Abstract
Background: The outcome of patients with Pseudomonas prosthetic joint infection (PS PJI) has not been well studied. The aim of this retrospective cohort study was to assess the outcome of patients with Pseudomonas PJI and to review risk factors associated with failure of therapy.
Methods: Between 1/1969 and 12/2012, 102 episodes of PS PJI in 91 patients were identified.
Results: The mean age at the time of diagnosis was 67.4 years; forty three percent had knee involvement. Over 40 percent had either diabetes mellitus or a history of gastrointestinal or genitourinary surgery. Nearly half (48 out of 102 episodes) received aminoglycoside monotherapy, while 25% received an anti-pseudomonal cephalosporin. The 2-year cumulative survival free from failure was 69% (95% CI, 56%-82%). Patients treated with resection arthroplasty, two-stage exchange, and debridement with implant retention had a 2-year cumulative survival free from failure of 80% (95% CI, 66%-95%), 83% (95% CI, 60%-100%), and 26% (95% CI, 23%-29%) respectively (P=0.0001).
Conclusions: PS PJI's are associated with a high failure rate. Patients treated with debridement and implant retention had a worse outcome.
Keywords: prosthetic joint infection, Pseudomonas, arthroplasty, debridement and implant retention.
Introduction
In an effort to improve the quality of life of patients with underlying connective tissue and degenerative joint disease, joint prosthesis placement is becoming more prevalent in the United States. It is estimated that by 2030, 4 million total knee and hip arthroplasty procedures will be performed yearly 5, 10. Commensurate with the increasing number of prosthetic joints being implanted, the total number of patients diagnosed and treated for prosthetic joint infections (PJI) is rising 4, 10, 12, 14. PJI is among the most common causes of failure of total joint arthroplasty, resulting in additional procedures, increased health care costs, and increased morbidity 3, 8, 14. Although the Infectious Diseases Society of America published guidelines in 2012 10 to help guide clinicians in the diagnosis and management of PJI, these guidelines primarily focus on the commonly encountered pathogens associated with PJI, namely Staphylococcus and Streptococcus species, and do not as comprehensively address the management of less commonly encountered organisms associated with PJI such as Pseudomonas sp..
Relatively less common, PJI caused by Gram-negative bacilli are a devastating complication, resulting in prolonged hospitalization and increased medical costs 4, 9, 12, 18. Pseudomonas sp. are challenging organisms to prevent and treat, owing to their strong association with nosocomial environments and high propensity for drug resistance. Pseudomonas sp., most notably P. aeruginosa, have a propensity to attach to bone and fibrocartilaginous articular structures, and are associated with osteomyelitis, septic arthritis, and less commonly, PJI 13, 17. There are sparse data in the literature describing the demographic characteristics, treatment options and outcomes of patients with PS PJI 4, 6, 9, 13, 17, 18. Lora-Tomayo et al. described 33 patients with early, late-chronic or hematogenous P. aeruginosa PJI (PS PJI), reporting an overall success rate of 81% 7. Ascione, et al. reported an overall treatment success rate of 80% with debridement and implant retention and 85% with 2-stage exchange surgery in the setting of late infection; as part of this study, 11 patients with PS PJI were incorporated 1. A study from the Netherlands on 12 patients with early PS PJI treated with debridement and implant retention followed by antimicrobial therapy reported a success rate of 66% 16.
To help address the paucity of data associated with PS PJI, we conducted a retrospective cohort study to determine the clinical characteristics, outcome and risk factors for management failure in patients with PS PJI. We anticipate that this information will help guide health care providers to effectively assess and manage patients with PS PJI.
Materials and Methods
Study population. The medical records of all episodes of Pseudomonas sp. total hip or knee arthroplasty infection (PS PJI) seen at the Mayo Clinic in Rochester, MN between January 1, 1969 and December 31, 2012 were reviewed. All episodes were followed from the date of PJI diagnosis until treatment failure, prosthesis removal, death or the end of the study period.
Data collection. Data were obtained from the electronic and written medical records and transferred onto a structured data sheet, using the REDCap™ database. The data was then analyzed using JMP® (version 10.0.0) statistical program software. Data on date of birth, gender, medical comorbidities, type of surgery, and antimicrobial therapy was abstracted.
Definitions.
PS PJI was said to be present if at least two separate cultures grew Pseudomonas sp. obtained from intra-articular aspiration or surgical specimens, or there was at least one positive culture from a joint source, along with any of the following: (a) purulence surrounding the prosthesis, which was observed at the time of periprosthetic aspiration or during surgery; (b) acute inflammation that was consistent with infection on histopathologic examination; or (c) the presence of a sinus tract, found on examination or during operative repair. This definition of PJI was adopted from the Infectious Disease Society of America's guidelines for the management of PJI 10.
Treatment failure was defined as the occurrence of PJI, as outlined above, caused by Pseudomonas sp. or any other organism at any time after the original therapy episode.
The original therapy episode was defined as treatment that was undertaken during the initial hospitalization after diagnosis of PJI was established.
Prosthetic joint infections in this study were defined as early if they occurred within 3 months of implantation, delayed if they occurred between 3 months and 2 years from the date of implantation and late if infection occurred greater than 2 years from the date of implantation.
Stratification of data. In order to account for the changes in medical and surgical practices that occurred during the duration of the study period, we performed a subset analysis in which we stratified patients according to two different time periods (1969 to 1989 and 1990 to 2012). The 2 time periods were selected in part based on shifts in medical and surgical practices that were observed in the Mayo Joint Registry, which contains patient information since 1969.
Statistical methods. The number and percentage of episodes in each of the medical and surgical groups were calculated. Differences in demographic factors, clinical variables, and microbiology between the groups were compared using the chi-square test for categorical variables, and Wilcoxon-sum rank analysis for continuous variables. The cumulative incidence of treatment failure and 95% confidence intervals were estimated by using Kaplan-Meier survival analysis. Comparisons of the overall efficacy between the medical and surgical treatment strategies were performed by the use of the Petot-Petot Wilcoxon test.
Results
Study population. Table 1 outlines the major demographic characteristics amongst the 91 patients (102 episodes) identified with PS PJI between January 1, 1969 and December 31, 2012, at the Mayo Clinic in Rochester, MN. Eleven patients had more than one episode, due to treatment failure. Fifteen of the 102 episodes (15%) were classified as early infection, occurring within 3 months of the implantation procedure. Forty five of the episodes (44%) were late infections, and forty two (41%) of the episodes were classified as delayed. Twenty patients (22%) had a history of gastrointestinal/genitourinary surgery prior to their diagnosis of PS PJI (repair of colovesicular fistula, appendectomy, femoral/hiatal/diaphragmatic hernia repair, cholecystectomy, prostatectomy, hysterectomy and renal stone extraction). Five patients (5%) had a history of renal pathology, which included chronic kidney disease or a history of recurrent renal stones. Four patients (4%) had recurrent urinary tract infections, with 2 having an established history of Pseudomonas aeruginosa UTI. Fifteen patients (16%) were on immunosuppressive medications during the time of their PJI; the majority of these were taking prednisone at doses of less than 20 mg daily. One patient was on methotrexate, and three were on prednisone doses of greater than 20 mg daily.
Table 1.
Demographic characteristics of 91 patients presenting with Pseudomonas PJI between 1969-2012.
| Demographic data | Results |
|---|---|
| Total patients (# episodes) | 91 (102) |
| Mean age at time of PJI dx, in years (range) | 67.4 (25-90) |
| Site of infection (% of total patients) | 39 knee (43%) |
| 52 hip (57%) | |
| Gender (% of total patients) | 49 females (54%) |
| 42 males (46%) | |
| Race (% of total patients) | 88 Caucasian (97%) |
| 3 African American (3%) | |
| Underlying Joint Disease (% of total patients) | 53 DJD (58%) |
| 20 Rheumatoid arthritis (22%) | |
| 18 Trauma (20%) | |
| 5 Malignancy (6%) | |
| 5 Aseptic necrosis of bone (6%) | |
| 3 Congenital bone disease (3%) | |
| Diabetes mellitus (% of total patients) | 20 (22%) |
| GI/GU Surgery (% of total patients) | 20 (22%) |
| Smoking history (% of total patients) | 10 (11%) |
| Chronic lymphedema/cellulitis (% of total patients) | 13 (14%) |
| Use of immunosuppressive medications (% of total patients) | 15 (16%) |
Diagnostic methods. Eighty three of the 102 episodes (81%) of PS PJI were diagnosed based on the presence of at least two positive cultures for Pseudomonas sp. obtained either from periprosthetic tissue or joint fluid. Among the remaining 19 episodes, the diagnosis was made on the basis of a single positive intraoperative culture and the presence of a sinus tract (9 episodes, 9% of total episodes) or the presence of purulence around the joint space (10 episodes, 10% of total episodes). Only three episodes had sonication cultures performed. Sixty four patient episodes (63%) had an elevated erythrocyte sedimentation rate and/or C-reactive protein. The predominant causative organism was P. aeruginosa (99% of all episodes), while only a single episode of infection was caused by Pseudomonas pickettii (1%). The most common organisms other than Pseudomonas that were isolated in polymicrobial infections were Staphylococcus aureus (10 episodes) Group D, G and viridans group streptococci (8 episodes) and coagulase negative Staphylococcus species (6 episodes).
Antibiogram patterns. Table 2 summarizes the total number of Pseudomonas isolates that underwent in vitro susceptibility testing in this study, based on time period.
Table 2.
In vitro susceptibilities of selected antimicrobials against Pseudomonas isolates in 91 patients with PS PJI, by time period.
| Antimicrobial Agent | Time Period | |
|---|---|---|
| 1969-1989 | 1990-2012 | |
| Gentamicin | 61 (100%) | 33 (91%) |
| Amikacin | 24 (100%) | 31 (97%) |
| Tobramycin | 24 (96%) | 8 (88%) |
| Cefepime | 1 (100%) | 30 (93%) |
| Ceftazidime | 9 (100%) | 31 (94%) |
| Piperacillin-tazobactam | 0 | 34 (85%) |
| Aztreonam | 5 (100%) | 2 (50%) |
| Imipenem | 4 (100%) | 23 (91%) |
| Meropenem | 0 | 25 (100%) |
| Ciprofloxacin | 2 (100%) | 34 (85%) |
| Levofloxacin | 0 | 26 (85%) |
Surgical intervention. Among the 102 patient episodes, fifty seven (56%) were treated with resection arthroplasty. Twenty three (23%) episodes were treated with a two-stage exchange procedure, with a mean duration between staged procedures of 222 days (range 30-973 days). Eighteen (18%) were treated with debridement and implant retention. Two episodes (2%) received chronic suppressive therapy without any surgical interventions, and two episodes (2%) received no surgical or antimicrobial interventions. In one of these episodes, the patient suffered from refractory heart failure making prosthetic joint surgery too risky, and in the second episode, surgical therapy was deferred until the patient's immunosuppressed state (Felty's Syndrome) had resolved after splenectomy; however subsequent aspiration of this patient's joint revealed Staphylococcus aureus (MSSA), resulting in a change in planned therapy.
Medical therapy. Of the 102 patient episodes, 47 (46%) received only an aminoglycoside as their primary initial therapy, 25 (25%) received therapy with either a third or fourth generation cephalosporin, 5 (5%) received carbapenem therapy, 5 (5%) received fluoroquinolone therapy and 4 (4%) received other β-lactam therapy (piperacillin-tazobactam, aztreonam). Of those that received aminoglycoside therapy, all were before the 1990-2012 time period. Fourteen episodes (14%) involved combination therapy, with the most common combinations used being cefepime and ciprofloxacin (4 episodes), ticarcillin and tobramycin (2 episodes) and ceftazidime and gentamicin (2 episodes). One patient was not initiated on any medical therapy due to a pending surgical procedure (splenectomy for Felty's Syndrome, as noted above). The mean duration of initial therapy was 34.5 days (range, 10-180 days). Twenty two patient episodes (22%) received oral suppressive therapy following their initial therapy, of which ciprofloxacin and levofloxacin were the most commonly used agents (91% of total cases); a combination of trovafloxacin with rifampin were used in one patient, and tetracycline suppressive therapy was used in the remaining case. Amongst the 23 episodes treated with 2-stage exchange, antimicrobial-loaded polymethylmethacrylate spacers were placed in 21 episodes [vancomycin spacer (10) vancomycin/gentamicin spacer (8) gentamicin spacer (1) tobramycin and amphotericin B spacer (1) vancomycin/tobramycin (1)]. Two patients had non-antibiotic impregnated spacers placed.
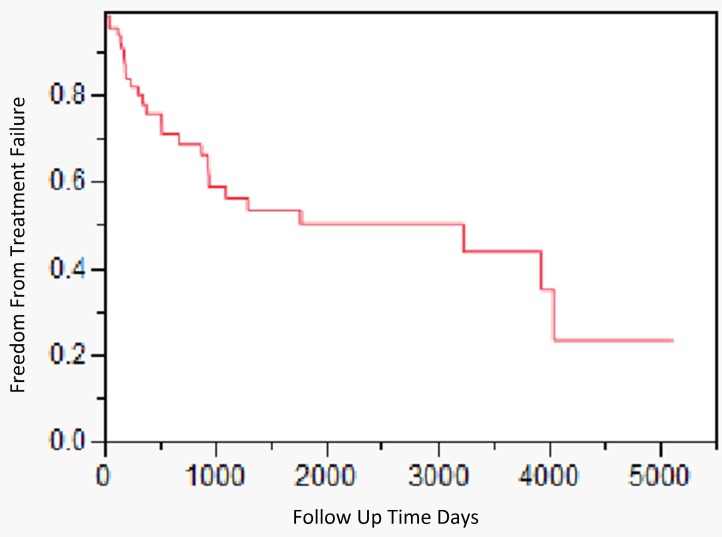
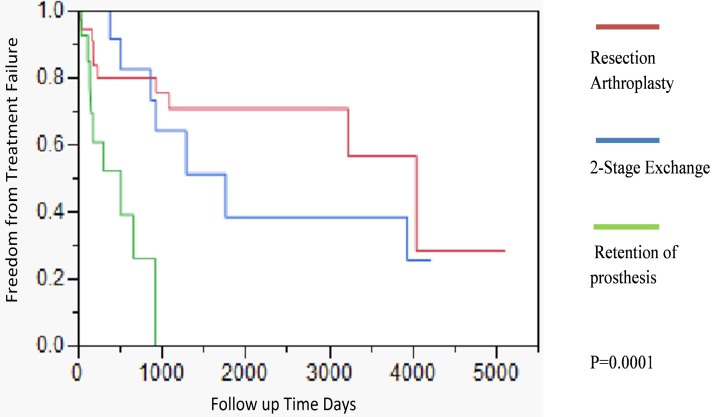
Outcome and survival analysis. Figure 1 outlines the outcome of all patients in this study. The median duration of followup was 8.8 years (range 0-13.9 years). The 2 and 5 year survival free from failure was 69% (95% CI, 56% -82%) and 50.5% (95% CI, 35%-66%) respectively. The outcome of patients treated with different parenteral antimicrobial regimens (aminoglycosides, 3rd/4th generation cephalosporins, carbapenems, and combination therapy) was similar (data not shown) (P=0.79). Patients treated with debridement and implant retention had a worse outcome when compared to resection arthroplasty or two stage exchange [2-year cumulative incidence of success 26% (95% CI, 23% -29%)] (P=0.0001). By 3 years of follow up, all patients treated with debridement and implant retention had failed therapy. Only 2 of the 18 episodes treated with prosthesis retention had symptoms occurring within two weeks of PJI diagnosis; the rest of the patients had chronic symptoms, occurring for more than 2 weeks' time. The 2 and 5 year survival free from failure for patients treated with resection arthroplasty was 80% (95% CI, 66%-95%), and 71% (95% CI 53-88%), respectively. The 2 and 5 year survival free from failure for patients treated with 2-stage exchange was 83% (95% CI, 60%-100%), and 39% (95% CI 35-42%) respectively (Figure 2). The 5 year survival free from failure for patients with or without diabetes mellitus was 31.4% (95%CI, 30.6%-31.3%) and 55% (95%CI, 54.8%-55.2%) respectively (P=0.05). There were no significant differences in outcome among patients who had polymicrobial infections vs. those that presented with a monomicrobial PS PJI (P=0.60).
Figure 1.
Kaplan-Meier time dependent analysis assessing the outcome of 102 Pseudomonas PJI episodes seen at the Mayo Clinic between 1969 and 2012.
Figure 2.
Kaplan-Meier time dependent analysis of Pseudomonas PJI, by type of surgical therapy. Patients with debridement and implant retention of their prosthesis had a significantly worse outcome when compared to resection arthroplasty and 2-stage exchange.
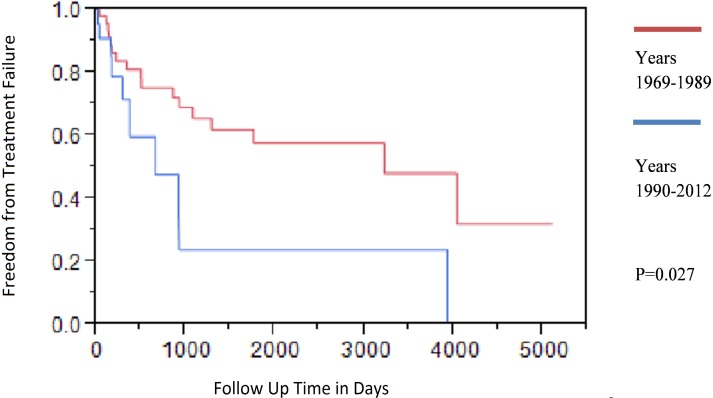
Secular trends. In order to account for the changes in medical and surgical practices that occurred during the study period, we stratified our patient episodes into two time periods based on the diagnosis of PJI. Comparison of both time groups by outcome showed that patients treated between 1969-1989 time period had overall better outcomes when compared to patients treated during the 1990-2012 time period (Figure 3, P=0.027). Table 3 compares selected demographics, medical and surgical factors in both groups. There were a significantly greater proportion of patients with diabetes mellitus (41%, vs 11%, P=0.003) during 1990 to 2012 period when compared to the 1969 to 1989 period. A greater proportion of patients after 1990 underwent debridement with implant retention (32%) compared to those patients treated before 1990 (9%). In addition, resection arthroplasty was far more common during 1969-1989 time period. Seventy two percent of patients treated during the 1969-1989 time period received aminoglycoside therapy.
Figure 3.
Kaplan-Meier time dependent analysis of Pseudomonas PJI, based on time period. Patients treated between 1969-1989 had a better outcome than those treated between 1990-2012 time period.
Table 3.
Characteristics of episodes of Pseudomonas PJIs based on time period
| 1969-1989 N=65 |
1990-2012 N=37 |
P value | |
|---|---|---|---|
| Mean Age | 67. 2 years (range 25-87) | 67.66 years (range 32-90) | -- |
|
Gender Female Male |
34/65 (52%) 31/65 (48%) |
19/37 (51%) 18/37 (49%) |
0.95 |
|
Site of Joint Involvement Knee Hip |
30/65 (46%) 35/65 (54%) |
19/37 (51%) 18/37 (49%) |
0.96 |
| Smoking | 6/65 (9%) | 7/37 (19%) | 0.73 |
| Diabetes mellitus | 7/65 (11%) | 15/37 (41%) | 0.003 |
| Immunosuppressive medicationsˡ | 10/65 (15%) | 9/37 (24%) | 0.44 |
| Malignancy | 5/65 (8%) | 9/37 (2.4%) | 0.03 |
| Primary therapy IV aminoglycoside | 47/65 (72%) | 0/37 (0%) | <0.05 |
| Primary therapy IV β-lactam | 8/65 (12%) | 26/37 (70%) | <0.05 |
| Primary therapy Fluoroquinolone | 2/65 (3%) | 3/37 (8%) | 0.35 |
| Primary therapy combination (β-lactam/aminoglycoside therapy) | 6/65 (9%) | 8/37 (22%) | 0.13 |
| Irrigation/debridement with retention of prosthesis | 6/65 (9%) | 12/37 (32%) | <.005 |
| Resection arthroplasty | 45/65 (69%) | 12/37 (32%) | 0.0004 |
| 2-stage exchange | 4/65 (6%) | 19/37 (51%) | <0.05 |
*ˡ Immunosuppressive medications included prednisone (<20 mg vs >20 mg/daily) and methotrexate
The analysis of in vitro antimicrobial susceptibility testing is outlined in Table 2.
Discussion
To our knowledge, this is the largest study of patients with Pseudomonas sp. prosthetic joint infections reported in the literature. In this study, we adopted the IDSA definition of PJI, in favor of the Musculoskeletal Infection Society definition since the majority of the patients did not have a joint aspiration cell count performed prior to surgery 10, 11.
We observed a high failure rate in patients with PS PJI, with a 2 year cumulative incidence of failure of 31%. We did not observe a significant difference in the outcome amongst various classes of anti-pseudomonal agents. Significant difference in outcomes was noted between patients treated with different surgical modalities. Our study showed a poor outcome in patients treated with debridement and implant retention, with a 2 year cumulative survival free from failure of only 26%. It should be noted that he majority of patients who underwent this procedure had chronic symptoms exceeding 2 or more weeks, supporting the notion that patients with chronic symptoms and PS PJI do poorly with implant retention. In contrast, Lora-Tomayo et al. reported an 81% success rate when debridement, antimicrobial therapy and implant retention was performed. However, the mean followup time for those patients that did not fail debridement and implant retention was only 347 days (range 92-692 days), In this study, the median followup period was more than 3 years, allowing a better capture of failure of therapy. 7, 16. This study suggests that need for adequate antimicrobial therapy and removal of all foreign bodies in order to optimize the chances of treatment success. This is further supported by the findings in Ascione's study, where the recommendation was made to remove the joint prosthesis in order to maximize eradication of infection, especially in cases of delayed infection 1.
Sixteen percent of patients in this study were receiving immunosuppressive therapy and 22% of patients had underlying diabetes mellitus. Furthermore, 18% had rheumatoid arthritis, and 11% of patients had a history of smoking. These comorbidities have been associated with other types of Pseudomonas infections 2, 15. Pseudomonas is often associated with the nosocomial environment, and we hypothesize that acquisition of this organism occurred during prior surgeries or procedures.
The majority of tested isolates from this study were susceptible to 3rd and 4th generation cephalosporins, including ceftazidime and cefepime. All of the tested isolates were susceptible to meropenem, and 91% of the isolates tested were imipenem susceptible after the 1990 time period. Interestingly, there was an approximately 20% resistance rate associated with anti-pseudomonal fluoroquinolones (ciprofloxacin and levofloxacin). Although susceptibility to levofloxacin was not routinely tested at our institution before 1991, ciprofloxacin susceptibilities were tested both between 1980-1989 and from 1990-2012. Our study does suggest an increasing rate of resistance to quinolones over time, making quinolones less desirable to use as empiric choices.
Our survival analysis of the two time periods revealed that patients diagnosed with PS PJI during 1969-1989 had a better outcome when compared to those patients diagnosed between 1990-2012 (Figure 3). We subsequently attempted to determine if differences were present between the two groups with respect to comorbidities, demographic factors, antimicrobial therapy or surgical therapies. There were a significantly higher proportion of patients treated with debridement and implant retention during the later time period, likely confounding the worse outcome noted during this time period. Furthermore, the poor outcome observed in patients managed between 1990 and 2012 time period may be also driven by a higher percentage of comorbidities in addition to choices of surgical regimens used during this time period.
This study has several limitations that are inherent to retrospective cohort studies, such as the lack of an established treatment protocol and the inability to assess all potential variables that may have led to differences in treatment outcome. In addition, because there were fewer patients treated with 4th generation cephalosporins and carbapenems, we were unable to assess the outcome of these episodes of PJI. Finally, a number of patients with prolonged symptoms were treated with debridement and implant retention, potentially limiting our ability to generalize the poor outcomes of patients treated with this approach to patients with more acute symptoms in whom it might be more successful. In conclusion, the majority of Pseudomonas isolates in patients with PS PJI were susceptible to the higher generation cephalosporins, carbapenems and aminoglycosides. Episodes treated with prosthesis retention have worse outcomes than episodes treated with resection arthroplasty or 2-stage exchange. Future cohort studies are warranted to confirm these findings.
References
- 1.Ascione T, Pagliano P, Mariconda M, Rotondo R, Balato G, Toro A, Barletta V, Conte M, Esposito S. Factors related to outcome of early and delayed prosthetic joint infections. J Infect. 2015;70:30–36. doi: 10.1016/j.jinf.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 2.Dantas RC, Ferreira ML, Gontijo-Filho PP, Ribas RM. Pseudomonas aeruginosa bacteraemia: independent risk factors for mortality and impact of resistance on outcome. J Med Microbiol. 2014;63:1679–1687. doi: 10.1099/jmm.0.073262-0. [DOI] [PubMed] [Google Scholar]
- 3.Galliera E, Drago L, Marazzi MG, Romano C, Vassena C, Corsi Romanelli MM. Soluble urokinase-type plasminogen activator receptor (suPAR) as new biomarker of the prosthetic joint infection: Correlation with inflammatory cytokines. Clin Chim Acta. 2015;441:23–28. doi: 10.1016/j.cca.2014.11.029. [DOI] [PubMed] [Google Scholar]
- 4.Hsieh PH, Lee MS, Hsu KY, Chang YH, Shih HN, Ueng SW. Gram-negative prosthetic joint infections: risk factors and outcome of treatment. Clin Infect Dis. 2009;49:1036–1043. doi: 10.1086/605593. [DOI] [PubMed] [Google Scholar]
- 5.Kurtz SM, Ong KL, Schmier J, Mowat F, Saleh K, Dybvik E, Karrholm J, Garellick G, Havelin LI, Furnes O, Malchau H, Lau E. Future clinical and economic impact of revision total hip and knee arthroplasty. J Bone Joint Surg Am. 2007;89(Suppl 3):144–151. doi: 10.2106/JBJS.G.00587. [DOI] [PubMed] [Google Scholar]
- 6.Lepetit C, Le Gal S, Michon J, Collon S, Tillou X. Pseudomonas aeruginosa septic trapezo-metacarpal arthritis after prostate laser vaporization. Infection; 2015. [DOI] [PubMed] [Google Scholar]
- 7.Lora-Tamayo J, Euba G, Ribera A, Murillo O, Pedrero S, Garcia-Somoza D, Pujol M, Cabo X, Ariza J. Infected hip hemiarthroplasties and total hip arthroplasties: Differential findings and prognosis. J Infect. 2013;67:536–544. doi: 10.1016/j.jinf.2013.07.030. [DOI] [PubMed] [Google Scholar]
- 8.Marculescu CE, Berbari EF, Hanssen AD, Steckelberg JM, Harmsen SW, Mandrekar JN, Osmon DR. Outcome of prosthetic joint infections treated with debridement and retention of components. Clin Infect Dis. 2006;42:471–478. doi: 10.1086/499234. [DOI] [PubMed] [Google Scholar]
- 9.Marculescu CE, Cantey JR. Polymicrobial prosthetic joint infections: risk factors and outcome. Clin Orthop Relat Res. 2008;466:1397–1404. doi: 10.1007/s11999-008-0230-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Osmon DR, Berbari EF, Berendt AR, Lew D, Zimmerli W, Steckelberg JM, Rao N, Hanssen A, Wilson WR. Executive summary: diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2013;56:1–10. doi: 10.1093/cid/cis966. [DOI] [PubMed] [Google Scholar]
- 11.Parvizi J. New definition for periprosthetic joint infection. Am J Orthop (Belle Mead NJ) 2011;40:614–615. [PubMed] [Google Scholar]
- 12.Sculco TP. The economic impact of infected joint arthroplasty. Orthopedics. 1995;18:871–873. [PubMed] [Google Scholar]
- 13.Seyman D, Ozen NS, Inan D, Ongut G, Ogunc D. Pseudomonas aeruginosa septic arthritis of knee after intra-articular ozone injection. New Microbiol. 2012;35:345–348. [PubMed] [Google Scholar]
- 14.Trampuz A, Zimmerli W. Diagnosis and treatment of implant-associated septic arthritis and osteomyelitis. Curr Infect Dis Rep. 2008;10:394–403. doi: 10.1007/s11908-008-0064-1. [DOI] [PubMed] [Google Scholar]
- 15.Tumbarello M, De Pascale G, Trecarichi EM, Spanu T, Antonicelli F, Maviglia R, Pennisi MA, Bello G, Antonelli M. Clinical outcomes of Pseudomonas aeruginosa pneumonia in intensive care unit patients. Intensive Care Med. 2013;39:682–692. doi: 10.1007/s00134-013-2828-9. [DOI] [PubMed] [Google Scholar]
- 16.Veltman ES, Vos FJ, Meis JF, Goosen JH. Debridement, antibiotics and implant retention in early postoperative infection with Pseudomonas aeruginosa. J Infect. 2015;70:307–309. doi: 10.1016/j.jinf.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 17.Yilmaz M, Arslan F, Mert A. Community Acquired Chronic Arthritis due to Pseudomonas aeruginosa in a Previously Healthy Pregnant Woman. Case Rep Infect Dis. 2014;2014:272306. doi: 10.1155/2014/272306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zmistowski B, Fedorka CJ, Sheehan E, Deirmengian G, Austin MS, Parvizi J. Prosthetic joint infection caused by gram-negative organisms. J Arthroplasty. 2011;26:104–108. doi: 10.1016/j.arth.2011.03.044. [DOI] [PubMed] [Google Scholar]