Abstract
Pseudomonas oryzihabitans is a saprophytic gram-negative microorganism usually found in damp environments, only occasionally responsible for human pathology. Infection mainly occurs in malnourished, immunocompromised individuals with indwelling catheters. There is no previous published record of infection after joint arthroplasty. To enhance the literature, in this article we report a patient with a Pseudomonas oryzihabitans infected total hip arthroplasty, and discuss the diagnosis and management of this unusual infection.
Keywords: Total hip arthroplasty, Periprosthetic joint infection, Pseudomonas oryzihabitans.
Introduction
Pseudomonas oryzihabitans is a gram-negative, non-fermenting, oxidase-negative, rod-shaped bacterium. It typically produces transparent or yellow-stained, rough, wrinkled colonies after 48 hours' incubation in agar media 1, 2. It is commonly isolated in moist environment such as soil and rice paddles 3. In the hospital setting, it has been recovered from sink drains and respiratory equipment 4. Pseudomonas oryzihabitans is only a rare cause of human infection. It has been reported to cause sepsis in children, bacteremia in critically ill or immunocompromised patients, peritonitis in patients undergoing ambulatory peritoneal dialysis, endophthalmitis, as well as central-venous-catheter-related infections, with the latter being the most frequently reported 5-9. The literature on this infection is scarse, represented mainly by infrequent case reports. To our knowledge, there is no documented case of periprosthetic joint infection (PJI) associated with Pseudomonas oryzihabitans in related literature. Therefore, to enhance the literature and increase the awareness of the treating physicians on this bacterial isolate infection, we report a patient with a Pseudomonas oryzihabitans infected total hip arthroplasty (THA) and discuss the diagnosis and management of this unusual infection.
Case report
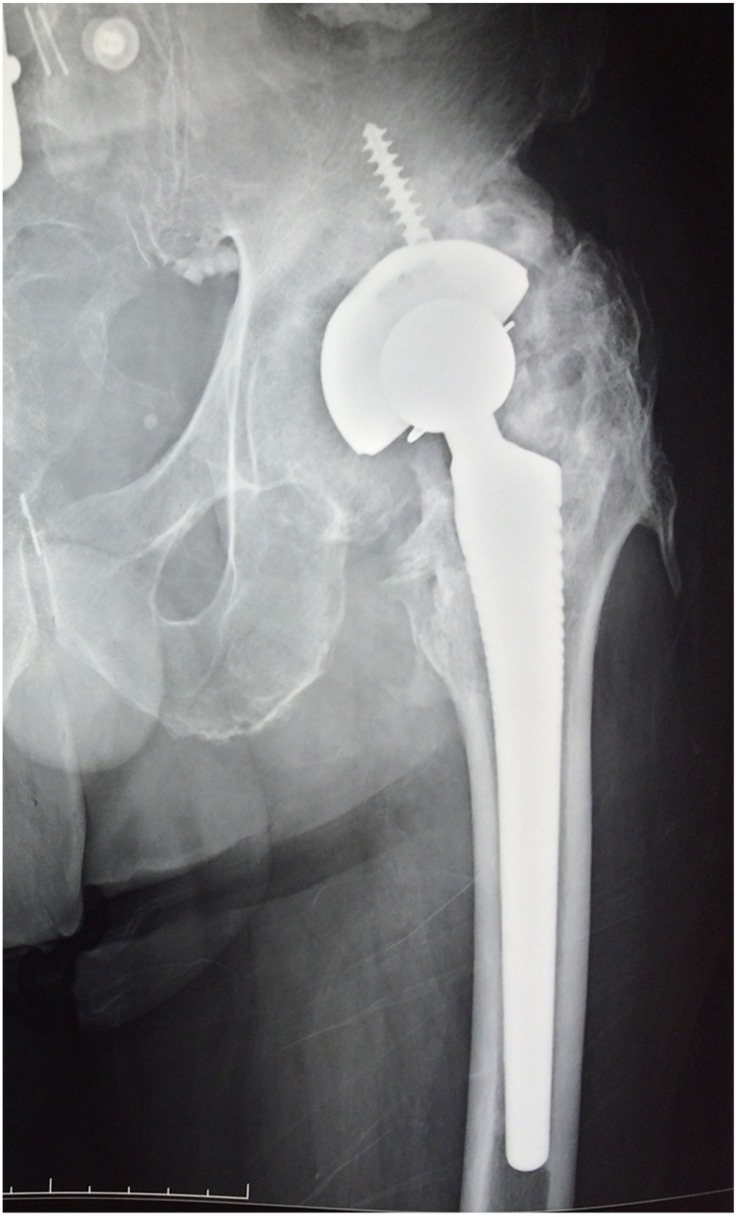
A 72-year-old man presented with left hip pain and intermittent low grade fever, as well as occasional yellowish drainage from a sinus tract at the surgical incision of a cementless THA performed 4 years before at another hospital. Patient's past medical history was also significant for a 2-vessel coronary artery bypass graft (CABG), performed one year prior to the current presentation. Prior use of steroids, a previous state of immunosuppression, or recent travel was not reported. The patient reported multiple prior hospital admissions due to drainage, allegedly attributed to a superficial infection and suppressed multiple times with multiple courses of per os and/or intravenous antibiotics including vancomycin, clindamycin, ciprofloxacin and cotrimoxazole, only to recur a few months after each episode and antibiotics regimen. Anteroposterior (AP) pelvis, AP and lateral hip radiographs as well as laboratory findings were consistent with PJI, as well as heterotopic ossification adjacent to the left hip joint (Fig. 1). Preoperative cultures collected with wound swabs showed Staphylococcus hominis and Staphylococcus capitis isolates of debatable clinical significance; cultures of synovial fluid aspiration were negative. With the assumptive diagnosis of PJI and septic loosening of the implants a joint decision was obtained for a staged THA revision.
Figure 1.
Preoperative anteroposterior radiograph of the left hip shows the infected THA and the adjacent heterotopic ossification.
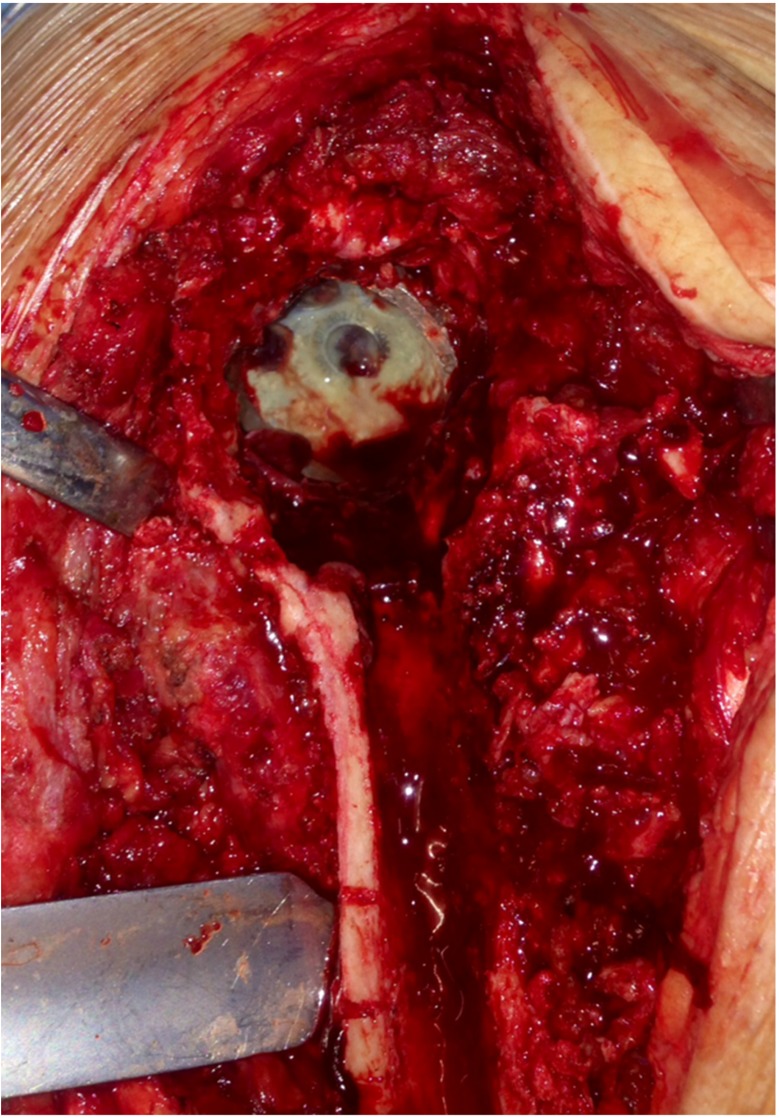
The patient was scheduled for the first stage THA revision 4 weeks after cessation of any antibiotics. Through a posterior hip approach, the femoral component was removed via a Paprosky-style extended proximal femoral osteotomy 10. Next, the acetabular cup and supporting screw were removed (Fig. 2). Eight tissue samples were obtained from the bone, joint capsule and femoral canal for cultures, and the implants were sent for sonication. We routinely use sonication to identify bacteria in the biofilm on explanted implants, as this method has been found to be significantly more sensitive than tissue biopsy, while maintaining almost identical specificity 11, 12. A custom-made polymethyl-methacrylate (PMMA) hip spacer was fashioned and placed in the femoral lumen. We used gentamicin-loaded Palacos R+G® bone cement (Zimmer, Inc. Warsaw, IN). When using an antibiotic loaded bone cement, we routinely admixture a second antibiotic (preferably vancomycin or other based on preoperative cultures and antibiogram, if available) to increase the local concentration of antibiotics and to obtain a synergistic effect, as adding a second antibiotic seems to increase the elution of both antibiotics 13, 14. The osteotomy was repaired with cerclage wires (Fig. 3). The sinus tract was resected. The patient was given 600mg of teicoplanin iv intraoperatively and was subsequently started on a standing daily dose of the same regimen.
Figure 2.
Intraoperative photograph shows the infected acetabular cup. The femoral stem has been removed via Paprosky osteotomy.
Figure 3.
Postoperative anteroposterior radiograph after first stage revision shows the custom PMMA spacer that was fashioned and placed in the femoral lumen and the Paprosky osteotomy repair with cerclage wires.
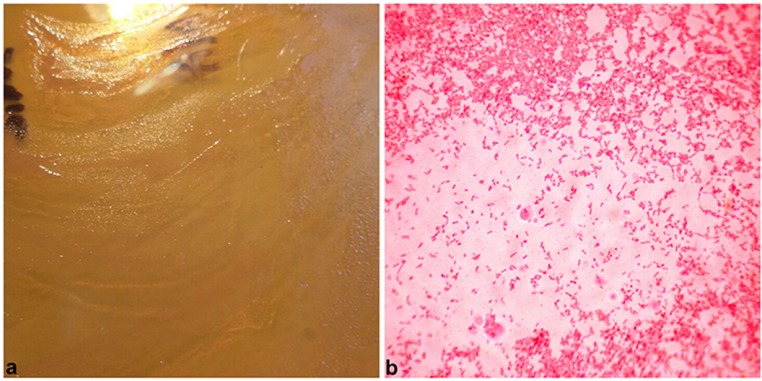
Postoperative recovery was uneventful and the patient was afebrile without any wound healing problems. Partial weight bearing with a walker was started on postoperative day two, and progressed as tolerated. Intraoperative tissue cultures were negative. Sonication of the implant at 15 days showed Pseudomonas oryzihabitans bacterial isolate (Fig. 4). Based on antibiotic susceptibilities, intravenous teicoplanin 600 mg and piperacillin/tazobactam 4.5 g qid was administered for 3 weeks, followed by ciprofloxacin 750 mg bid and clindamicin 600 mg qid for another four weeks, aiming for a second stage revision surgery. However, because of cardiovascular comorbidities, the patient was informed for the high risk associated with a second operation and did not consent for further surgery.
Figure 4.
Transparent, rough, wrinkled colonies, cultured from material obtained with sonication of the removed prosthesis (a); Gram stain (400x) reveals small, rod-shaped, gram-negative bacteria (b).
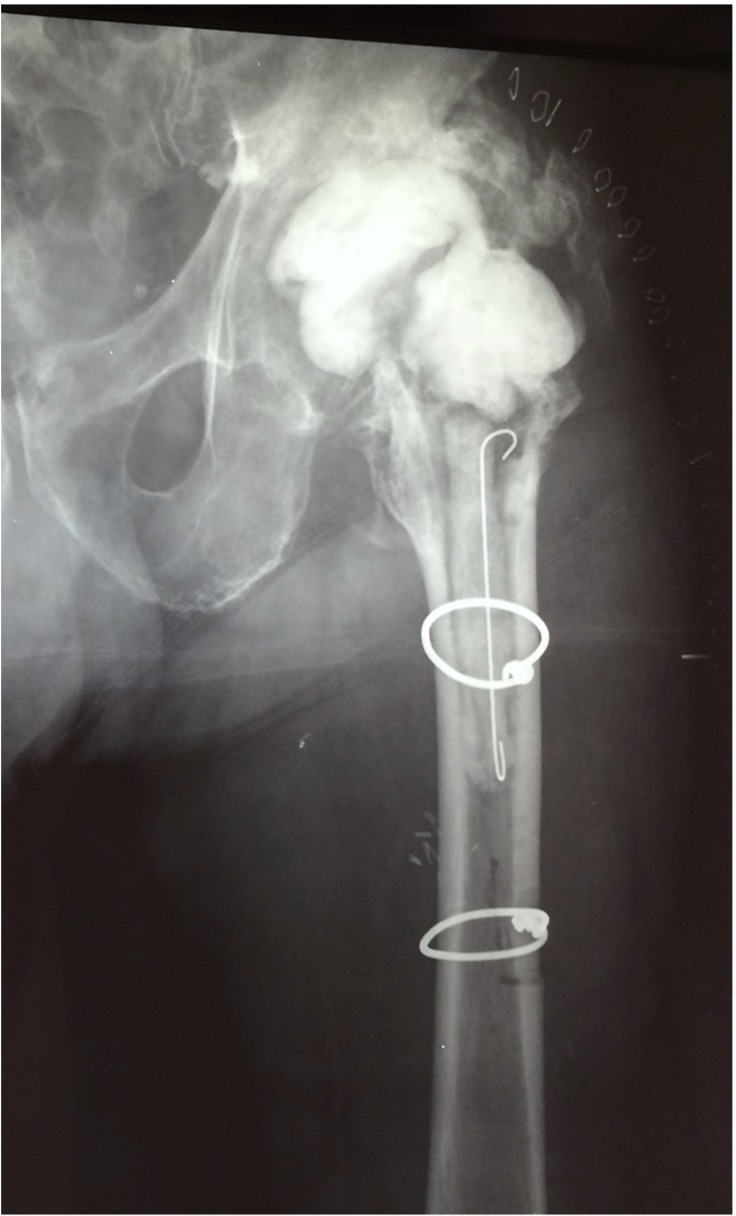
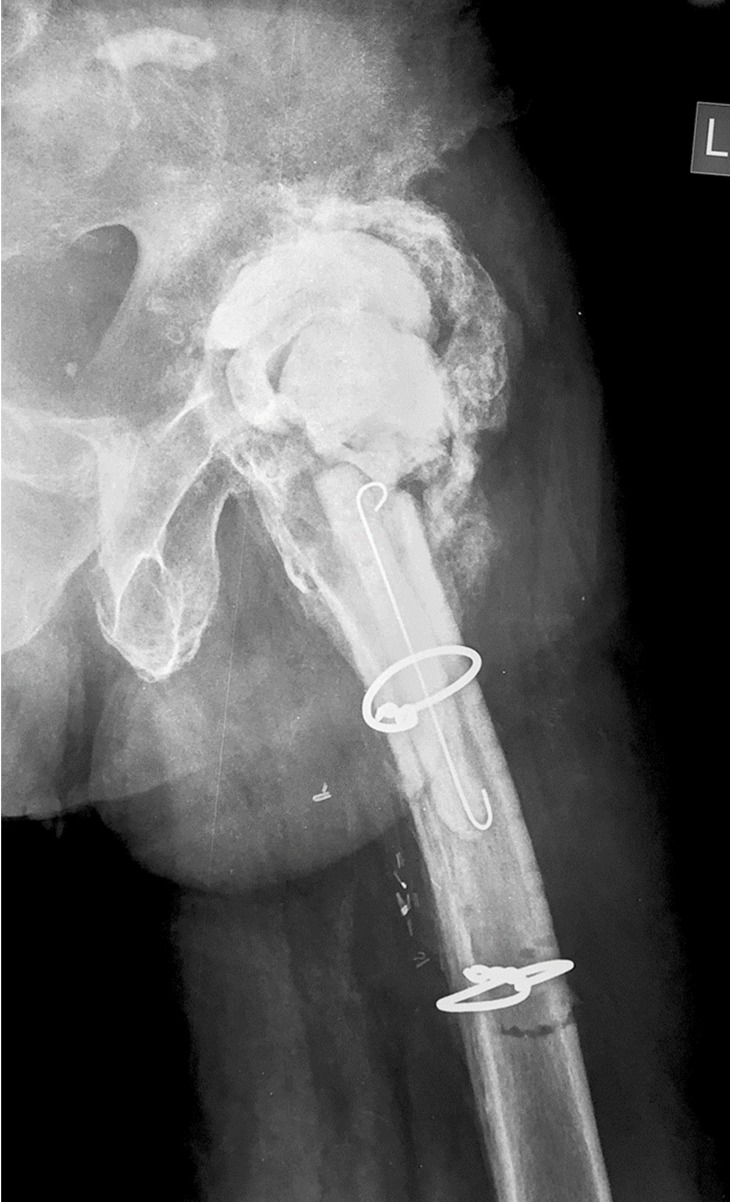
At the last follow-up, 24 months after the first stage THA revision, the patient is well, without any clinical, laboratory or imaging evidence of infection; his wound healed completely, he could ambulate with crutches for short distances with mild pain, and inflammatory markers had reduced to normal values. Radiographs of the left hip joint showed a stable PMMA spacer, without any evidence of osteolysis or loosening, and with extensive periarticular heterotopic bone formation that obviously provided for the stability of the joint (Fig. 5). The patient denied further surgery and remains at close follow-up for the risk of recurrent Pseudomonas oryzihabitans infection.
Figure 5.
Anteroposterior radiograph of the left hip at the last follow-up shows a stable PMMA spacer, without any evidence of osteolysis or loosening, and with extensive periarticular heterotopic bone formation.
Discussion
The genus Pseudomonas was originally described in 1894 15, 16. The species oryzihabitans was first identified by Dresel and Stickl in 1928 in urine and gastrointestinal specimens 17, 18. At first, as the organism closely resembled the bacteria that caused enteric fever, it was given the name Chromobacterium typhiflavuum 17. The name change to Pseudomonas oryzihabitans occurred in 1985, after Kodama et al. 3 isolated the microorganism from rice fields in Japan, and were able to demonstrate phenotypic similarities to other Pseudomonas species. However, in 1987, Holmes et al. 19 proposed yet another name, Flavimonas oryzihabitans, as these bacteria are oxidase negative and show little DNA homology to other Pseudomonas strains.
Little is known regarding the pathogenesis of Pseudomonas oryzihabitans and the circumstances under which it becomes virulent. Patients are generally likely to have an underlying disease, have already been hospitalized for a long time, have a compromised immune system, and have implanted prosthetic material such as indwelling central catheters, ambulatory peritoneal dialysis catheters and port-a-caths, or have consistent contact with contaminated respiratory equipment 20-22. Administration of steroids, AIDS and hematological malignancies are also known predisposing factors 20. Among the clinical manifestations of the disease, a literature search can yield cases of bacteremia in malnourished children, sepsis in premature neonates, biliary tract infections, meningitis, pneumonia, indwelling catheter-related infections, peritonitis, endophthalmitis, subdural empyema, sinusitis, soft-tissue and urinary tract infections 5-8, 21, 23. To the best of our knowledge, there is no previous report of a Pseudomonas oryzihabitans PJI.
Pseudomonas oryzihabitans is rarely resistant to antibiotic therapy and thus fairly easy to eradicate. These bacteria are usually susceptible to fluoroquinolones, aminoglycosides and carbapenems, whereas occassional resistance has been reported to ampicillin, amoxicillin-clavulanic acid and cefazolin 7, 21. Even though antibiotics should clear up the infection most of the time, they do not as often obviate the need for prosthetic material removal, usually deemed necessary for complete eradication 6. In the patient presented herein, implant removal was done, followed by a short (approximately 7 weeks) of antibiotics aiming for the second stage revision surgery. At the last follow-up, the patient was feeling well, with mild pain and no evidence of recurrent infection. He had a stable joint, which we attribute to the periarticular heterotopic bone formation, and therefore, he denied further surgical treatment. In the present patient, although antibiotics were administered for a short time, we believe that implant removal was beneficial for eradication of his infection. In our practice, as in the present patient, we are always in favor of complete implant removal for PJIs.
References
- 1.Rahav G, Simhon A, Mattan Y, Moses AE, Sacks T. Infections with Chryseomonas luteola (CDC group Ve-1) and flavimonas oryzihabitans (CDC group Ve-2) Medicine. 1995;74:83–8. doi: 10.1097/00005792-199503000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Woo KS, Choi JL, Kim BR, Kim JE, Kim KH, Kim JM. et al. Outbreak of pseudomonas oryzihabitans pseudobacteremia related to contaminated equipment in an emergency room of a tertiary hospital in Korea. Infection & chemotherapy. 2014;46:42–4. doi: 10.3947/ic.2014.46.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kodama K, Kimura N, Komagata K. Two New Species of Pseudomonas: P. oryzihabitans Isolated from Rice Paddy and Clinical Specimens and P. luteola Isolated from Clinical Specimens. Int J Syst Bacteriol. 1985;35:467–74. [Google Scholar]
- 4.Steinberg JP, Rio CD. Other gram-negative and gram-variable bacilli. In: Mandell GL, Bennett JE, Dolin R, editors. Principles and Practices of Infectious Diseases. New York, NY: Churchill-Livingstone; 2005. [Google Scholar]
- 5.Freney J, Hansen W, Etienne J, Vandenesch F, Fleurette J. Postoperative infant septicemia caused by Pseudomonas luteola (CDC group Ve-1) and Pseudomonas oryzihabitans (CDC group Ve-2) J Clin Microbiol. 1988;26:1241–3. doi: 10.1128/jcm.26.6.1241-1243.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Esteban J, Martin J, Ortiz A, Santos-O'Connor F, Cabria F, Reyero A. Pseudomonas oryzihabitans peritonitis in a patient on continuous ambulatory peritoneal dialysis. Clinical microbiology and infection: the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2002;8:607–8. doi: 10.1046/j.1469-0691.2002.00447.x. [DOI] [PubMed] [Google Scholar]
- 7.Marin M, Garcia de Viedma D, Martin-Rabadan P, Rodriguez-Creixems M, Bouza E. Infection of hickman catheter by Pseudomonas (formerly flavimonas) oryzihabitans traced to a synthetic bath sponge. J Clin Microbiol. 2000;38:4577–9. doi: 10.1128/jcm.38.12.4577-4579.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsai CK, Liu CC, Kuo HK. Postoperative endophthalmitis by Flavimonas oryzihabitans. Chang Gung medical journal. 2004;27:830–3. [PubMed] [Google Scholar]
- 9.Reed RP. Flavimonas oryzihabitans sepsis in children. Clin Infect Dis. 1996;22:733–4. doi: 10.1093/clinids/22.4.733. [DOI] [PubMed] [Google Scholar]
- 10.Younger TI, Bradford MS, Magnus RE, Paprosky WG. Extended proximal femoral osteotomy. A new technique for femoral revision arthroplasty. J Arthroplasty. 1995;10:329–38. doi: 10.1016/s0883-5403(05)80182-2. [DOI] [PubMed] [Google Scholar]
- 11.Trampuz A, Piper KE, Jacobson MJ, Hanssen AD, Unni KK, Osmon DR. et al. Sonication of removed hip and knee prostheses for diagnosis of infection. N Engl J Med. 2007;357:654–63. doi: 10.1056/NEJMoa061588. [DOI] [PubMed] [Google Scholar]
- 12.Ahmad SS, Shaker A, Saffarini M, Chen AF, Hirschmann MT, Kohl S. Accuracy of diagnostic tests for prosthetic joint infection: a systematic review. Knee surgery, sports traumatology, arthroscopy: official journal of the ESSKA; 2016. [DOI] [PubMed] [Google Scholar]
- 13.Penner MJ, Masri BA, Duncan CP. Elution characteristics of vancomycin and tobramycin combined in acrylic bone-cement. J Arthroplasty. 1996;11:939–44. doi: 10.1016/s0883-5403(96)80135-5. [DOI] [PubMed] [Google Scholar]
- 14.Anagnostakos K, Kelm J. Enhancement of antibiotic elution from acrylic bone cement. Journal of biomedical materials research Part B, Applied biomaterials. 2009;90:467–75. doi: 10.1002/jbm.b.31281. [DOI] [PubMed] [Google Scholar]
- 15.Peix A, Ramirez-Bahena MH, Velazquez E. Historical evolution and current status of the taxonomy of genus Pseudomonas. Infection, genetics and evolution: journal of molecular epidemiology and evolutionary genetics in infectious diseases. 2009;9:1132–47. doi: 10.1016/j.meegid.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 16.Migula N. Arbeiten aus dem Bakteriologischen Institut der Technischen Hochschule zu Karlsruhe. 1894;: 235-8.
- 17.Lucas KG, Kiehn TE, Sobeck KA, Armstrong D, Brown AE. Sepsis caused by Flavimonas oryzihabitans. Medicine. 1994;73:209–14. doi: 10.1097/00005792-199407000-00003. [DOI] [PubMed] [Google Scholar]
- 18.Dresel EG, Stickl O. Ueber reversible Mutationsformen der Typhusbazillen beim Menschen. Deutsche Medizinische Wohenschrift; 1928. pp. 517–9. [Google Scholar]
- 19.Holmes B, Steigerwalt AG, Weaver RE, Brenner DJ. Chryseomonas luteola comb. nov. and Flavimonas oryzihabitans gen. nov, comb. nov, Pseudomonas-like species from human clinical specimens formerly known, respectively, as groups Ve-1 and Ve-2. Int J Syst Bacteriol. 1987:245–50. [Google Scholar]
- 20.Giacometti A, Cirioni O, Quarta M, Schimizzi AM, Del Prete MS, Scalise G. Unusual clinical presentation of infection due to Flavimonas oryzihabitans. European journal of clinical microbiology & infectious diseases: official publication of the European Society of Clinical Microbiology. 1998;17:645–8. doi: 10.1007/BF01708348. [DOI] [PubMed] [Google Scholar]
- 21.Lejbkowicz F, Belavsky L, Kudinsky R, Gery R. Bacteraemia and sinusitis due to Flavimonas oryzihabitans infection. Scandinavian journal of infectious diseases. 2003;35:411–4. doi: 10.1080/00365540310012208. [DOI] [PubMed] [Google Scholar]
- 22.Dussart-Baptista L, Bodilis J, Barray S, Frebourg N, Fournier M, Dupont JP. et al. Recurrent recovery of Pseudomonas oryzihabitans strains in a karstified chalk aquifer. Water research. 2007;41:111–7. doi: 10.1016/j.watres.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 23.Lin RD, Hsueh PR, Chang JC, Teng LJ, Chang SC, Ho SW. et al. Flavimonas oryzihabitans bacteremia: clinical features and microbiological characteristics of isolates. Clin Infect Dis. 1997;24:867–73. doi: 10.1093/clinids/24.5.867. [DOI] [PubMed] [Google Scholar]