a pilot study. J Comp Psychol. 1999;113(1):59–65. doi: 10.1037/0735-7036.113.1.59. [DOI] [PubMed] [Google Scholar]
- Barrett LF, Bliss-Moreau E. Affect as a Psychological Primitive. Adv Exp Soc Psychol. 2009;41:167–218. doi: 10.1016/S0065-2601(08)00404-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berntson GG, Bigger JT, Jr, Eckberg DL, Grossman P, Kaufmann PG, Malik M, … van der Molen MW. Heart rate variability: origins, methods, and interpretive caveats. Psychophysiology. 1997;34(6):623–648. doi: 10.1111/j.1469-8986.1997.tb02140.x. [DOI] [PubMed] [Google Scholar]
- Bliss-Moreau E. Constructing nonhuman animal emotion. (submitted) [Google Scholar]
- Bliss-Moreau E, Machado CJ, Amaral DG. Macaque cardiac physiology is sensitive to the valence of passively viewed sensory stimuli. PLoS One. 2013;8(8):e71170. doi: 10.1371/journal.pone.0071170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacioppo JT, Bernston GG, Larsen JT, Poehlmann KM, Ito TA. The Psychophysiology of Emotion. In: Lewis M, Haviland-Jones JM, editors. Handbook of Emotions. 2. New York: Guilford Press; 2000. pp. 173–191. [Google Scholar]
- Capitanio JP. Behavioral pathology. In: Mitchell G, Erwin J, editors. Comparative Primate Biology: Volume 2A. Behavior, Conservation, and Ecology. New York: Alan R. Liss; 1986. pp. 411–454. [Google Scholar]
- Capitanio JP, Mason WA, Mendoza SP, DelRosso L, Robers JA. Nursery rearing and biobehavioral organization. In: Sackett GP, Ruppenthal G, Elizas K, editors. Nursery Rearing of nonhuman Primates in the 21st Century. New York: Springer; 2006. pp. 191–213. [Google Scholar]
- Chamove AS, Rosenblum LA, Harlow HF. Monkeys (Macaca mulatta) raised only with peers. A pilot study. Anim Behav. 1973;21(2):316–325. doi: 10.1016/s0003-3472(73)80073-9. [DOI] [PubMed] [Google Scholar]
- Champoux M, Bennett A, Shannon C, Higley JD, Lesch KP, Suomi SJ. Serotonin transporter gene polymorphism, differential early rearing, and behavior in rhesus monkey neonates. Mol Psychiatry. 2002;7(10):1058–1063. doi: 10.1038/sj.mp.4001157. [DOI] [PubMed] [Google Scholar]
- Champoux M, Metz B, Suomi SJ. Behavior of Nursery Peer-Reared and Mother-Reared Rhesus-Monkeys from Birth through 2 Years of Age. Primates. 1991;32(4):509–514. doi: 10.1007/Bf02381941. [DOI] [Google Scholar]
- Craig AD. Interoception: the sense of the physiological condition of the body. Curr Opin Neurobiol. 2003;13(4):500–505. doi: 10.1016/s0959-4388(03)00090-4. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Nagai Y. How Emotions Are Shaped by Bodily States. Emotion Review. 2012;4(2):163–168. doi: 10.1177/1754073911430132. [DOI] [Google Scholar]
- Dettmer AM, Novak MA, Suomi SJ, Meyer JS. Physiological and behavioral adaptation to relocation stress in differentially reared rhesus monkeys: hair cortisol as a biomarker for anxiety-related responses. Psychoneuroendocrinology. 2012;37(2):191–199. doi: 10.1016/j.psyneuen.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golub MS, Hogrefe CE, Widaman KF, Capitanio JP. Iron deficiency anemia and affective response in rhesus monkey infants. Developmental Psychobiology. 2009;51:47–59. doi: 10.1002/dev.20345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb DH, Capitanio JP. Latent variables affecting behavioral response to the human intruder test in infant rhesus macaques (Macaca mulatta) Am J Primatol. 2013;75(4):314–323. doi: 10.1002/ajp.22107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb DH, Capitanio JP, McCowan B. Risk factors for stereotypic behavior and self-biting in rhesus macaques (Macaca mulatta): animal’s history, current environment, and personality. Am J Primatol. 2013;75(10):995–1008. doi: 10.1002/ajp.22161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow HF, Harlow MK. The Effect of Rearing Conditions on Behavior. Int J Psychiatry. 1965;1:43–51. [PubMed] [Google Scholar]
- Karere GM, Kinnally EL, Sanchez JN, Famula TR, Lyons LA, Capitanio JP. What is an “adverse” environment? Interactions of rearing experiences and MAOA genotype in rhesus monkeys. Biol Psychiatry. 2009;65(9):770–777. doi: 10.1016/j.biopsych.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogan A, Oveis C, Carr EW, Gruber J, Mauss IB, Shallcross A, … Keltner D. Vagal activity is quadratically related to prosocial traits, prosocial emotions, and observer perceptions of prosociality. J Pers Soc Psychol. 2014;107(6):1051–1063. doi: 10.1037/a0037509. [DOI] [PubMed] [Google Scholar]
- Novak MA. Self-injurious behavior in rhesus monkeys: new insights into its etiology, physiology, and treatment. Am J Primatol. 2003;59(1):3–19. doi: 10.1002/ajp.10063. [DOI] [PubMed] [Google Scholar]
- Oveis C, Cohen AB, Gruber J, Shiota MN, Haidt J, Keltner D. Resting respiratory sinus arrhythmia is associated with tonic positive emotionality. Emotion. 2009;9(2):265–270. doi: 10.1037/a0015383. [DOI] [PubMed] [Google Scholar]
- Porges SW. The polyvagal perspective. Biological Psychology. 2007;74(2):116–143. doi: 10.1016/j.biopsycho.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley KS, Stifter CA. A comparative validation of sympathetic reactivity in children and adults. Psychophysiology. 2006;43(4):357–365. doi: 10.1111/j.1469-8986.2006.00405.x. [DOI] [PubMed] [Google Scholar]
- Ruppenthal GC, Arling GL, Harlow HF, Sackett GP, Suomi SJ. A 10-year perspective of motherless-mother monkey behavior. J Abnorm Psychol. 1976;85(4):341–349. doi: 10.1037//0021-843x.85.4.341. [DOI] [PubMed] [Google Scholar]
- Sackett GP, Ruppenthal GC, Davis AE. Survival, growth, health, and reproduction following nursery rearing compared with mother rearing in pigtailed monkeys (Macaca nemestrina) Am J Primatol. 2002;56(3):165–183. doi: 10.1002/ajp.1072. [DOI] [PubMed] [Google Scholar]
- Shively CA, Mietus JE, Grant KA, Goldberger AL, Bennett AJ, Willard SL. Effects of chronic moderate alcohol consumption and novel environment on heart rate variability in primates (Macaca fascicularis) Psychopharmacology. 2007;192(2):183–191. doi: 10.1007/s00213-007-0709-z. [DOI] [PubMed] [Google Scholar]
- Suomi SJ, Collins ML, Harlow HF, Ruppenthal GC. Effects of maternal and peer separations on young monkeys. J Child Psychol Psychiatry. 1976;17(2):101–112. doi: 10.1111/j.1469-7610.1976.tb00382.x. [DOI] [PubMed] [Google Scholar]
- Wang Z, Lu W, Qin R. Respiratory sinus arrhythmia is associated with trait positive affect and positive emotional expressivity. Biol Psychol. 2013;93(1):190–196. doi: 10.1016/j.biopsycho.2012.12.006. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Noble PL, Lyons CK, Sterk SM, Insel TR. Rearing effects on cerebrospinal fluid oxytocin concentration and social buffering in rhesus monkeys. Neuropsychopharmacology. 2003;28(5):910–918. doi: 10.1038/sj.npp.1300128. [DOI] [PubMed] [Google Scholar]