Abstract
Photoactive preparations were obtained by treatment of photosynthetic membranes with lipid micelles and deoxycholate.
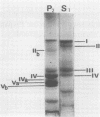
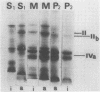
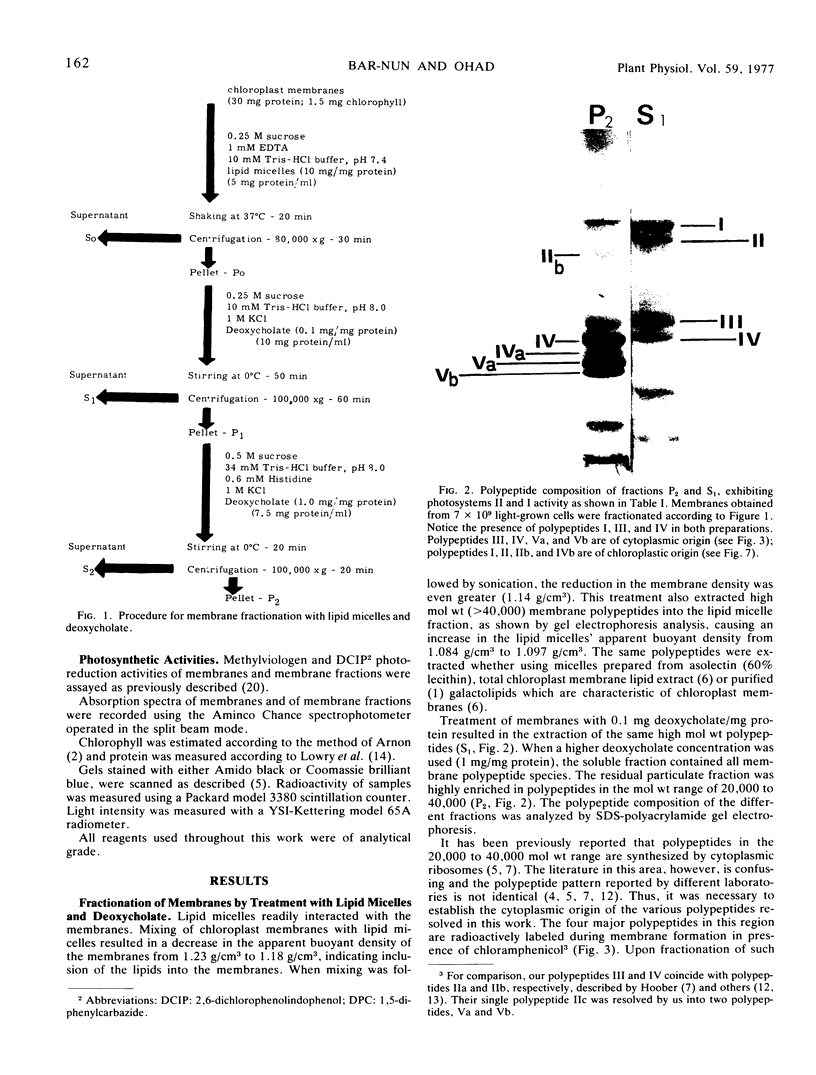
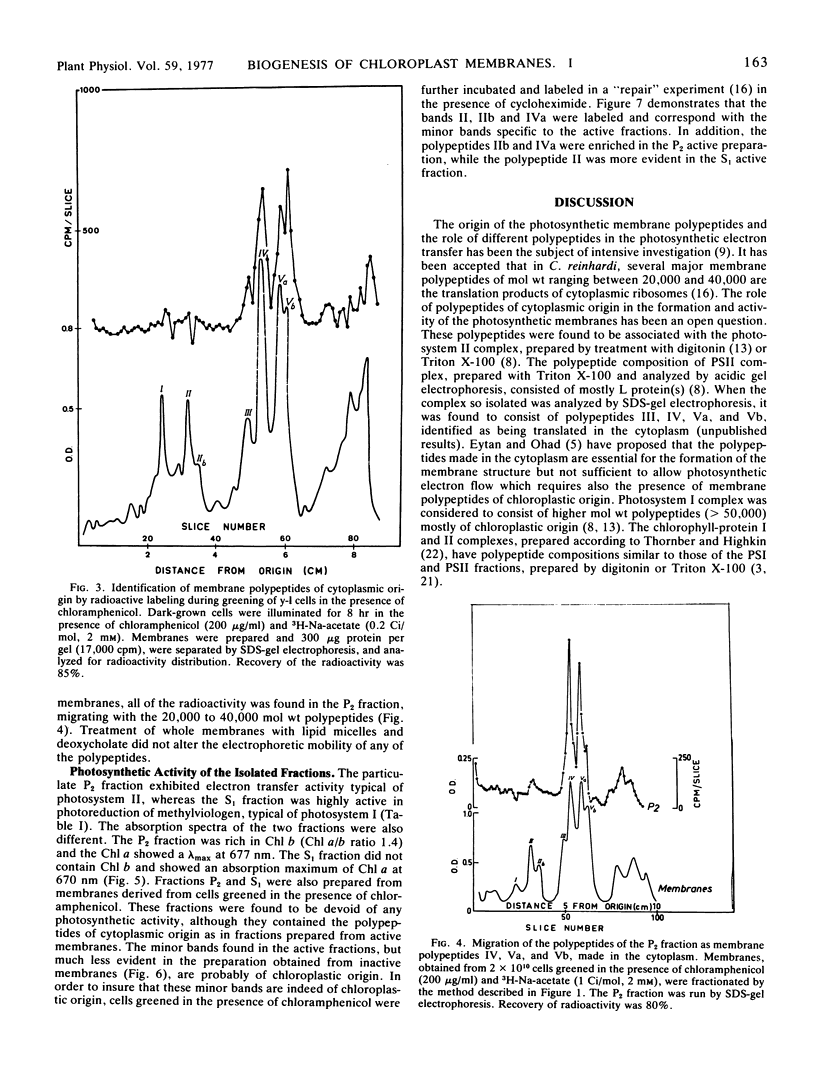
A photosystem I preparation can be extracted from the membranes by both lipid micelles and deoxycholate, whereas the photosystem II preparation remains in a particulate state.
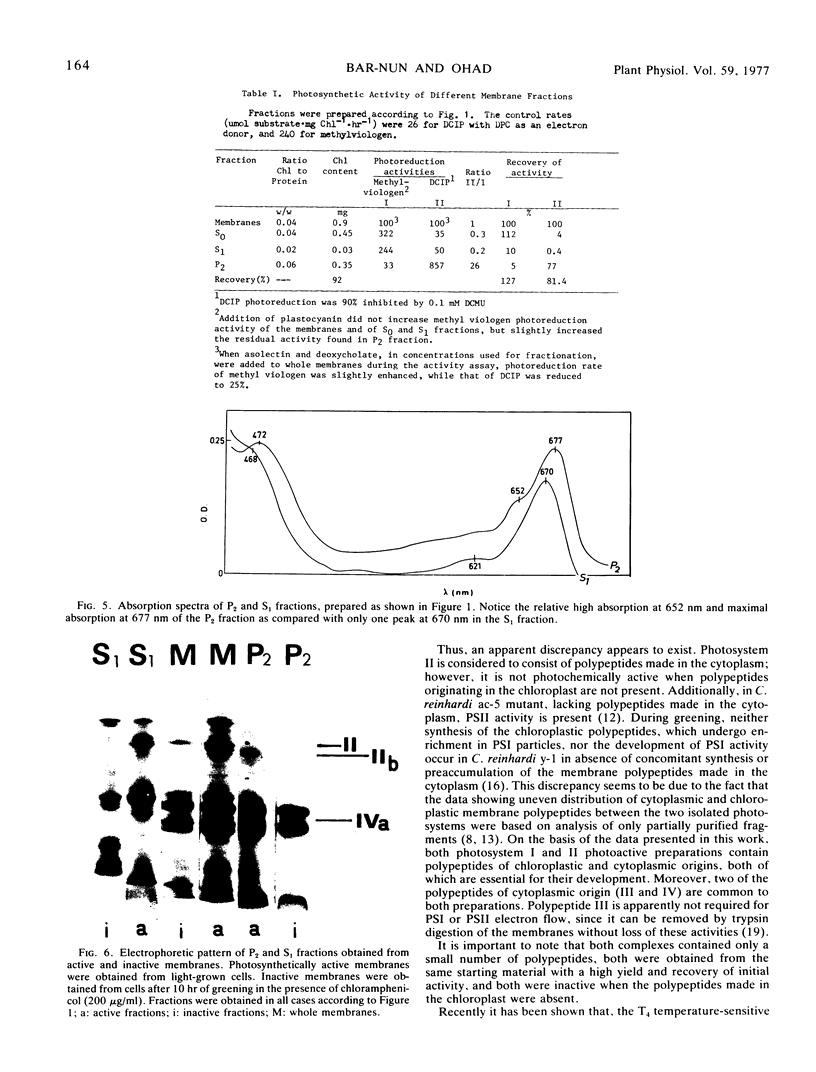
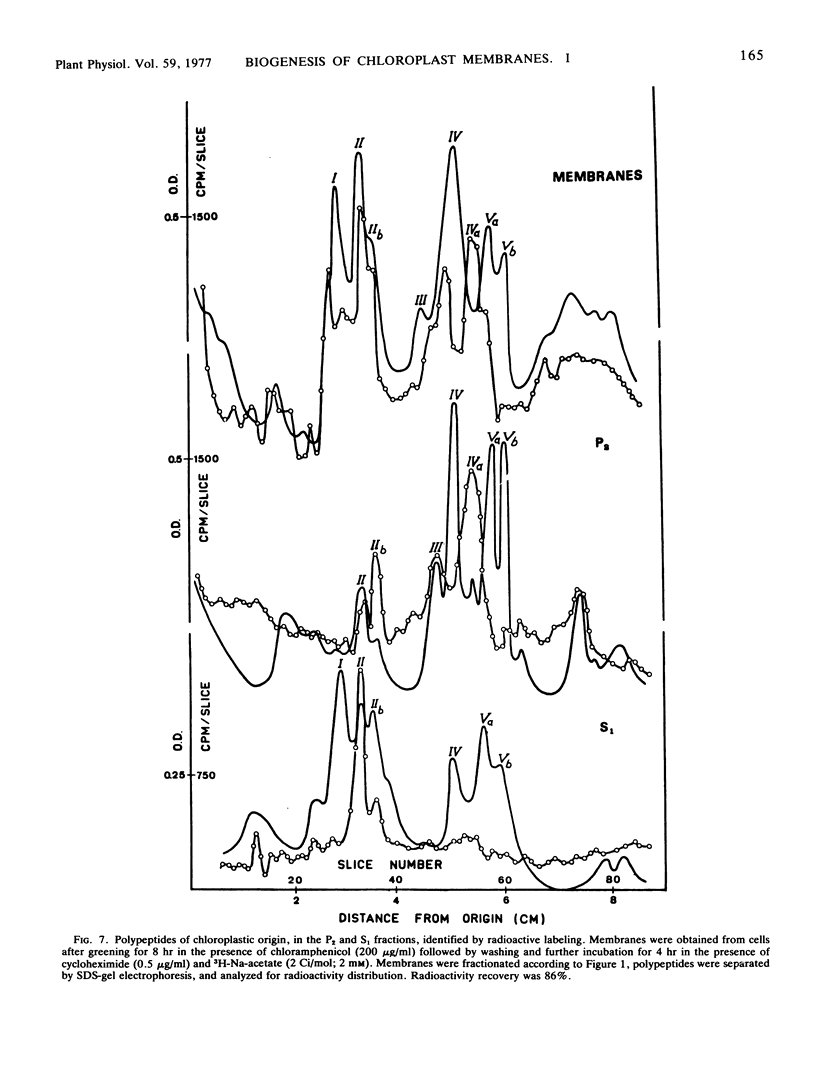
The photosystem II preparation exhibits electron transfer activity measured by photoreduction of 2,6-dichlorophenolindophenol with 1,5-diphenylcarbazide as an electron donor and has a ratio of chlorophyll a/b of 1.4. This preparation contains three major polypeptides of cytoplasmic origin and two of chloroplastic origin.
The photosystem I preparation exhibits high rates of methylviologen photoreduction with ascorbate and 2,6-dichlorophenolindophenol as electron donors and does not contain chlorophyll b. This preparation contains two major polypeptides of chloroplastic origin and one of cytoplasmic origin. These results indicate that both cytoplasm and chloroplast contribute polypeptides for the formation of photosystems I and II in Chlamydomonas reinhardi.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua N. H., Bennoun P. Thylakoid membrane polypeptides of Chlamydomonas reinhardtii: wild-type and mutant strains deficient in photosystem II reaction center. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2175–2179. doi: 10.1073/pnas.72.6.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg I., Ohad I. Biogenesis of chloroplast membranes. IV. Lipid and pigment changes during synthesis of chloroplast membranes in a mutant of Chlamydomonas reinhardi y-1. J Cell Biol. 1970 Mar;44(3):563–571. doi: 10.1083/jcb.44.3.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoober J. K. Sites of synthesis of chloroplast membrane polypeptides in Chlamydomonas reinhardi y-1. J Biol Chem. 1970 Sep 10;245(17):4327–4334. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levine R. P., Burton W. G., Duram H. A. Membrane polypeptides associated with photochemical systems. Nat New Biol. 1972 Jun 7;237(75):176–177. doi: 10.1038/newbio237176a0. [DOI] [PubMed] [Google Scholar]
- Levine R. P., Duram H. A. The polypeptides of stacked and unstacked Chlamydomonas reinhardi chloroplast membranes and their relation to photsystem II activity. Biochim Biophys Acta. 1973 Dec 14;325(3):565–572. doi: 10.1016/0005-2728(73)90216-8. [DOI] [PubMed] [Google Scholar]
- MacLennan D. H. Purification and properties of an adenosine triphosphatase from sarcoplasmic reticulum. J Biol Chem. 1970 Sep 10;245(17):4508–4518. [PubMed] [Google Scholar]
- Ohad I., Siekevitz P., Palade G. E. Biogenesis of chloroplast membranes. I. Plastid dedifferentiation in a dark-grown algal mutant (Chlamydomonas reinhardi). J Cell Biol. 1967 Dec;35(3):521–552. doi: 10.1083/jcb.35.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohad I., Siekevitz P., Palade G. E. Biogenesis of chloroplast membranes. II. Plastid differentiation during greening of a dark-grown algal mutant (Chlamydomonas reinhardi). J Cell Biol. 1967 Dec;35(3):553–584. doi: 10.1083/jcb.35.3.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regitz G., Ohad I. Trypsin-sensitive photosynthetic activities in chloroplast membranes from Chlamydomonas reinhardi, y-1. J Biol Chem. 1976 Jan 10;251(1):247–252. [PubMed] [Google Scholar]
- Schantz R., Bar-Nun S., Ohad I. Preparation of Antibodies against Specific Chloroplast Membrane Polypeptides Associated with the Formation of Photosystems I and II in Chlamydomonas reinhardi y-1. Plant Physiol. 1977 Feb;59(2):167–172. doi: 10.1104/pp.59.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornber J. P., Highkin H. R. Composition of the photosynthetic apparatus of normal barley leaves and a mutant lacking chlorophyll b. Eur J Biochem. 1974 Jan 3;41(1):109–116. doi: 10.1111/j.1432-1033.1974.tb03250.x. [DOI] [PubMed] [Google Scholar]
- Toson G., Contessa A. R., Bruni A. Solubilization of mitochondrial ATPase by phospholipids. Biochem Biophys Res Commun. 1972 Jul 25;48(2):341–347. doi: 10.1016/s0006-291x(72)80056-1. [DOI] [PubMed] [Google Scholar]