Abstract
Background: Implant-related infections remain a major complication after orthopaedic surgery. Antibacterial coating of implants may prevent bacterial adhesion and biofilm formation. However, in spite of extensive preclinical research in the field, antibacterial coatings to protect orthopaedic implants in the clinical setting remain particularly few. The aim of the present study is to evaluate the safety of a calcium-based, antibiotic-loaded bone substitute as an antibacterial coating of cementless joint prosthesis.
Methods: From March 2013 to August 2015, 20 consecutive patients scheduled for cementless or hybrid two-stage revision surgery for peri-prosthetic joint infection were included in this prospective, observational, pilot study. Cerament G or Cerament V, a gentamicin or vancomycin-loaded calcium-based resorbable bone substitute (60% calcium sulphate, 40% hydroxyapatite), was applied at surgery on the stem surface of hip (n=7) or knee (n=13) revision prosthesis. After surgery, all patients underwent clinical (HHS or KSS and SF-12 score), laboratory and radiographic evaluation at 3, 6 and 12 months and yearly thereafter.
Results: At a minimum of 12 months follow-up, 19/20 (95%) patients showed no recurrence of infection and no signs of radiographic loosening of the stem. No adverse events were associated with the use of Cerament G or V.
Conclusions: This is the first pilot clinical study on the short-term safety of using a calcium-based, gentamicin or vancomycin-loaded bone substitute as a surface coating on cementless prosthetic implants. If confirmed by larger studies and at longer follow-ups, these findings may open a new prospective to protect intra-operatively orthopedic implants from bacterial adhesion, through the use of resorbable, osteoconductive, antibiotic carriers.
Keywords: Peri-prosthetic infection, Coating, Calcium sulphate, Hydroxyapatite.
Introduction
Periprosthetic joint infection (PJI) remains one of the most feared complications after orthopaedic surgery 1. Factors that are associated with an increased risk of post-surgical infection include the presence of an implanted biomaterial 2, while host's related factors, such as diabetes, renal failure, peripheral vasculopathy and smoking, may raise the risk by more than 20 times, compared to the general population, in spite of current antibiotic prophylaxis 3.
The ability of microorganisms to adhere to the implant and to immediately produce a protective biofilm layer is currently considered one of the main reasons why the treatment of implant-related infection remains particularly challenging 4, 5, 6. As a result, any strategy aimed at protecting the implant at the time of surgery, to prevent bacterial adhesion and biofilm formation on the implant surface, can be extremely helpful in reducing PJIs, especially in high risk patients 7-9.
In the last decade, several synthetic calcium-based bone substitutes have been developed to deliver various local antibiotics 10, 25, and represent an established valid alternative to autologous or allograft bone grafts as a method to treat bone loss 11. Among the several advantages, compared to bone grafts, synthetic bone substitutes offer unlimited availability, avoid donor site morbidity, and provide immediate mechanical support and osteoconduction to fill bone defects 12. Moreover, they may be pre-loaded with antibiotics, thus acting as local antibacterial carriers. Antibiotic impregnation allows high local concentration of antibiotics, limiting systemic side effects and ensuring the delivery of antibiotic where needed in a way that is not dependent on vascular supply 13, 14.
Cerament (BoneSupport AG, Lund, Sweden) is an injectable bone substitute, composed of 60% w/w fast resorbing calcium sulphate, which is intended to be quickly replaced by newly formed bone, and 40% w/w of calcium hydroxyapatite, that acts as a long-lasting scaffold to allow further bone ingrowth 15. Cerament G and Cerament V incorporate gentamicin sulphate (175 mg/10 mL) and vancomycin (66 mg/mL), respectively, and preclinical studies have demonstrated their efficacy in treating infected bone defects 16 and no detrimental effects on osteointegration when used as a coating on implants 17.
The aim of the present prospective, observational pilot clinical study was to evaluate the use of Cerament G or Cerament V applied intra-operatively as a coating of cementless joint prosthesis, in order to provide additional local antibacterial protection, without interfering with implant osteointegration.
Materials and Methods
From March 2013 to August 2015, 20 consecutive patients scheduled for two-stage hip or knee prosthesis reimplantation for PJI or septic osteoarthritis, were included in this single center, prospective, observational study (Table 1). Inclusion criteria were the need for joint reimplantation using a cementless or hybrid revision prosthesis, and previous infection caused by microorganism(s) sensitive to gentamicin or vancomycin. Exclusion criteria were the need of a fully cemented joint prosthesis, psychiatric or neurological disorders, pregnancy or breastfeeding at the time of surgery and hypersensitivity to aminoglycosides. Our local Ethical Committee approved the study. All patients were informed and gave their consent to participation to this study.
Table 1.
Demographics, pre-clinical data, diagnosis, surgical procedures and results of included patients.
| N. | Sex | Age | Relevant co-morbidities | Diagnosis | Primary Organisms | Cerament G or V | Follow-up (months) | Outcome | Complications | Adverse Events | SF12 (P +M) | KSS | HHS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 78 | Type 2 diabetes | TKA infected | Streptococcus Gallolyticus | Cerament G | 36 | No infection | None | 85,6 | 86 | NA | |
| 2 | F | 65 | None | THA infected | None Isolated | Cerament G | 34 | No infection | None | None | 96 | NA | 88 |
| 3 | M | 89 | None | THA infected | Staphylococcus Epidermidis | Cerament G | 32 | No infection | None | None | 90.5 | NA | 91 |
| 4 | F | 77 | Chronic renal insufficiencyCongestive heart failure | THA infected | Staphylococcus Epidermidis | Cerament G | 30 | No infection | Heterotopic ossification (Brooker grade II) | None | 88 | NA | 81 |
| 5 | F | 71 | None | TKA infected | None Isolated | Cerament G | 29 | No infection | None | None | 95 | 76 | NA |
| 6 | F | 59 | Thyroiditis | THA infected | Paenibacillus Spp. MS | Cerament G | 29 | No infection | None | None | 99,7 | NA | 86 |
| 7 | F | 71 | Type 2 diabetes | THA infected | Stenotrophomonas spp | Cerament G | 28 | No infection | None | None | 87,3 | NA | 70 |
| 8 | M | 71 | Type 2 diabetes | TKA infected | Staphylococcus Aureus | Cerament G | 24 | No infection | None | None | 90.8 | 86 | NA |
| 9 | F | 88 | None | TKA infected | None Isolated | Cerament G | 20 | No infection | None | None | 84 | 72 | NA |
| 10 | F | 65 | None | Septic knee osteoarthitis after exposed proximal tibia fracture | Pseudomonas Aeruginosa | Cerament G | 20 | Infection | Surgical wound dehiscence | None | 69,1 | 64 | NA |
| 11 | M | 51 | None | TKA infected | None Isolated | Cerament G | 12 | No infection | None | None | 98 | 85 | NA |
| 12 | M | 40 | None | TKA infected | Staphylococcus Epidermidis | Cerament V | 12 | No infection | None | None | 100 | 80 | NA |
| 13 | F | 49 | None | THA infected | Staphylococcus Aureus | Cerament V | 12 | No infection | None | None | 88 | NA | 80 |
| 14 | F | 68 | None | THA infected | Staphylococcus Lugdunensis | Cerament V | 12 | No infection | None | None | 90.5 | NA | 82,6 |
| 15 | F | 77 | Bradiarrhytmia, Psoriasis | TKA infected | Enterococcus FaecalisEscherichia ColiStaphylococcus Aureus | Cerament V | 12 | No infection | None | None | 77,2 | 77 | NA |
| 16 | M | 68 | Prostatic cancer with bone metastasis | TKA infected | Staphylococcus EpidermidisStaphylococcus LentusEnterococcus Casseliflavus Group D | Cerament V | 12 | No infection | None | None | 73,8 | 72 | NA |
| 17 | F | 70 | None | TKA loosening | None Isolated | Cerament G | 12 | No infection | None | None | 87,7 | 80 | NA |
| 18 | M | 63 | Hypertension, ischemic heart disease | TKA infected | None Isolated | Cerament V | 12 | No infection | None | None | 80 | 75,5 | NA |
| 19 | M | 81 | None | TKA infected | None Isolated | Cerament G | 12 | No infection | None | None | 76 | 75 | NA |
| 20 | M | 57 | Hyper tension,Type 2 diabetes | Septic non union distal femur and knee septic osteoarthritis | Staphylococcus capitis | Cerament V | 12 | No infection | None | None | 77 | 77 | NA |
All patients underwent a first stage procedure, including removal of all foreign infected material and complete debridement of bone and soft tissues, followed by implantation of a preformed hip or knee gentamicin-loaded spacer (Spacer G or Spacer K, Tecres SpA, Sommacampagna, Italy). During surgery, four to six samples of periprosthetic tissue were collected from different sites. Solid tissue samples from periprosthetic tissue were placed into sterile cases, and were subsequently cultured in anaerobic and aerobic agar media and in thyoglicolate broth enriched with vitamin K and hemin. After an incubation time of ten days, positive cultures were sent for organism identification and sensitivity testing.
After an interval period ranging from 8 to 12 weeks, all patients underwent revision surgery with a hip (A-Aequa, AdlerOrtho Srl, Milan, Italy) or knee (TC3, DePuy Inc., Warsaw, USA or Endomodel, Waldemar-Link GmbH, Hamburg, Germany) revision prosthesis.
At the time of implantation, the prosthetic stem and cup (for hip prosthesis) was fully coated using 10 to 20 mL of Cerament G or Cerament V (Figure 1). Cerament was prepared according to manufacturers guidelines during the surgical procedure. Four minutes after the completion of the preparation process, Cerament was directly applied with a disposable syringe onto the surface of the prosthesis stem, which was immediately implanted according to our standard practice. The hip prostheses were completely cementless, whilst the epiphyseal part of the knee implants were secured with a tobramycin-loaded bone cement (Simplex, Stryker Inc., Mahwah, NJ, USA).
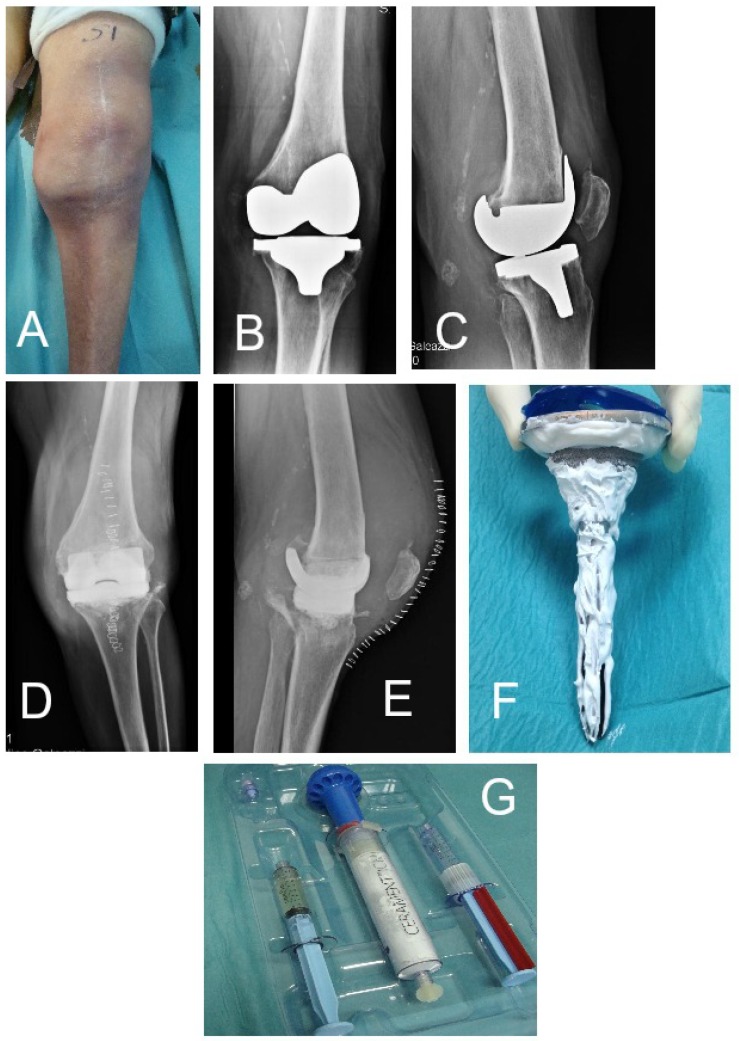
Figure 1.
Patient n. 3 (see Table 1). (A) pre-operative clinical aspect, (B,C) pre-operative radiographic findings with implant loosening and osteolysis and (D,E) after antibiotic-loaded spacer implanted. (F,G) Cerament G preparation kit and the coated implant at the time of reimplantation.
Follow-up consisted of clinical, laboratory and radiographic examination at approximately 3, 6 and 12 months after surgery and yearly thereafter. The average follow up time was 18.1 months ± 6.7 (range 12-36).
Harris Hip Scores (HHS) or Knee Society Scores (KSS), and short form (SF)-12 were collected at every follow-up visit. Recurrence of infection, defined as the presence of clinical signs of inflammation/infection at the surgical site and an elevation of C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR) serum levels, was recorded.
Radiographic bone healing, the presence of radiolucent lines, and implant loosening were monitored at follow-ups by an independent radiologist; local or systemic clinical side effects or the need for unplanned further surgery were also recorded.
Results
A total of 20 patients (8 males, 12 females) were included in the study. The mean age was 67.8 years old (range 40 - 89 years). Each patient completed a minimum follow up of 12 months (maximum 36 months, mean follow up 18.1 months ± 6.7). Microbiological data with isolated organisms and antibiotic regimen are reported in Table 1, together with KSS or HSS scores, SF-12 evaluation, infection recurrence and adverse effects.
At the latest follow-up, there was no clinical or laboratory evidence of infection recurrence in 19 patients (95%) and no radiographic signs of implant loosening (cf. Figure 2). In particular, non progressive radiolucent lines were observed in two patients (2 and 3) with hip prosthesis in Gruen zone 1 and 7. No detectable subsidence of the implants was detected at the latest follow-up.
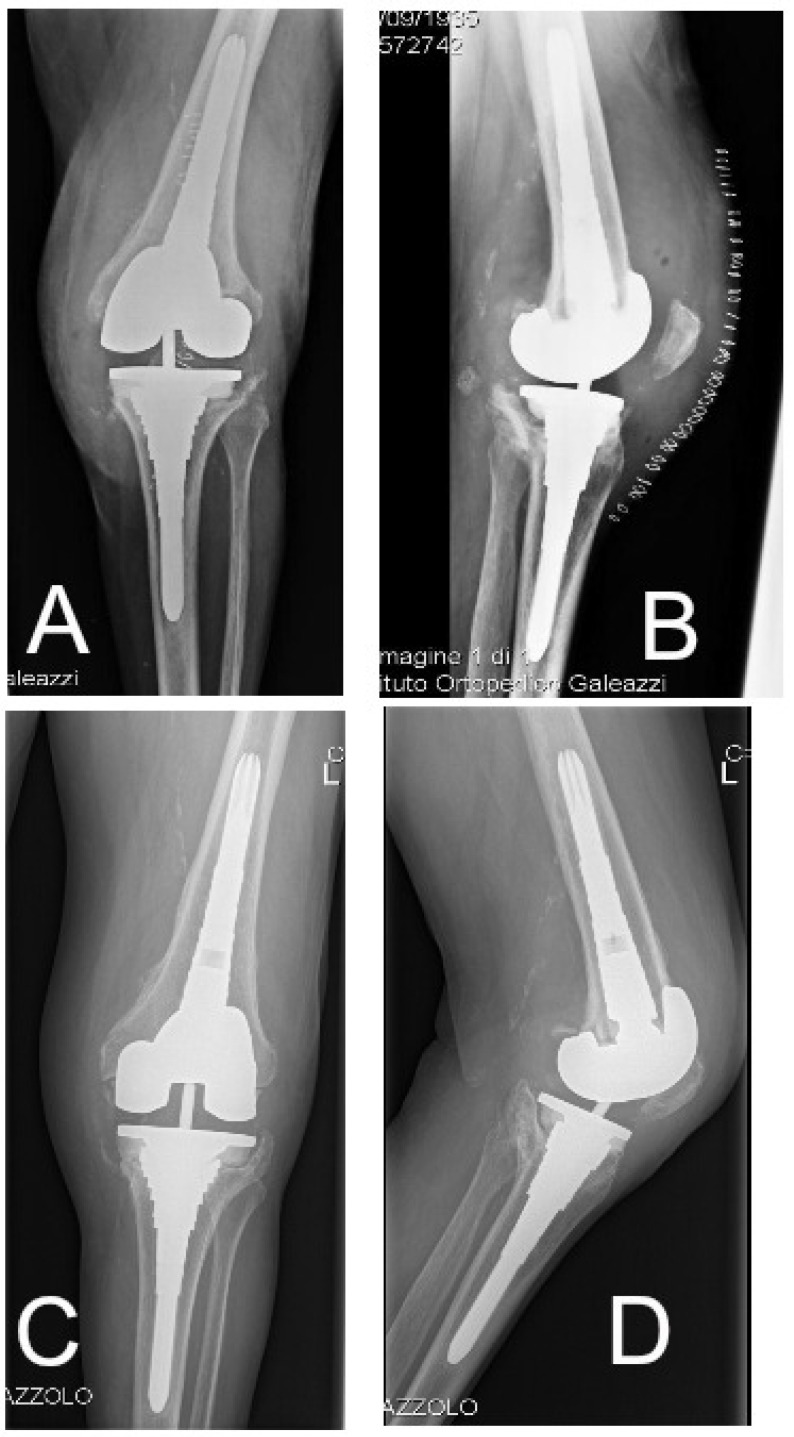
Figure 2.
Plain radiographs of the same patient described in Figure 1 immediately after surgery (A,B), at 12 months (C,D).
No adverse events were associated with the use of Cerament G or V.
Infection recurrence occurred in patient number 10. She was a 65 year old woman affected by a severe septic knee osteoarthritis, caused by P.aeruginosa after an exposed proximal third tibia fracture with associated extensive soft tissue damage and multiple scars. Following a two-stage knee joint prosthesis, with a Cerament G coated implant, she developed a skin necrosis with wound dehiscence that required early surgical debridement with partial revision of the prosthesis and a muscle flap. At the latest follow-up, she has a persistent small fistula, controlled by suppressive antibiotic treatments and she refused further surgery.
Discussion
To our knowledge, this is the first study reporting the use of a calcium-based, antibiotic-loaded bone substitute used as a coating on cementless or hybrid joint implants. It is well recognized that implant features, including size, shape, material and surface finishing play an important role in the onset or prevention of PJI 18. Several antibacterial coating technologies are currently under review, but only few are currently commercially available for clinical use 9, 25.
Allogenic antibiotic-loaded cancellous bone graft has been shown to be effective for delivering local antibiotics when used during total joint arthroplasty 20. However, their limited availability, risk of disease transmission and costs prevent more widespread use of this resource. To overcome these limitations, various synthetic bone substitutes have been introduced to the market,21-24 but their application as a coating on implants has not been previously reported.
Cerament was developed as an injectable bone substitute, acting both as a temporary filler and a scaffold for bone remodeling 15. In a recent study, Cerament was used as a filler in tibial plateau fractures, showing satisfactory radiological and clinical outcome with a mean follow up of 44 months. However, the presence of infection was an exclusion criteria in this study 26.
Cerament G and Cerament V, the more recently available version of Cerament, can be utilized in septic bone defects, providing local antibiotic elution with a peak level in the first 3 hours and sustained release above minimal inhibitory concentration (MIC) for up to 28 days. [BoneSupport data on file] The semi-liquid formulation, that lasts several minutes after the preparation process before hardening, enables it to be easily spread onto a surface.
This pilot study has shown for the first time that Cerament G or V can be safely applied at the time of orthopaedic surgery as a coating on a cementless or hybrid implant. However, our findings do have some limitations; histological analysis was not undertaken to confirm osteointegration of the coated implants, nor were microbiological investigations performed to assess the ability of the Cerament G or V to prevent bacterial adhesion and biofilm formation when acting as a coating. There is a need for a larger, prospective, comparative study to further analyze its efficiency, with a longer follow-up.
Conclusions
This is the first pilot clinical study on the short-term safety of using a calcium-based, gentamicin or vancomycin-loaded bone substitute as a surface coating on cementless and hybrid prosthetic implants. Our data, although in a limited case series, demonstrates the safety of this application with promising results in infection prevention. If confirmed by larger studies, these findings may open a new prospective to protect intra-operatively orthopedic implants from bacterial adhesion, through the use of resorbable, osteoconductive, antibiotic carriers.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards
References
- 1.Romano CL, Romano D, Logoluso N, Drago L. Bone and joint infections in adults: a comprehensive classification proposal. Eur Orthop Traumatol. 2011;1(6):207–17. doi: 10.1007/s12570-011-0056-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lentino JR. Prosthetic joint infections: bane of orthopedists, challenge for infectious disease specialists. Clin Infect Dis. 2003;36(9):1157–61. doi: 10.1086/374554. [DOI] [PubMed] [Google Scholar]
- 3.Pruzansky JS, Bronson MJ, Grelsamer RP, Strauss E, Moucha CS. Prevalence of modifiable surgical site infection risk factors in hip and knee joint arthroplasty patients at an urban academic hospital. J Arthroplasty. 2014;29(2):272–6. doi: 10.1016/j.arth.2013.06.019. [DOI] [PubMed] [Google Scholar]
- 4.Gristina AG, Shibata Y, Giridhar G, Kreger A, Myrvik QN. The glycocalyx, biofilm, microbes, and resistant infection. Semin Arthroplasty. 1994;5(4):160–70. [PubMed] [Google Scholar]
- 5.Gristina AG. Biomaterial-centered infection: microbial adhesion versus tissue integration. Science. 1987;237(4822):1588–95. doi: 10.1126/science.3629258. [DOI] [PubMed] [Google Scholar]
- 6.Gristina AG, Naylor P, Myrvik Q. Infections from biomaterials and implants: a race for the surface. Med Prog Technol. 1988;14(3-4):205–24. [PubMed] [Google Scholar]
- 7.Jamsen E, Furnes O, Engesaeter LB. et al. Prevention of deep infection in joint replacement surgery. Acta Orthop. 2010;81(6):660–6. doi: 10.3109/17453674.2010.537805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van de Belt H, Neut D, Schenk W, van Horn JR, van der Mei HC, Busscher HJ. Infection of orthopedic implants and the use of antibiotic-loaded bone cements. A review. Acta Orthop Scand. 2001;72(6):557–71. doi: 10.1080/000164701317268978. [DOI] [PubMed] [Google Scholar]
- 9.Romano CL, Scarponi S, Gallazzi E, Romano D, Drago L. Antibacterial coating of implants in orthopaedics and trauma: a classification proposal in an evolving panorama. J Orthop Surg Res. 2015;10(1):157. doi: 10.1186/s13018-015-0294-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hake ME, Young H, Hak DJ, Stahel PF, Hammerberg EM, Mauffrey C. Local antibiotic therapy strategies in orthopaedic trauma: Practical tips and tricks and review of the literature. Injury. 2015;46(8):1447–56. doi: 10.1016/j.injury.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 11.Kurien T, Pearson RG, Scammell BE. Bone graft substitutes currently available in orthopaedic practice: the evidence for their use. Bone Joint J. 2013;95b(5):583–97. doi: 10.1302/0301-620X.95B5.30286. [DOI] [PubMed] [Google Scholar]
- 12.Campana V, Milano G, Pagano E. et al. Bone substitutes in orthopaedic surgery: from basic science to clinical practice. J Mater Sci Mater Med. 2014;25(10):2445–61. doi: 10.1007/s10856-014-5240-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hanssen AD. Local antibiotic delivery vehicles in the treatment of musculoskeletal infection. Clin Orthop Relat Res. 2005;437:91–6. doi: 10.1097/01.blo.0000175713.30506.77. [DOI] [PubMed] [Google Scholar]
- 14.Zalavras CG, Patzakis MJ, Holtom P. Local antibiotic therapy in the treatment of open fractures and osteomyelitis. Clin Orthop Relat Res. 2004;427:86–93. doi: 10.1097/01.blo.0000143571.18892.8d. [DOI] [PubMed] [Google Scholar]
- 15.Abramo A, Geijer M, Kopylov P, Tägil M. Osteotomy of distal radius fracture malunion using a fast remodeling bone substitute consisting of calcium sulphate and calcium phosphate. Applied Biomaterials. 2010;92B(1):281–6. doi: 10.1002/jbm.b.31524. [DOI] [PubMed] [Google Scholar]
- 16.Dvorzhinskiy A, Perino G, Chojnowski R, Van Der Meulen M, Ross F, Bostrom M, Yang N. Cerament bone void filler with gentamicin increases bone formation and decreases detectable infection in a rat model of debrided osteomyelitis. Bone Joint J. 2015;97B:9. doi: 10.5194/jbji-6-283-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zampelis V, Tagil M, Lidgren L, Isaksson H, Atroshi I, Wang JS. The effect of a biphasic injectable bone substitute on the interface strength in a rabbit knee prosthesis model. J Orthop Surg Res. 2013;8:25. doi: 10.1186/1749-799X-8-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moriarty TF, Schlegel U, Perren S, Richards RG. Infection in fracture fixation: can we influence infection rates through implant design? J Mater Sci Mater Med; 2010. p. 21. [DOI] [PubMed] [Google Scholar]
- 19.Cats-Baril W, Gehrke T, Huff K, Kendoff D, Maltenfort M, Parvizi J. International consensus on periprosthetic joint infection: description of the consensus process. Clin Orthop Relat Res. 2013;471:4065–75. doi: 10.1007/s11999-013-3329-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anagnostakos K, Schroder K. Antibiotic-impregnated bone grafts in orthopaedic and trauma surgery: a systematic review of the literature. Int J Biomater. 2012;2012:538061. doi: 10.1155/2012/538061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garcia-Gareta E, Coathup MJ, Blunn GW. Osteoinduction of bone grafting materials for bone repair and regeneration. Bone. 2015;81:112–21. doi: 10.1016/j.bone.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 22.Watson JT. The use of an injectable bone graft substitute in tibial metaphyseal fractures. Orthopedics. 2004;27(1 Suppl):s103–7. doi: 10.3928/0147-7447-20040102-04. [DOI] [PubMed] [Google Scholar]
- 23.Jansen J, Ooms E, Verdonschot N, Wolke J. Injectable calcium phosphate cement for bone repair and implant fixation. Orthop Clin North Am. 2005;36(1):89–95. doi: 10.1016/j.ocl.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 24.Geurts J, Chris Arts JJ, Walenkamp GH. Bone graft substitutes in active or suspected infection. Contra-indicated or not? Injury. 2011;42(Suppl 2):S82–6. doi: 10.1016/j.injury.2011.06.189. [DOI] [PubMed] [Google Scholar]
- 25.Kanellakopoulou K, Giamarellos-Bourboulis EJ. Carrier systems for the local delivery of antibiotics in bone infections. Drugs. 2000;59(6):1223–32. doi: 10.2165/00003495-200059060-00003. [DOI] [PubMed] [Google Scholar]
- 26.Iundusi R, Gasbarra E, D'Arienzo M, Piccioli A, Tarantino U. Augmentation of tibial plateau fractures with an injectable bone substitute: Cerament. Three year follow-up from a prospective study. BMC Musculoskelet Disord. 2015;16:115. doi: 10.1186/s12891-015-0574-6. [DOI] [PMC free article] [PubMed] [Google Scholar]