Abstract
Sortilin 1 (Sort1) is a trafficking receptor that has been implicated in the regulation of plasma cholesterol in humans and mice. Here, we use metabolomics and hyperinsulinemic-euglycemic clamp approaches to obtain further understanding of the in vivo effects of Sort1 deletion on diet-induced obesity as well as on adipose lipid and glucose metabolism. Results show that Sort1 knockout does not affect Western diet-induced obesity or adipose fatty acid and ceramide concentrations. Under the basal fasting state, chow-fed Sort1 knockout mice have decreased adipose glycolytic metabolites, but Sort1 deletion does not affect insulin-stimulated tissue glucose uptake during the insulin clamp. These results suggest that Sort1 loss-of-function in vivo does not affect obesity development, but differentially modulates adipose glucose metabolism under fasting and insulin-stimulated states.
Keywords: Obesity, diabetes, hyperlipidemia, cholesterol
Introduction
Sortilin 1 (Sort1) is one of the several vacuolar protein sorting 10 protein (VPS10P)-domain receptors. The VPS10P-domain receptors are single transmembrane receptors that bind various functionally–unrelated proteins and mediate their intracellular vesicular trafficking to the endocytic pathway either from the trans-Golgi apparatus or after endocytosis (1). Recent genome-wide association studies (GWAS) revealed that SORT1 gene was strongly associated with plasma low-density lipoprotein cholesterol concentrations and the risk of cardiovascular disease in large human populations (2, 3). In experimental models, hepatic Sort1 has been shown to modulate hepatic VLDL production and plasma lipoprotein clearance (4–10). Furthermore, Sort1 knockout mice showed reduced atherosclerosis, which may be attributed to attenuated macrophage activation (11, 12) and vascular calcification (13).
Sort1 is highly expressed in adipocytes. Previous studies identified Sort1 as an essential component of the GLUT4 storage vesicle (GSV) (14, 15). The N-terminal luminal domain of Sort1 interacts with Glut4, while the cytoplasmic tail of Sort1 interacts with adaptor proteins to facilitate GSV formation (16–21). It has been shown in both adipocytes and myocytes that Sort1 was required for insulin-dependent glucose uptake (20, 22–24). However, no studies so far have investigated the in vivo impact of Sort1 deficiency on glucose metabolism in fat tissues. Paradoxically, a more recent study showed that Sort1 KO mice were protected against high fat diet-induced obesity and showed improved insulin sensitivity in an insulin tolerance test, which was partially attributed to lower acid sphingomyelinase (aSMase) activity in the liver and the adipose tissue (25). Because obesity and adipose lipid and glucose metabolism profoundly impact hepatic lipid production and plasma lipid concentration, there is a clear need to clarify the role of Sort1 in the regulation of obesity and adipose lipid and glucose metabolism under physiological and pathological settings in vivo. Such knowledge is needed to improve current understanding of the novel role of Sort1 in the regulation of lipid metabolism and inflammation in health and disease processes.
In this study, we investigated the impact of Sort1 loss-of-function on obesity, adipose lipid and glucose metabolism by using metabolite analysis and hyperinsulinemic-euglycemic clamp study. Findings from this study showed that Sort1 KO mice had decreased adipose glycolytic metabolites at basal fasting state. However, Sort1 deficiency did not affect Western diet-induced obesity and adipose fatty acid and sphingolipid concentration, and had no significant effect on insulin-stimulated tissue glucose uptake during the hyperinsulinemic-euglycemic clamp study under either chow-fed condition or after diet-induced obesity.
Materials and Methods
Reagents
The antibodies against Sort1 (ab16640; Lot: GR64653-1), Glut4 (ab654; Lot: GR101575-1) and α-Tubulin (ab7291; Lot: GR122217-1) were purchased from Abcam (Cambridge, MA). The antibody against Histone 3 (9717; Lot: 8) was purchased from Cell Signaling Technology (Danvers, MA). The antibody against Actin (A5441; Lot: 063M4808) was purchased from Sigma (St. Louis, MO).
Mice
Global Sort1 KO mice were purchased from Taconic Biosciences Inc. as described previously (10). The Sort1 gene was silenced by gene trapping technology that inserted a stop codon into the second intron of the Sort1 gene. Mice were obtained on mixed background (129/SvEv/C57BL/6) and were backcrossed to C57BL/6J (The Jackson Lab) for multiple generations. Male F7 and F8 generations of Sort1 KO and WT littermates were used for this study. Western diet feeding was initiated when mice were 10 weeks of age. All mice were maintained on a standard chow diet and water ad libitum. The Western diet (TD.88137, Harlan Teklad) contains 21% milk fat (w/w) and 0.2% cholesterol. All animal protocols were approved by the Institutional Animal Care and Use Committee.
Western blot
Tissue homogenates were prepared in 1X RIPA buffer containing 1% SDS and protease inhibitor cocktail, and was incubated for 1 h on ice, followed by brief sonication. Protein concentrations were determined by a BCA assay kit (Rockford, IL). A representing blot is shown. Relative band intensity (normalized to loading controls) was determined by ImageJ software. Average band intensity was expressed as mean ± S.E.M..
Glucose tolerance test
Mice were fasted overnight and received a single intra-peritoneal injection of glucose at 2g/kg body weight. A drop of blood was collected from the tail and glucose was measured with a glucose monitor.
Analysis of tissue metabolites
This was performed by Metabolon Inc (Durham, NC). Several recovery standards were added based on tissue weight prior to sample extraction for quality control and data normalization. Extract was analyzed on four UPLC-MS/MS systems, utilizing: 1) positive ion mode, early elution; 2) positive ion mode, late elution; 3) negative ion mode and 4) polar-negative ion mode. All methods utilized a Waters ACQUITY UPLC and a Thermo Scientific Q-Exactive high resolution/accurate mass spectrometer interfaced with a heated electrospray ionization (HESI-II) source and Orbitrap mass analyzer operated at 35,000 mass resolution. Peaks were quantified using area-under-the-curve. Two-way ANOVA and t-test were used for statistical analysis.
Hyperinsulinemic-euglycemic clamp
Insulin clamps were performed at the Mouse Metabolic Phenotyping Center (MMPC) at the Vanderbilt University. Mice were maintained on chow or Western diet for 8 weeks. Catheters were implanted into a carotid artery and a jugular vein for sampling and infusions respectively five days before the study. The study was initiated in mice after fasting for 5 h. [3-3H]-glucose was continuously infused at 0.075 μCi/min for a 90 min equilibration and basal sampling period. [3-3H]-glucose was mixed with the non-radioactive glucose infusate (infusate specific activity of 0.5 μCi/mg glucose) during the 2 h clamp period. Arterial glucose was clamped using a variable rate of glucose infusion. Baseline plasma variables were calculated as the mean of values obtained in blood samples collected at 15 and 5 min prior insulin infusion (2.5 mU/Kg/min for chow-fed mice or 4 mU/kg/min for Western diet-fed mice). Blood was taken from 80–120 min for the determination of [3-3H]-glucose. Clamp insulin was determined at 100 min and 120 min. At 120 min, an intravenous bolus of 13 μCi 2-[14C]-deoxyglucose was administered. Blood was taken from 2–25min for determination of 2-[14C]-deoxyglucose. After the last sample, mice were anesthetized. Plasma insulin and c-peptide were determined by RIA. Radioactivity of [3-3H]-glucose, 2-[14C]-deoxyglucose and 2-[14C]-deoxyglucose -6-phosphate in plasma or tissues were determined by liquid scintillation counting. Glucose appearance (Ra) and disappearance (Rd) rates were determined using steady-state equations (26). Endogenous glucose appearance (endoRa) was determined by subtracting the glucose infusion rate (GIR) from total Ra. The glucose metabolic index (Rg) was calculated as previously described (27).
Statistical analysis
Results were expressed as mean ± S.E.M.. Statistical analysis was performed by Student’s t-test or ANOVA. A p < 0.05 was considered statistically significant.
Results
Sortilin 1 knockout did not affect Western diet-induced obesity, adipose fatty acid and sphingolipid metabolism
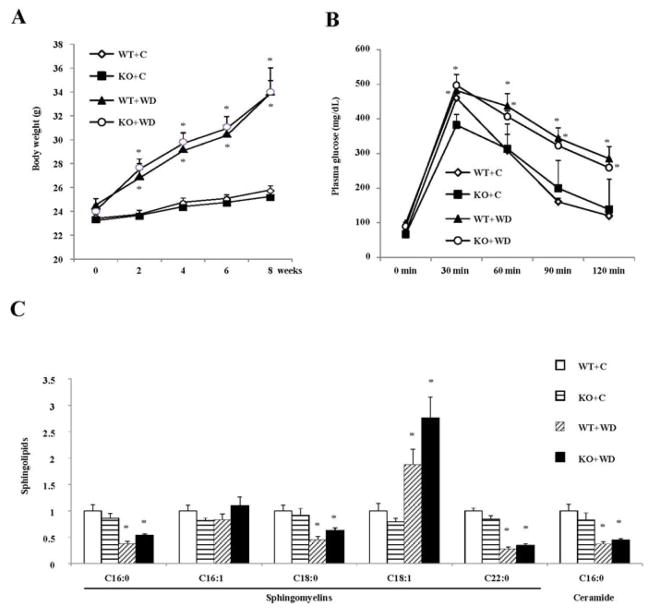
To investigate the role of Sort1 on diet-induced obesity, we fed WT and Sort1 KO mice a chow or a Western diet. We found that WT mice and Sort1 KO mice gained similar amount of body weight over the 8-week feeding period (Fig 1A). Glucose tolerance test also did not show significant difference in glucose tolerance between WT and Sort1 KO mice on either chow diet or Western diet (Fig 1B). These results are inconsistent with a recent study reporting that high fat diet-fed Sort1 KO mice showed lower body weight and decreased adipose aSMase enzyme activity (25). Ceramide signaling contributes to insulin resistance and impaired tissue glucose metabolism in obesity (28, 29). A previous in vitro study showed that Sort1 played a redundant role in addition to the mannose 6-phosphate receptor (M6PR) in mediating the lysosomal trafficking of aSMase that hydrolyzes sphingomyelins to ceramides (30). Thus it is thought that Sort1 deficiency may decrease the aSMase-mediated ceramide production in lysosomes. Here we showed that the relative concentrations of C16:0-ceramide, the principle ceramide species mediating the development of insulin resistance and obesity (28, 29), and its precursor C16:0 sphingomyelin were not different between Sort1 KO mice and WT controls under either chow-fed or Western diet-fed conditions (Fig 1C).
FIGURE 1. Sort1 knockout did not affect diet-induced obesity or adipose ceramide concentration in mice.
Mice were fed a chow diet (C) or a Western diet (WD) for 8 week. Mice were fasted overnight before sacrifice. A. Body weight. (n=4–9). B. Glucose tolerance test. (n=4–9). C. Metabolites were measured in epididymal fat of mice fed a chow or a Western diet for 8 week. Mice were fasted overnight before sacrifice. Y-axis: relative area under the curve with control (WT+C) set as “1”. n=4–6. All results are expressed as mean ± SEM. “*”, p< 0.05. vs. WT+C group.
Sortilin 1 knockout decreased glycolytic metabolites in adipose tissue under chow-fed fasting condition
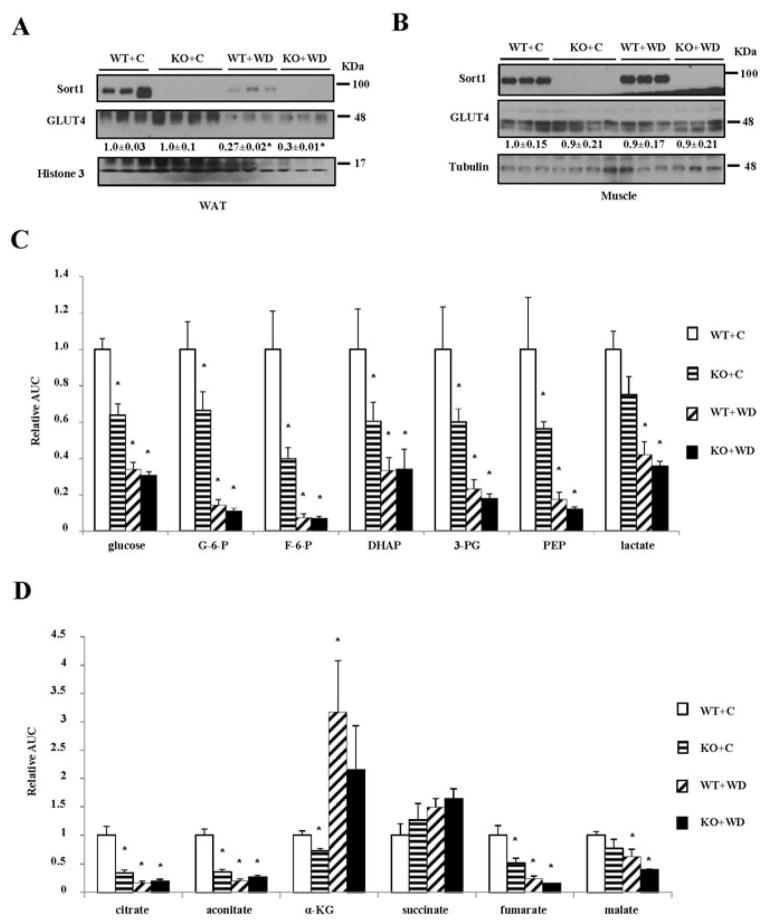
We next studied the impact of Sort1 loss-of-function on adipose glucose metabolism in mice. Consistent with a previous report (24), Western diet feeding decreased adipose Sort1 protein (Fig 2A). However, muscle Sort1 protein was not altered in Western diet-fed mice (Fig 2B). Glut4 protein was reduced in white adipose tissue but not muscle of Western diet-fed WT mice (Fig 2A, 2B), which was a known phenomenon (31). WT and Sort1 KO mice showed similar Glut4 protein levels in adipose and skeletal muscle (Fig 2A, 2B), suggesting that Sort1 loss-of-function did not affect Glut4 protein abundance in vivo.
FIGURE 2. Sort1 KO mice showed reduced adipose glycolytic and TCA cycle intermediates.
Mice were fed a chow or a Western diet for 8 week. Mice were fasted overnight before sacrifice. A, B. Western blot of Sort1 and Glut4 protein in epididymal fat and soleus muscle of WT and Sort1 KO mice fed a chow or a Western diet (WD). C. D. Relative concentrations of metabolites in the epididymal adipose tissue. Y-axis: relative area under the curve with control (WT+C) set as “1”. n=4–6. Results are expressed as mean ± SEM. “*”, p< 0.05. vs. WT+C group. G-6-P: glucose-6-phosphate; DHAP: dihydroxyacetone phosphate; 3-PG: glyceraldehyde 3-phosphate; PEP: phosphoenolpyruvate; α-KG: α-ketoglutarate.
Next, we measured the relative concentrations of glycolytic metabolites in the adipose of overnight fasted mice, which may reflect basal glucose metabolism under non-insulin stimulated state. Chow-fed Sort1 KO mice showed significantly reduced relative concentrations of glucose and all detected glycolytic intermediates except lactate (Fig 2C). Such changes closely mimicked the significantly decreased adipose glycolytic metabolites in Western diet-fed WT mice (Fig 2C), which was in line with impaired adipose glucose uptake in obesity. After Western diet feeding, WT and Sort1 KO mice no longer showed differential levels of glucose and glycolytic intermediates (Fig 2C), which could be due to the predominant reduction of the glycolytic metabolites caused by Western diet-induced obesity. To seek further evidence of reduced basal glycolysis in adipose of Sort1 KO mice, we measured the relative concentrations of the TCA cycle intermediates since the end glycolytic metabolite is a substrate for TCA cycle. Results in Fig 2D showed that the relative concentration of citrate, the product of the first reaction in the TCA cycle, was reduced by ~70% in chow-fed Sort1 KO mice compared to WT. In addition, the relative concentrations of several downstream TCA cycle intermediates aconitate, α-ketoglutarate and fumarate were also significantly decreased in chow-fed Sort1 KO mice (Fig 2D). Western diet feeding resulted in more reduction of citrate, aconitate, fumarate and malate in both WT and Sort1 KO mice (Fig 2D), which suggested that obesity was associated with decreased TCA cycle metabolites in the adipose tissues.
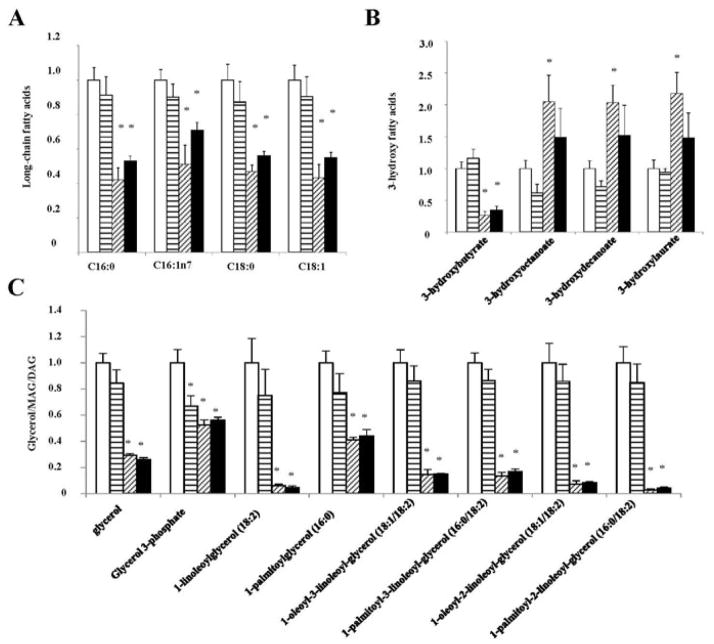
Altered cellular glycolysis may also affect fatty acid metabolism in the adipose tissues. However, no genotype-dependent differences in the relative concentrations of long-chain fatty acids, glycerol, monoacylglycerol (MAG) and diacylglycerol (DAG) were observed between WT and Sort1 KO mice under chow-fed or Western diet-fed conditions (Fig 3A, 3C). On the other hand, glycerol 3-phosphate, a significant amount of which is derived from glycolysis, was reduced in chow-fed Sort1 KO mice and also in Western diet-fed WT and Sort1 KO mice (Fig 3C). It is worth noting that Western diet significantly decreased ketone body 3-hydroxylbutyrate but increased other 3-hydroxy fatty acid species in WT mice (Fig 3B), which, in the absence of increased fatty acids, indicated impaired mitochondria fatty acid oxidation. Species of 3-hydroxy fatty acids and ketone body 3-hydroxybutyrate were not different between chow-fed WT and Sort1 KO mice (Fig 3B). These results suggest that, despite altered glycolytic activity, Sort1-deficiency did not affect adipose fatty acid metabolism. In addition, Western diet feeding strongly and consistently decreased major C16 and C18 long chain fatty acids as well as MAG, DAG and glycerol to similar levels in WT and Sort1 KO mice (Fig 3A, 3C), which may reflect a metabolic shift to increased triglyceride synthesis and fat storage as an adaptive response to higher dietary fat intake.
FIGURE 3. Sort1 knockout did not affect adipose fatty acid metabolism in mice.
Mice were fed a chow or a Western diet for 8 week. Mice were fasted overnight before sacrifice. A. Long chain fatty acid species. B. 3-hydroxy fatty acids. C. Glycerol, glycerol 3-phosphate, monoacylglyerol (MAG) and diacylglycerol (DAG). Y-axis: relative area under the curve with control (WT+C) set as “1”. n=4–6. Results are expressed as mean ± SEM. “*”, p< 0.05. vs. WT+C group.
Sort1 deficiency did not affect insulin-stimulated glucose uptake during insulin clamp in mice
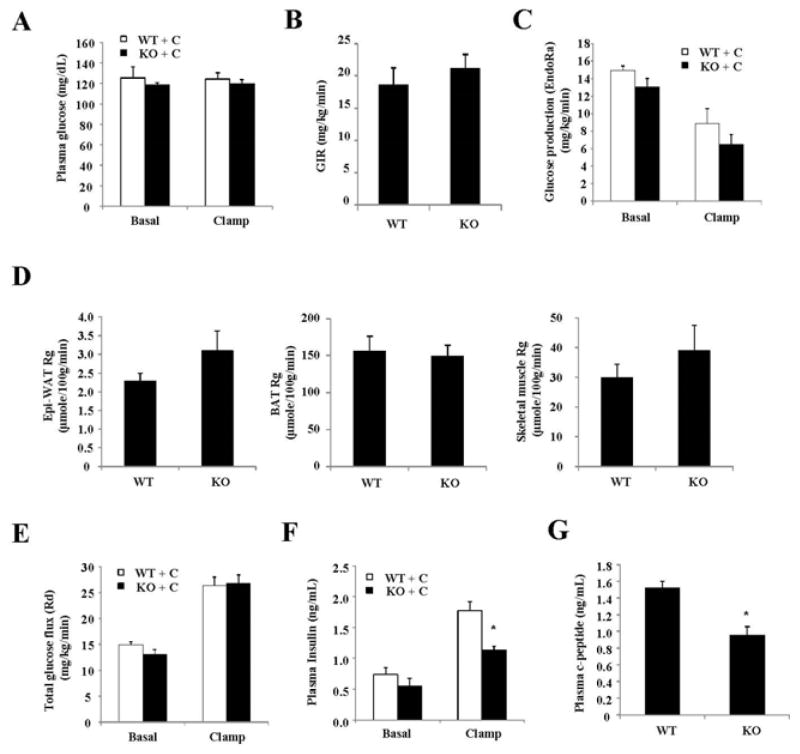
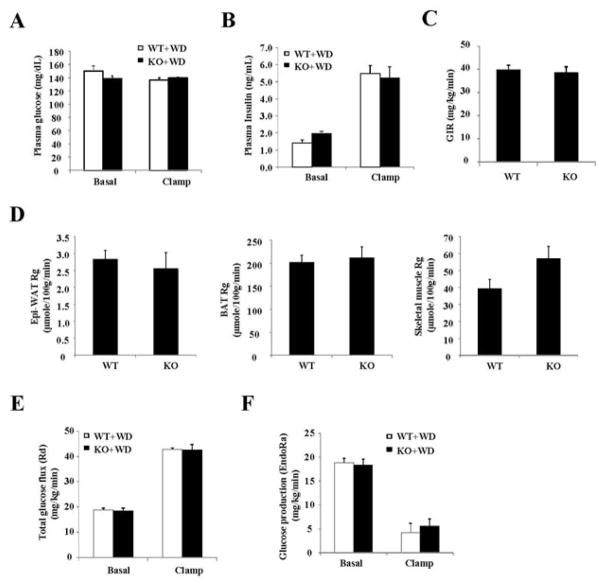
Next, hyperinsulinemic-euglycemic clamp was used to further determine the effects of Sort1 loss-of-function on in vivo insulin action and tissue-specific glucose handling in mice. During the clamp, the total glucose infusion rate was similar between chow-fed WT and Sort1 KO mice (Fig 4A, 4B). The endogenous glucose production (EndoRa) was similar between WT and Sort1 KO mice (Fig 4C). The insulin-stimulated glucose uptake into muscle and white and brown adipose tissues was not different between WT and Sort1 KO mice (Fig 4D). Interestingly, despite well-maintained euglycemia during the clamp (Fig 4A), the clamp insulin levels were significantly lower in Sort1 KO mice (Fig 4F). During the insulin clamp, the plasma insulin represented the mixture of exogenously infused insulin and endogenously secreted insulin by the β cells. Fig 4G showed that plasma C-peptide during clamp was also significantly reduced in Sort1 KO mice, suggesting lower clamp insulin was likely due to decreased endogenous insulin secretion but not increased liver insulin clearance in Sort1 KO mice. The endogenous insulin production in the presence of exogenous glucose and insulin infusion during insulin clamp is still a poorly understood process. The underlying cause of decreased clamp insulin in chow-fed Sort1 KO mice still requires further investigation. In Western diet-fed mice, the clamp glucose was well maintained and the clamp insulin levels were similar in WT and Sort1 KO mice (Fig 5A, 5B). Under these conditions, Sort1 KO mice and WT mice exhibited similar total glucose infusion rate (GIR) (Fig 5C). Insulin-stimulated tissue glucose uptake into the muscle and white and brown fat were similar between the groups (Fig 5D). Total glucose flux (Rd) and endogenous glucose production (endoRa) were also similar between the two groups (Fig 5E, 5F).
FIGURE 4. Hyperinsulinemic-euglycemic clamp study of chow-fed mice.
The experimental procedure is described in the Materials and Method. Hyperinsulinemic-euglycemic clamp parameters. GIR: glucose infusion rate. Rg: tissue glucose uptake. Skeletal muscle: Soleus muscle. Epi-WAT: Epididymal fat. BAT: brown adipose tissue. (n=8). Results are expressed as mean ± SEM. “*”, p< 0.05 vs. WT.
FIGURE 5. Hyperinsulinemic-euglycemic clamp study of 8-week Western diet-fed mice.
The experimental procedure is described in the Materials and Method. Hyperinsulinemic-euglycemic clamp parameters. GIR: glucose infusion rate. Rg: tissue glucose uptake. Skeletal muscle: Soleus muscle. Epi-WAT: Epididymal fat. BAT: brown adipose tissue. n=8–9. Results are expressed as mean ± SEM.
Discussion
This study characterized the impact of Sort1 loss-of-function on adipose glucose metabolism in mouse models. Such in vivo studies are currently lacking in the field but are clearly needed to better define the pathophysiological function of Sort1 and its implication in the pathogenesis of obesity and diabetes. Our studies showed that Sort1 loss-of-function differentially affected adipose glucose metabolism under basal fasting state and insulin-stimulated state. By studying the in vivo insulin action and insulin-stimulated organ-specific glucose uptake with hyperinsulinemic-euglycemic clamp, we found that chow-fed Sort1 KO mice generally showed normal overall glucose flux and tissue glucose uptake during insulin clamp, suggesting that, at least under the clamp condition, Sort1 deficiency did not significantly impair insulin – stimulated glucose uptake into muscle and adipose. If lower clamp insulin is taken into consideration, the overall insulin sensitivity may be actually slightly higher in Sort1 KO mice. The adipose and muscle Glut4 protein abundance was also similar between Sort1 KO mice and WT. This is different from genetic deletion of another key GSV component insulin-regulated aminopeptidase (IRAP) in mice, which resulted in decreased Glut4 protein abundance in muscle and adipose (32). Similarly, deletion of the GSV component low density lipoprotein receptor – related protein 1 (LRP1) in adipose tissue also reduced adipose Glut4 protein abundance in mice (33). Given that Sort1 knockdown reduced Glut4 protein abundance and impaired insulin-dependent glucose uptake in cultured adipocytes, a more plausible explanation may be that the congenital loss of Sort1 function in vivo can be compensated by other GSV components. In addition to IRAP and LRP1, M6PR and sorting-related receptor with type-A repeats (SorLA) were identified as components of purified GSVs from rat adipocytes (33, 34). M6PR, SorLA and LRP1 can also interact with and recruit the Golgi-localized γ-ear-containing Arf-binding proteins (GGA) of coat adaptor proteins that are required for intracellular vesicle trafficking (35). The existence of such functional redundancy is possible as loss of insulin-dependent glucose uptake may be detrimental to many cell types.
In contrast to insulin-stimulated glucose uptake, we found consistently decreased glycolytic and TCA cycle intermediate metabolites in the adipose of chow-fed fasted Sort1 KO mice. The altered glycolytic and TCA cycle metabolite profile closely resembled the changes caused by Western diet feeding to WT mice, which was expected to cause adipose insulin resistance that impaired cellular glucose uptake and metabolism. These genotype-dependent changes of glycolytic metabolites under chow-fed conditions thus suggest that there is a causal link between Sort1 deficiency and decreased glucose metabolism in the adipose tissue. Under fasting, adipocytes are exposed to relatively lower concentrations of circulating insulin. Since chow-fed WT and Sort1 KO mice had similar adipose GLUT4 protein, reduced glycolytic intermediates in chow-fed Sort1 KO mice could be a result of reduced GLUT4 membrane translocation, which would be consistent with the required role of Sort1 in conferring the insulin responsiveness of GSV demonstrated in cultured adipocytes (20, 22). However, such changes may have been masked after strong exogenous insulin stimulation during the insulin clamp. Reduced basal glucose uptake has also been reported in adipocytes isolated from mice lacking another GSV component IRAP ex vivo (32). It was not surprising to see that Sort1 KO mice had normal fasting glucose levels, because both mice lacking Glut4 and mice lacking IRAP generally maintained normal glycaemia (32, 36). When fed a Western diet, Sort1 deficiency did not further decrease adipose glycolytic metabolites. One possible explanation may be that Western diet-induced obesity may have a much stronger effect in decreasing adipose glucose metabolism, which therefore masked the genotype-dependent effects in Western diet-fed WT and Sort1 KO mice.
In our study, Sort1 deficiency did not affect Western diet-induced weight gain. This finding is inconsistent with a previous study showing that Sort1 KO mice were resistant to high fat diet-induced obesity (25). This study showed that high fat diet-fed Sort1 KO mice had lower adipose aSMase activity than high fat diet-fed WT mice, but adipose ceramide concentrations were not directly quantified (25). Cellular ceramides can be produced through other aSMase-independent pathways. Our metabolite analysis revealed no difference in ceramide levels between WT and Sort1 KO mice on either chow diet or Western diet. Unfortunately, the study did not report the adipose aSMase activity in mice under the chow-fed condition without body weight difference between the two groups (25), which would be more informative because obesity is known to increase aSMase activity (37). Although the underlying cause of such discrepancy is still not clear, we would like to note that the Sort1 KO mice used in the two studies were generated independently with different targeting strategies (10, 38). In addition, the fat-enriched diets used in the two studies were different. The previous study used a diet contained 60% fat calories from lard (25), while the Western diet used in our study contained 42% fat calories from milk fat. Therefore we cannot rule out the possibility that the fat content or the source of dietary fat may potentially influence the obesity phenotypes in Sort1 KO mice and WT controls. To our knowledge, there have been no other independent studies reporting the effect of Sort1 ablation on diet-induced weight gain in mice. A few previous studies have employed high fat diet feeding to Sort1 KO mice on Ldlr−/−, ApoE−/− or Apobec1−/−/hAPOB-tg genetic background (4, 11–13). However, these studies did not report data on weight gain in these mice. Obesity, adipocyte dysfunction and insulin resistance are early events associated with obesity development, which critically contributes to the pathogenesis of hepatic steatosis and hyperlipidemia. Determining whether adipose Sort1 deficiency affects obesity development and adipose lipid and glucose metabolism is important in improving our understanding of the mechanistic link between Sort1 function and plasma lipid concentrations.
Acknowledgments
This work is supported in part by the American Diabetes Association Junior Faculty Award (T.L.), NIH grant 1R01DK102487-01 (T.L), and the NIGMS grants P20GM103549 and P30GM118247.
Abbreviations
- Sort1
Sortilin 1
- VPS10P
vacuolar protein sorting 10 protein
- aSMase
acid sphingomyelinase
- SorLA
sorting-related receptor with type-A repeats
- GSV
Glut4 storage vesicles
- KO
knockout
- GIR
glucose infusion rate
- MAG
monoacylglycerol
- DAG
diacylglycerol
- IRAP
insulin-regulated aminopeptidase
- LRP1
low density lipoprotein receptor – related protein 1
- M6PR
mannose 6-phosphate receptor
- GGA
Golgi-localized γ-ear-containing Arf-binding proteins
Footnotes
Author Contribution. Jibiao Li, David J. Matye and Yifeng Wang designed and performed research, and analyzed data. Tiangang Li designed research, analyzed data and wrote the paper. These authors declare no conflict of interest.
References
- 1.Hermey G. The Vps10p-domain receptor family. Cell Mol Life Sci. 2009;66:2677–2689. doi: 10.1007/s00018-009-0043-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kathiresan S, Melander O, Guiducci C, Surti A, Burtt NP, Rieder MJ, Cooper GM, Roos C, Voight BF, Havulinna AS, Wahlstrand B, Hedner T, Corella D, Tai ES, Ordovas JM, Berglund G, Vartiainen E, Jousilahti P, Hedblad B, Taskinen MR, Newton-Cheh C, Salomaa V, Peltonen L, Groop L, Altshuler DM, Orho-Melander M. Six new loci associated with blood low-density lipoprotein cholesterol, high-density lipoprotein cholesterol or triglycerides in humans. Nat Genet. 2008;40:189–197. doi: 10.1038/ng.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Myocardial Infarction Genetics C; Kathiresan S, Voight BF, Purcell S, Musunuru K, Ardissino D, Mannucci PM, Anand S, Engert JC, Samani NJ, Schunkert H, Erdmann J, Reilly MP, Rader DJ, Morgan T, Spertus JA, Stoll M, Girelli D, McKeown PP, Patterson CC, Siscovick DS, O'Donnell CJ, Elosua R, Peltonen L, Salomaa V, Schwartz SM, Melander O, Altshuler D, Ardissino D, Merlini PA, Berzuini C, Bernardinelli L, Peyvandi F, Tubaro M, Celli P, Ferrario M, Fetiveau R, Marziliano N, Casari G, Galli M, Ribichini F, Rossi M, Bernardi F, Zonzin P, Piazza A, Mannucci PM, Schwartz SM, Siscovick DS, Yee J, Friedlander Y, Elosua R, Marrugat J, Lucas G, Subirana I, Sala J, Ramos R, Kathiresan S, Meigs JB, Williams G, Nathan DM, MacRae CA, O'Donnell CJ, Salomaa V, Havulinna AS, Peltonen L, Melander O, Berglund G, Voight BF, Kathiresan S, Hirschhorn JN, Asselta R, Duga S, Spreafico M, Musunuru K, Daly MJ, Purcell S, Voight BF, Purcell S, Nemesh J, Korn JM, McCarroll SA, Schwartz SM, Yee J, Kathiresan S, Lucas G, Subirana I, Elosua R, Surti A, Guiducci C, Gianniny L, Mirel D, Parkin M, Burtt N, Gabriel SB, Samani NJ, Thompson JR, Braund PS, Wright BJ, Balmforth AJ, Ball SG, Hall A, Schunkert H, Erdmann J, Linsel-Nitschke P, Lieb W, Ziegler A, Konig I, Hengstenberg C, Fischer M, Stark K, Grosshennig A, Preuss M, Wichmann HE, Schreiber S, Schunkert H, Samani NJ, Erdmann J, Ouwehand W, Hengstenberg C, Deloukas P, Scholz M, Cambien F, Reilly MP, Li M, Chen Z, Wilensky R, Matthai W, Qasim A, Hakonarson HH, Devaney J, Burnett MS, Pichard AD, Kent KM, Satler L, Lindsay JM, Waksman R, Knouff CW, Waterworth DM, Walker MC, Mooser V, Epstein SE, Rader DJ, Scheffold T, Berger K, Stoll M, Huge A, Girelli D, Martinelli N, Olivieri O, Corrocher R, Morgan T, Spertus JA, McKeown P, Patterson CC, Schunkert H, Erdmann E, Linsel-Nitschke P, Lieb W, Ziegler A, Konig IR, Hengstenberg C, Fischer M, Stark K, Grosshennig A, Preuss M, Wichmann HE, Schreiber S, Holm H, Thorleifsson G, Thorsteinsdottir U, Stefansson K, Engert JC, Do R, Xie C, Anand S, Kathiresan S, Ardissino D, Mannucci PM, Siscovick D, O'Donnell CJ, Samani NJ, Melander O, Elosua R, Peltonen L, Salomaa V, Schwartz SM, Altshuler D Wellcome Trust Case Control C. Genome-wide association of early-onset myocardial infarction with single nucleotide polymorphisms and copy number variants. Nat Genet. 2009;41:334–341. doi: 10.1038/ng.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kjolby M, Andersen OM, Breiderhoff T, Fjorback AW, Pedersen KM, Madsen P, Jansen P, Heeren J, Willnow TE, Nykjaer A. Sort1, encoded by the cardiovascular risk locus 1p13.3, is a regulator of hepatic lipoprotein export. Cell Metab. 2010;12:213–223. doi: 10.1016/j.cmet.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 5.Musunuru K, Strong A, Frank-Kamenetsky M, Lee NE, Ahfeldt T, Sachs KV, Li X, Li H, Kuperwasser N, Ruda VM, Pirruccello JP, Muchmore B, Prokunina-Olsson L, Hall JL, Schadt EE, Morales CR, Lund-Katz S, Phillips MC, Wong J, Cantley W, Racie T, Ejebe KG, Orho-Melander M, Melander O, Koteliansky V, Fitzgerald K, Krauss RM, Cowan CA, Kathiresan S, Rader DJ. From noncoding variant to phenotype via SORT1 at the 1p13 cholesterol locus. Nature. 2010;466:714–719. doi: 10.1038/nature09266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gustafsen C, Kjolby M, Nyegaard M, Mattheisen M, Lundhede J, Buttenschon H, Mors O, Bentzon JF, Madsen P, Nykjaer A, Glerup S. The hypercholesterolemia-risk gene SORT1 facilitates PCSK9 secretion. Cell Metab. 2014;19:310–318. doi: 10.1016/j.cmet.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 7.Butkinaree C, Canuel M, Essalmani R, Poirier S, Benjannet S, Asselin MC, Roubtsova A, Hamelin J, Marcinkiewicz J, Chamberland A, Guillemot J, Mayer G, Sisodia SS, Jacob Y, Prat A, Seidah NG. Amyloid precursor-like protein 2 and sortilin do not regulate the PCSK9 convertase-mediated low density lipoprotein receptor degradation but interact with each other. J Biol Chem. 2015 doi: 10.1074/jbc.M115.647180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li J, Bi L, Hulke M, Li T. Fish oil and fenofibrate prevented phosphorylation-dependent hepatic sortilin 1 degradation in Western diet-fed mice. J Biol Chem. 2014;289:22437–22449. doi: 10.1074/jbc.M114.548933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bi L, Chiang JY, Ding WX, Dunn W, Roberts B, Li T. Saturated fatty acids activate ERK signaling to downregulate hepatic sortilin 1 in obese and diabetic mice. J Lipid Res. 2013;54:2754–2762. doi: 10.1194/jlr.M039347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li J, Wang Y, Matye DJ, Chavan H, Krishnamurthy P, Li F, Li T. Sortilin 1 Modulates Hepatic Cholesterol Lipotoxicity in Mice via Functional Interaction with Liver Carboxylesterase 1. The Journal of biological chemistry. 2017;292:146–160. doi: 10.1074/jbc.M116.762005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mortensen MB, Kjolby M, Gunnersen S, Larsen JV, Palmfeldt J, Falk E, Nykjaer A, Bentzon JF. Targeting sortilin in immune cells reduces proinflammatory cytokines and atherosclerosis. J Clin Invest. 2014;124:5317–5322. doi: 10.1172/JCI76002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patel KM, Strong A, Tohyama J, Jin X, Morales CR, Billheimer J, Millar J, Kruth H, Rader DJ. Macrophage sortilin promotes LDL uptake, foam cell formation, and atherosclerosis. Circ Res. 2015;116:789–796. doi: 10.1161/CIRCRESAHA.116.305811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goettsch C, Hutcheson JD, Aikawa M, Iwata H, Pham T, Nykjaer A, Kjolby M, Rogers M, Michel T, Shibasaki M, Hagita S, Kramann R, Rader DJ, Libby P, Singh SA, Aikawa E. Sortilin mediates vascular calcification via its recruitment into extracellular vesicles. J Clin Invest. 2016 doi: 10.1172/JCI80851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin BZ, Pilch PF, Kandror KV. Sortilin is a major protein component of Glut4-containing vesicles. The Journal of biological chemistry. 1997;272:24145–24147. doi: 10.1074/jbc.272.39.24145. [DOI] [PubMed] [Google Scholar]
- 15.Morris NJ, Ross SA, Lane WS, Moestrup SK, Petersen CM, Keller SR, Lienhard GE. Sortilin is the major 110-kDa protein in GLUT4 vesicles from adipocytes. J Biol Chem. 1998;273:3582–3587. doi: 10.1074/jbc.273.6.3582. [DOI] [PubMed] [Google Scholar]
- 16.Zhu Y, Doray B, Poussu A, Lehto VP, Kornfeld S. Binding of GGA2 to the lysosomal enzyme sorting motif of the mannose 6-phosphate receptor. Science. 2001;292:1716–1718. doi: 10.1126/science.1060896. [DOI] [PubMed] [Google Scholar]
- 17.Nielsen MS, Madsen P, Christensen EI, Nykjaer A, Gliemann J, Kasper D, Pohlmann R, Petersen CM. The sortilin cytoplasmic tail conveys Golgi-endosome transport and binds the VHS domain of the GGA2 sorting protein. EMBO J. 2001;20:2180–2190. doi: 10.1093/emboj/20.9.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kato Y, Misra S, Puertollano R, Hurley JH, Bonifacino JS. Phosphoregulation of sorting signal-VHS domain interactions by a direct electrostatic mechanism. Nature structural biology. 2002;9:532–536. doi: 10.1038/nsb807. [DOI] [PubMed] [Google Scholar]
- 19.Pilch PF. The mass action hypothesis: formation of Glut4 storage vesicles, a tissue-specific, regulated exocytic compartment. Acta physiologica. 2008;192:89–101. doi: 10.1111/j.1748-1716.2007.01788.x. [DOI] [PubMed] [Google Scholar]
- 20.Shi J, Kandror KV. Sortilin is essential and sufficient for the formation of Glut4 storage vesicles in 3T3-L1 adipocytes. Dev Cell. 2005;9:99–108. doi: 10.1016/j.devcel.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 21.Watson RT, Khan AH, Furukawa M, Hou JC, Li L, Kanzaki M, Okada S, Kandror KV, Pessin JE. Entry of newly synthesized GLUT4 into the insulin-responsive storage compartment is GGA dependent. EMBO J. 2004;23:2059–2070. doi: 10.1038/sj.emboj.7600159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang G, Buckler-Pena D, Nauta T, Singh M, Asmar A, Shi J, Kim JY, Kandror KV. Insulin responsiveness of glucose transporter 4 in 3T3-L1 cells depends on the presence of sortilin. Mol Biol Cell. 2013;24:3115–3122. doi: 10.1091/mbc.E12-10-0765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsuchiya Y, Hatakeyama H, Emoto N, Wagatsuma F, Matsushita S, Kanzaki M. Palmitate-induced down-regulation of sortilin and impaired GLUT4 trafficking in C2C12 myotubes. J Biol Chem. 2010;285:34371–34381. doi: 10.1074/jbc.M110.128520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaddai V, Jager J, Gonzalez T, Najem-Lendom R, Bonnafous S, Tran A, Le Marchand-Brustel Y, Gual P, Tanti JF, Cormont M. Involvement of TNF-alpha in abnormal adipocyte and muscle sortilin expression in obese mice and humans. Diabetologia. 2009;52:932–940. doi: 10.1007/s00125-009-1273-3. [DOI] [PubMed] [Google Scholar]
- 25.Rabinowich L, Fishman S, Hubel E, Thurm T, Park WJ, Pewzner-Jung Y, Saroha A, Erez N, Halpern Z, Futerman AH, Zvibel I. Sortilin deficiency improves the metabolic phenotype and reduces hepatic steatosis of mice subjected to diet-induced obesity. J Hepatol. 2015;62:175–181. doi: 10.1016/j.jhep.2014.08.030. [DOI] [PubMed] [Google Scholar]
- 26.Steele R, Wall JS, De Bodo RC, Altszuler N. Measurement of size and turnover rate of body glucose pool by the isotope dilution method. The American journal of physiology. 1956;187:15–24. doi: 10.1152/ajplegacy.1956.187.1.15. [DOI] [PubMed] [Google Scholar]
- 27.Kraegen EW, James DE, Jenkins AB, Chisholm DJ. Dose-response curves for in vivo insulin sensitivity in individual tissues in rats. Am J Physiol. 1985;248:E353–362. doi: 10.1152/ajpendo.1985.248.3.E353. [DOI] [PubMed] [Google Scholar]
- 28.Turpin SM, Nicholls HT, Willmes DM, Mourier A, Brodesser S, Wunderlich CM, Mauer J, Xu E, Hammerschmidt P, Bronneke HS, Trifunovic A, LoSasso G, Wunderlich FT, Kornfeld JW, Bluher M, Kronke M, Bruning JC. Obesity-induced CerS6-dependent C16:0 ceramide production promotes weight gain and glucose intolerance. Cell Metab. 2014;20:678–686. doi: 10.1016/j.cmet.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 29.Raichur S, Wang ST, Chan PW, Li Y, Ching J, Chaurasia B, Dogra S, Ohman MK, Takeda K, Sugii S, Pewzner-Jung Y, Futerman AH, Summers SA. CerS2 haploinsufficiency inhibits beta-oxidation and confers susceptibility to diet-induced steatohepatitis and insulin resistance. Cell Metab. 2014;20:687–695. doi: 10.1016/j.cmet.2014.09.015. [DOI] [PubMed] [Google Scholar]
- 30.Ni X, Morales CR. The lysosomal trafficking of acid sphingomyelinase is mediated by sortilin and mannose 6-phosphate receptor. Traffic. 2006;7:889–902. doi: 10.1111/j.1600-0854.2006.00429.x. [DOI] [PubMed] [Google Scholar]
- 31.Leto D, Saltiel AR. Regulation of glucose transport by insulin: traffic control of GLUT4. Nat Rev Mol Cell Biol. 2012;13:383–396. doi: 10.1038/nrm3351. [DOI] [PubMed] [Google Scholar]
- 32.Keller SR, Davis AC, Clairmont KB. Mice deficient in the insulin-regulated membrane aminopeptidase show substantial decreases in glucose transporter GLUT4 levels but maintain normal glucose homeostasis. The Journal of biological chemistry. 2002;277:17677–17686. doi: 10.1074/jbc.M202037200. [DOI] [PubMed] [Google Scholar]
- 33.Jedrychowski MP, Gartner CA, Gygi SP, Zhou L, Herz J, Kandror KV, Pilch PF. Proteomic analysis of GLUT4 storage vesicles reveals LRP1 to be an important vesicle component and target of insulin signaling. J Biol Chem. 2010;285:104–114. doi: 10.1074/jbc.M109.040428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kandror KV, Pilch PF. The insulin-like growth factor II/mannose 6-phosphate receptor utilizes the same membrane compartments as GLUT4 for insulin-dependent trafficking to and from the rat adipocyte cell surface. The Journal of biological chemistry. 1996;271:21703–21708. doi: 10.1074/jbc.271.36.21703. [DOI] [PubMed] [Google Scholar]
- 35.Hou JC, Pessin JE. Ins (endocytosis) and outs (exocytosis) of GLUT4 trafficking. Current opinion in cell biology. 2007;19:466–473. doi: 10.1016/j.ceb.2007.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Katz EB, Stenbit AE, Hatton K, DePinho R, Charron MJ. Cardiac and adipose tissue abnormalities but not diabetes in mice deficient in GLUT4. Nature. 1995;377:151–155. doi: 10.1038/377151a0. [DOI] [PubMed] [Google Scholar]
- 37.Bikman BT, Summers SA. Ceramides as modulators of cellular and whole-body metabolism. J Clin Invest. 2011;121:4222–4230. doi: 10.1172/JCI57144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jansen P, Giehl K, Nyengaard JR, Teng K, Lioubinski O, Sjoegaard SS, Breiderhoff T, Gotthardt M, Lin F, Eilers A, Petersen CM, Lewin GR, Hempstead BL, Willnow TE, Nykjaer A. Roles for the pro-neurotrophin receptor sortilin in neuronal development, aging and brain injury. Nature neuroscience. 2007;10:1449–1457. doi: 10.1038/nn2000. [DOI] [PubMed] [Google Scholar]