Abstract
Local release of antibiotic has advantages in the treatment of chronic osteomyelitis and infected fractures. The adequacy of surgical debridement is still key to successful clearance of infection but local antibiotic carriers seem to afford greater success rates by targeting the residual organisms present after debridement and delivering much higher local antibiotic concentrations compared with systemic antibiotics alone. Biodegradable ceramic carriers can be used to fill osseous defects, which reduces the dead space and provides the potential for subsequent repair of the osseous defect as they dissolve away. A dissolving ceramic antibiotic carrier also raises the possibility of single stage surgery with definitive closure and avoids the need for subsequent surgery for spacer removal.
In this article we provide an overview of the properties of various biodegradable ceramics, including calcium sulphate, the calcium orthophosphate ceramics, calcium phosphate cement and polyphasic carriers. We summarise the antibiotic elution properties as investigated in previous animal studies as well as the clinical outcomes from clinical research investigating their use in the surgical management of chronic osteomyelitis.
Calcium sulphate pellets have been shown to be effective in treating local infection, although newer polyphasic carriers may support greater osseous repair and reduce the risk of further fracture or the need for secondary reconstructive surgery. The use of ceramic biocomposites to deliver antibiotics together with BMPs, bisphosphonates, growth factors or living cells is under investigation and merits further study.
We propose a treatment protocol, based on the Cierny-Mader classification, to help guide the appropriate selection of a suitable ceramic antibiotic carrier in the surgical treatment of chronic osteomyelitis.
Keywords: Osteomyelitis, ceramic, antibiotic carrier, biodegradable, calcium sulphate, calcium phosphate.
Introduction
Chronic osteomyelitis is characterised by the presence of sequestered bone. This becomes an infective nidus on which bacterial colonies may form a biofilm and significantly undermine the body's ability to mount an effective immune response 1,2. Effective treatment must include adequate excision of this dead bone and biofilm. This will produce a bone defect, or dead space. One of the central tenets of the surgical treatment of osteomyelitis is to ensure that the dead space left at the end of the surgery is appropriately managed 3,4.
At the end of debridement, the surgeon must assume that the whole operative field will be contaminated with bacteria disseminated during the surgery. Bone is an unyielding tissue so any defect left at the end of the surgery will remain and will fill with haematoma. This environment is low in oxygen and has a low pH, which provides an ideal environment for the propagation of these persisting planktonic bacteria and allows early biofilm development. Systemic antibiotics are administered in an effort to target these remaining organisms, but the perfusion of antibiotics into bone defects may be poor, resulting in low antibiotic penetration. A more attractive option would be to use an antibiotic carrier that can fill the void and deliver high concentrations of local antibiotics.
In this article, we will investigate the use of various ceramic biocomposites used to fill bone voids and evaluate the evidence for their use as biodegradable antibiotic carriers in the treatment of chronic osteomyelitis.
The argument for local antibiotic carriers
One of the clear advantages of using a local antibiotic carrier is the ability to achieve high local concentrations of antibiotic without systemic toxicity. The dosing of systemic antibiotics is limited by the potential toxic effects they may have on organs, such as the kidneys. Local antibiotic carriers can elute high concentrations of antibiotic locally, often in the order of 10-100 times the minimum inhibitory concentration for the organisms present 5,6 and potentially above the mean biofilm eradication concentration. The importance of performing a thorough debridement of the sequestered bone in osteomyelitis cannot be overstated, particularly when the presence of a biofilm can confer organism resistance to antibiotics in the order of a 1000 times higher than normal therapeutic concentrations 7. Following debridement, there will inevitably be planktonic organisms present throughout the operative field. If the debridement has been adequate, there will be very little biofilm present with static bacteria, so there is an opportunity to kill the residual planktonic bacteria before new biofilm and resistant colonies can be established. Local antibiotic carriers may be effective at eradicating even moderately resistant organisms in situations where systemic antibiotics would not, due to the limitations posed by systemic toxicity. Despite these high local levels, the systemic concentrations of antibiotic with the use of local carriers seem to remain low 8,9.
The other advantage of using an inorganic carrier to deliver local antibiotic is the fact that it can be biodegradable. This negates the need for further surgery to remove implanted antibiotic spacers once they have served their purpose, and makes single stage surgery for osteomyelitis a viable option.
Several animal studies have demonstrated that the combination of an antibiotic with a ceramic carrier is effective for treating infection 8,10-15. Several writers have also published their outcomes for treating chronic osteomyelitis in humans with ceramic antibiotic carriers 9,16-22.
Comparison of antibiotic elution between polymethylmethacrylate (PMMA) cement and bioceramics
Release of antibiotics contained within PMMA bone cement relies on surface diffusion. The rate of antibiotic release is therefore influenced by the surface area of the antibiotic spacer and the concentration gradient between its surface and the surrounding soft tissues 6. The antibiotic release with PMMA is initially high during the first 48-72 hours postoperatively but quickly falls to lower, sub-therapeutic levels for a sustained period. One study has shown that PMMA beads may continue to elute low levels of antibiotic even up to five years following implantation 23. This long release profile is a major drawback in osteomyelitis treatment. Prolonged low-level release of antibiotics below the minimum inhibitory concentration needed to eradicate organisms creates a selection pressure that may cause multi-drug resistant organisms to predominate. Furthermore, once the antibiotic levels are too low to kill organisms the PMMA itself can become colonized and organisms are able to form a biofilm upon its surface 23,24. This problem could be negated with a secondary surgical procedure to remove the PMMA, although it may be preferable to avoid further surgery in osteomyelitis treatment, particularly when the soft tissue envelope is often suboptimal.
By contrast, antibiotic containing ceramic preparations have the advantage of high local antibiotic elution for a finite release time, based on the speed at which the carrier dissolves. All antibiotics contained within ceramic carriers will be released, and there is no prolonged low-level release of antibiotics as seen with PMMA.
The ideal bone substitute
After infection eradication, the next priority is to support the osseous repair of the cavity left behind following debridement. The gold standard against which all bone graft substitutes must be compared remains autologous bone graft 25,26. This is able to provide three core attributes for bone healing: osteoconduction, osteoinduction and osteogenesis.
Osteoconduction is the provision of a biocompatible scaffold to act as a framework for the adhesion of osteogenic cells and the ingrowth of new blood vessels 27. Osteoinduction is the process that supports the mitogenesis of undifferentiated mesenchymal cells leading to the formation of osteoprogenitor cells with the capacity to form new bone 28. This property is in large part due to the influence of bone matrix protein of the transforming growth factor (TGF)-β superfamily, which includes the bone morphogenetic proteins (BMP) 27.
Osteogenesis exists when the graft material contains cells capable of synthesizing new bone. This property can only exist in autograft or when bone substitutes are enriched with cultured autologous cells 26,29. It remains unclear whether host cells remain viable with the use of non-vascularised autografts 30.
Ideal bone void filler in osteomyelitis
An ideal bone void filler must be:
biocompatible to avoid local reactions
bioabsorbable to avoid the need for removal surgery
able to elute high levels of local antibiotic
able to provide mechanical strength to support bone
osteoconductive to encourage new bone ongrowth and remodelling
Obliteration of any dead space in bone is important for reducing the risk of haematoma accumulation that can be a nidus for residual infection. Some void fillers have the additional advantage of being injectable while still in paste form, allowing the whole void to be filled. Some carriers have been developed that are polyphasic, containing more than one constituent, resulting in staggered resorption of the material and potentially providing inherent mechanical stability for longer periods of time. This phased resorption may also theoretically aid new bone formation as it provides a porous scaffold onto which bone may grow. This process is optimised if the pore size of the dissolving scaffold mirrors that of bone, in the region of 150-500μm 31-33.
Unlike autologous bone graft, ceramic bone substitutes are not in themselves osteoinductive or osteogenic. However, their use avoids the morbidity of collecting bone graft from distant sites to fill bone voids 34. Some recent animal studies have investigated the use of biological bioceramic composites with the addition of BMP and/or osteogenic cells to try to replicate these important attributes, which will be discussed later.
Finally, any material used in delivering local antibiotics must be evaluated for its biocompatibility and perform its desired function in the body without eliciting adverse local or systemic effects in the recipient 35.
Types of bioceramics
The principle types of biodegradable ceramics available for antibiotic delivery are based on either calcium sulphate or calcium phosphate. Within the calcium phosphate group, there are several different ceramics (Table 1) with the two principle types being tricalcium phosphate and hydroxyapatite.
Table 1.
Different forms of calcium phosphate.
| Form of Calcium Phosphate | Chemical formula |
|---|---|
| Monocalcium phosphate | |
| Monocalcium phosphate monohydrate | Ca(H2PO4)2•H2O |
| Monocalcium phosphate anhydrous | Ca(H2PO4)2 |
| Dicalcium phosphate | |
| Dibasic calcium phosphate dihydrate (Brushite) | CaHPO4•2H2O |
| Dibasic calcium phosphate hemihydrate | CaHPO4•0.5H2O |
| Dibasic calcium phosphate anhydrous (Monetite) | CaHPO4 |
| Tricalcium phosphate | Ca3(PO4)2 |
| α-Tricalcium phosphate | α-Ca3(PO4)2 |
| β-Tricalcium phosphate | β-Ca3(PO4)2 |
| Hydroxyapatite | |
| Hydroxyapatite | Ca10(PO4)6(OH)2 |
| Calcium-deficient hydroxyapatite | Ca10-x(HPO4)x(PO4)6-x(OH)2-x (0 < x < 1) |
| Biphasic Tricalcium Phosphate | Intimate mixture of tricalcium phosphate and hydroxyapatite |
| Tetracalcium phosphate | Ca4(PO4)2O |
| Octacalcium phosphate (Hilgenstockite) | Ca8(HPO4)2(PO4)4•5H2O |
There are also preparations combining more than one type of ceramic, often combining calcium sulphate with a ceramic from the calcium phosphate group. Biphasic tricalcium phosphate is actually a form of calcium phosphate containing an intimate mixture of β-tricalcium phosphate and hydroxyapatite.
Calcium sulphate
Calcium sulphate is an inorganic, naturally occurring compound with the formula CaSO4. It exists in several different hydrate forms, depending on how much water is incorporated into its crystal lattice. There are three principle forms that calcium sulphate can take:
The anhydrous state (known as anhydrite) with the formula CaSO4
The hemihydrate state with the formula CaSO4•0.5H2O
The dihydrate state (known as gypsum or 'plaster of Paris') with the formula CaSO4•2H2O.
The hemihydrate form of calcium sulphate can further be divided into two forms: an alpha-hemihydrate and a beta-hemihydrate. Although more or less chemically identical, these two forms of hemihydrate have differing porosities.
When water is added to the hemihydrate form, there is a slight exothermic reaction and gypsum is formed, which sets due to the formation of a solid crystal lattice.
Calcium sulphate has been used as a bone graft material since 1892 36. In all its forms, calcium sulphate is a white solid which is poorly soluble in water. When water is added to powdered calcium sulphate, a paste can be produced that sets at low temperatures.
Calcium sulphate has a compressive strength that is equivalent to cancellous bone 37. However, it is brittle and quickly loses its strength as it is hydrolysed. Its dissolution occurs relatively quickly; 3-6 weeks in soft tissues and 6-12 weeks in bone 38. It is therefore not effective as a structural void filler because it is not present for long enough to support new bone healing 39. In a series looking at the use of a calcium sulphate antibiotic carrier as a void filler following chronic osteomyelitis excision, there was only partial bone ingrowth in 59% of cases and none in 37% at final follow-up 21. Calcium sulphate is effective at delivering high levels of local antibiotic because it dissolves relatively quickly. Conversely, this mean it is unable to provide any significant long-term mechanical support or act as a scaffold for tissue regeneration 43.
As it dissolves, calcium sulphate produces an acidic microenvironment 40-42. This has been implicated in causing some local wound issues, with serous ooze in a proportion of cases 16,20,22, although this seems to be self-limiting 21.
Calcium phosphate
Calcium phosphate is the principle mineral found in bone and includes a family of minerals containing calcium ions (Ca2+) together with orthophosphates (PO43-). It exists in various forms (Table 1), with hydroxyapatite being the most abundant, making up around 70% of the mineral content of bone by weight. Other forms used in antibiotic carriers include tricalcium phosphate and dicalcium phosphate, of which the hemihydrate form, known as Brushite, is most widely used.
Mechanical properties of calcium orthophosphates
While tricalcium phosphate and hydroxyapatite have similar compressive strengths to calcium sulphate, monocalcium phosphate has mechanical compressive strength in the order of 4-10 times that of cancellous bone 37. However, the true mechanical strength in vivo is likely to be significantly less because the graft is relatively brittle and less able to withstand normal physiological tensile and shear forces within bone 37,44.
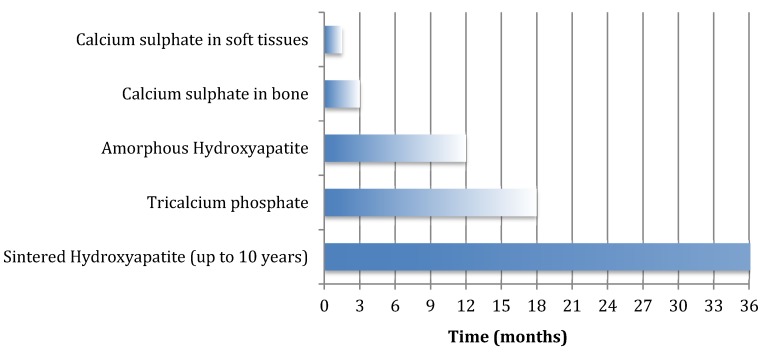
Another significant difference in comparison to calcium sulphate is the much longer resorption time seen in these phosphate ceramics (Figure 1 and Table 2) 36. The rate at which resorption occurs in orthophosphate ceramics is most predicted by their solubility in water. If the ceramic is less soluble than the mineral part of bone, it dissolves extremely slowly, and if it is more soluble, it dissolves more quickly 45.
Figure 1.
Duration of remodelling or dissolution of various ceramic biocomposites.
Table 2.
Resorption times for various ceramic carriers.
| Material | Resorption time | Compressive strength |
|---|---|---|
| Calcium sulphate | 3-6 weeks in soft tissues | Poor |
| 6-12 weeks in bone | ||
| Tricalcium phosphate | 6-18 months | Intermediate |
| Calcium phosphate | 6 months-10 years | Excellent |
| Hydroxyapatite | ||
| * Amorphous precipitated low temperature form * Sintered high temperature form |
6-12 months | Excellent |
| 10 years plus | Excellent | |
Tricalcium phosphate resorbs over a period lasting between 6-18 months 46,47, whereas monocalcium phosphate and hydroxyapatite can resorb over a period ranging from 6 months to 10 years 37. Given that their crystalline surface is compatible with osteoconduction, there is a greater potential for bone repair over time than with the use of calcium sulphate in isolation. Hydroxyapatite is the most osteoconductive material in this group.
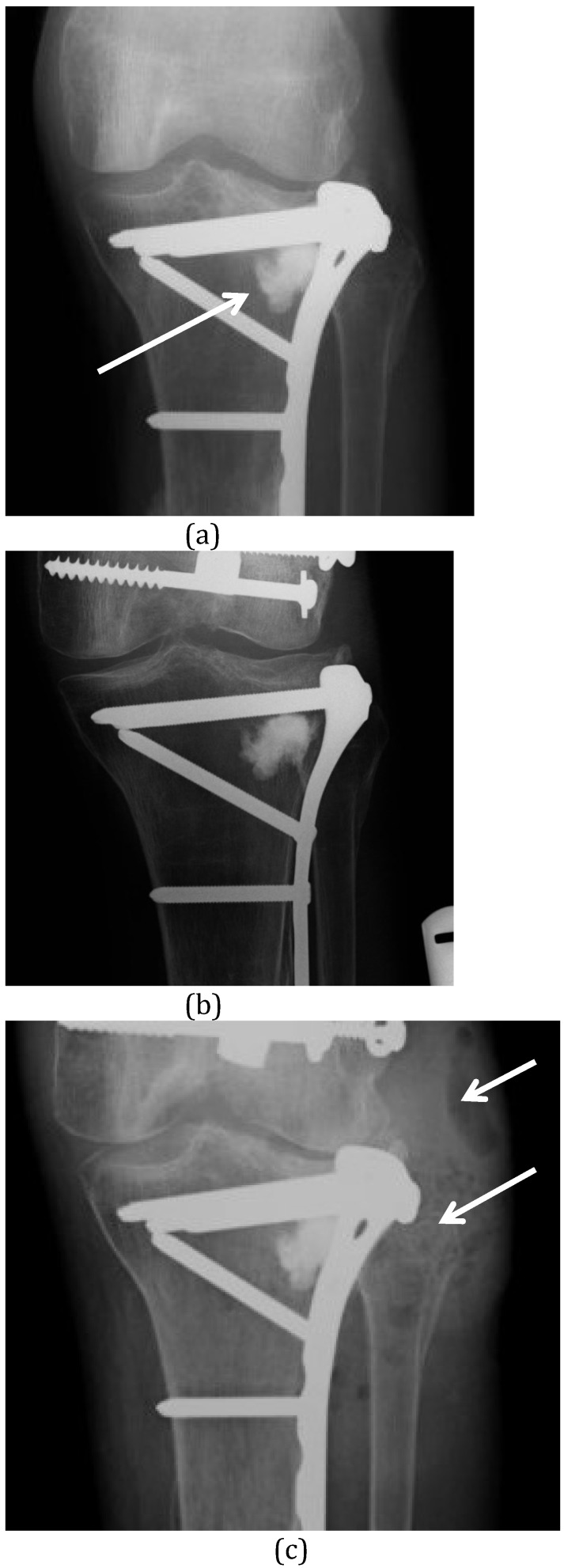
All calcium phosphate ceramics are formed in one of two ways: i) as precipitations out of an aqueous solution at low temperatures or ii) through sintering at high temperature, often above 800°C. Calcium phosphate cements (which are discussed below) are all produced through low temperature reactions, but many of the other orthophosphate ceramics come as solids, prepared using the high temperature reaction of sintering. These sintered ceramics are highly crystalline which makes them osteoconductive, but also highly insoluble and very slow to dissolve. The low temperature orthophosphates may be more appropriate in the treatment of osteomyelitis, as they have intermediate remodelling times. For example, the amorphous hydroxyapatite produced though low temperature precipitation in calcium phosphate cements may dissolve over 6 to 12 months. In contrast, the highly crystalline hydroxyapatite produced through sintering may remain present for decades 48. Indeed, its dissolution is not a passive process because, unlike the other orthophosphates, hydroxyapatite relies entirely on osteoclastic cellular activity for its breakdown 49. In some situation, this slow dissolution of ceramic can cause problems as a nidus for future infection (Figure 2).
Figure 2.
(A) This proximal tibial plateau fracture was treated with open reduction and internal fixation. Elevation of the depressed plateau created a bone void in the lateral condyle which was filled with a composite of α-tricalcium phosphate, calcium carbonate and monocalcium phosphate (Norian™) (white arrow). (B) Radiograph taken 4 years later shows no remodelling of this very stable ceramic. (C) The patient presented again at 6.5 years after implantation of the ceramic with an abscess over the lateral tibia (white arrows) and septic arthritis of the knee. The ceramic material remains unchanged and is now involved in the infection. It was difficult to remove at surgery.
Calcium phosphate cement
Calcium phosphate can be made into a cement producing a workable material that can be molded or injected into a defect 50. It hardens isothermically, allowing heat sensitive antibiotics to be added without being destroyed 51. The cement contains an aqueous solution and a fine powder containing one or more groups of calcium phosphate compounds. As the powder is added to a premeasured volume of fluid, a reaction occurs in which the ceramic initially forms a fluid paste that can be used to fill bone defects completely. This is an advantage in preventing further fluid re-accumulation following debridement surgery. As the reaction continues, crystals precipitate and as they grow, they interdigitate with one another forming a solid-setting cement 52-54. Once it has solidified, it continues to harden and has a compressive strength equal to cancellous bone by 48 hours 27.
These calcium orthophosphate cements fall into two different categories depending on what the final product of this crystallization reaction is: i) calcium deficient hydroxyapatite or ii) Brushite 54.
Although mechanically strong, the downside of calcium phosphate cements is that they only degrade layer-by-layer, limiting new bone ingrowth because the pore sizes present remains too small to support bone infiltration 45.
In practice, calcium phosphate cements are rarely pure as they often contain other compounds. The calcium phosphate bone substitute Norian™ (DePuy Synthes, Warsaw, Indiana, USA) consists of a combination of α-tricalcium phosphate (85%), calcium carbonate (12%) and monocalcium phosphate (3%) 44.
Although a variety of antibiotics can be added to ceramic carriers, their addition to calcium orthophosphate cements can have major impacts on setting times and mechanical strength 50,55.
Polyphasic bioceramics
Combining differing types of ceramics into one formulation gives the potential of having more than one phase of resorption. A faster resorbing component, such as calcium sulphate, can dissolve quickly, allowing high early release of antibiotic and leaving behind a porous scaffold offering more prolonged structural stability and bone ingrowth 56. There is a fine balance in optimising new bone formation: if resorption is too slow, the ceramic will obstruct bone healing; if resorption is too fast, gaps will form between the ceramic and the bone which are too wide to bridge 49.
The optimal resorption rate can be better achieved through the combination of calcium sulphate with calcium orthophosphates. For example, by combining calcium sulphate and amorphous hydroxyapatite in a ceramic cement, a more porous hydroxyapatite scaffold is left behind after the calcium sulphate undergoes early dissolution. This higher porosity provides a greater available surface area for breakdown by osteoclastic activity, accelerating the resorption rate.
Antibiotic elution profiles of ceramic antibiotic carriers
Antibiotic release has been studied in a number of in vitro and animal studies. A breakdown of some of these studies is given in Table 3.
Table 3.
Papers investigating in vitro antibiotic elution times for ceramic local antibiotic carriers.
| Paper | Material | Contents | Model | Antibiotic | Elution time |
|---|---|---|---|---|---|
| Hamanishi et al. 1996 (51) | Calcium orthophosphate cement | Tetracalcium phosphate Dicalcium phosphate dihydrate |
Laboratory | a) Vancomycin 1% b) Vancomycin 2% c) Vancomycin 5% |
a) 2 weeks b) 4 weeks c) >9 weeks |
| Turner et al. 2005 (8) | Not stated | Calcium sulphate | Canine | Tobramycin | 14-28 days |
| Rauschmann et al. 2005 (57) | Not stated | Calcium sulphate Hydroxyapatite |
Laboratory | Vancomycin Gentamicin |
10 days |
| Webb et al. 2008 (59) | Not stated | Calcium sulphate | Laboratory | Daptomycin | Up to 28 days |
| Scharer et al. 2009 (60) | Prodense | Calcium sulphate Tricalcium phosphate Dibasic calcium phosphate hemihydrate (Brushite) |
Laboratory | Vancomycin | 21 days |
| Wang et al. 2011 (61) | Not stated | Calcium sulphate BMP-2 |
New Zealand White Rabbit | Vancomycin | 21 days |
| Maier et al. 2013 (58) | Cerasorb | Tricalcium phosphate | Laboratory | Vancomycin Gentamycin | 4-6 days |
The reported antibiotic elution profiles of different bioceramics remain fairly similar, with the majority of carriers delivering antibiotics above the minimum inhibitory concentrations for between 3-4 weeks. The two principle exceptions to this are the studies of Rauschmann et al. 57 and Maier et al. 58, where the antibiotic elution was significantly shorter, tailing off at 10 and 6 days respectively. This difference might be explained by the fact that in these papers, the antibiotics were added to the pre-formed ceramic pellets rather that during the ceramic mixing phase before final setting. If the antibiotic is added to the final ceramic solid then its release is governed by diffusion alone. Conversely, if the antibiotic is added before the ceramic has set, then a proportion of the antibiotic may be trapped within the ceramic and only become available for release on the dissolution of the carrier, which takes more time. Hamanishi et al. 51 added varying concentrations of vancomycin to a calcium phosphate cement and found that the release profile was prolonged when higher concentrations of antibiotics were used.
Concerns regarding the cytotoxicity of antibiotics
Kwong et al. showed that exposure of cultured stem cells taken from femoral bone marrow to tobramycin at high levels demonstrated a negative effect on the osteogenic potential of the stem cells 62. Another study looking at a gentamicin coating to titanium implants found no negative impact on osteoblast function 63.
Rathbone et al. investigated the in vitro effect of various antibiotics on human osteoblast cell numbers and activity indirectly by measuring osteoblast DNA and alkaline phosphtase (ALP) levels after 10-14 days of antibiotic exposure 64. This group found that there was a significant variability of effect noted with different antibiotics. Antibiotics with the greatest decrement include ciprofloxacin, doxycycline, minocycline, rifampicin, colistin methanesulfonate, gentamicin, and penicillin. In contrast, vancomycin, amikacin and tobramycin, were the least cytotoxic and did not appreciably affect cell numbers and activity until very high concentrations were used. The authors point out that it is not clear how well the measurement of osteoblast DNA and ALP levels translate into in vivo impairment of bone formation.
One observation from this study is that at very high doses, all antibiotics demonstrated a suppressive effect on osteoblast numbers. Therefore, these negative antibiotic effects on osteogenesis must be weighted up against the need for eradication of local infection. While bone recovery is desirable, the initial priority is the clearance of infection and this must take precedence over any reconstruction considerations. In practice, several antibiotics have been successfully used in the treatment of chronic osteomyelitis but this work may well inform choices about antibiotic selection if several options are available.
Current commercially available ceramic antibiotic carriers
Much experimental work has been done looking at combining various antibiotics with ceramics, but at present, there are only a few commercially available antibiotic carriers licensed for the treatment of osteomyelitis (Table 4). Of these, PerOssal (aap Implantate AG, Berlin, Germany) and Stimulan (Biocomposites, Keele, UK) do not contain antibiotics. They are mixed with antibiotics at the time of implantation. However, currently the addition of antibiotics to Stimulan has not been assessed by a European Medicines Competent Authority and is considered off-label usage.
Table 4.
Commercially available ceramic antibiotic carriers.
| Product | Composition | Form | Antibiotic |
|---|---|---|---|
| Osteoset T | α-hemihydrate calcium sulphate | Pellets | Tobramycin |
| Herafill G | Calcium sulphate Calcium carbonate Triglyceride |
Pellets | Gentamicin |
| PerOssal | Nanocrystalline Hydroxyapatite (51.5%) Calcium sulphate (48.5%) |
Pellets | |
| Stimulan | Calcium sulphate | Pellets or injectable paste | |
| Cerament G | Calcium sulphate Hydroxyapatite (40wt%) |
Injectable Cement | Gentamicin (175mg per 10mls) |
| Cerament V | Calcium sulphate Hydroxyapatite (40wt%) |
Injectable Cement | Vancomycin |
Of these, the most extensively investigated in the surgical management of chronic osteomyelitis is Osteoset T (Wright Medical, Memphis, Tennessee, USA) 16-21. Cerament G (Bonesupport, Lund, Sweden) is a biocomposite containing calcium sulphate and hydroxyapatite that has a flowable delivery system. Once mixed, it forms a paste that can be injected into bone defects, completely filling the cavity and excluding any dead space, which obliterates any areas that may harbour residual bacteria or small fragments of biofilm 65. The high level of a bacteriocidal antibiotic released acts at an important time, when most residual bacteria will be in planktonic form after adequate debridement. A comparison of the outcomes for Osteoset T and Cerament G in the surgical treatment of chronic osteomyelitis showed there to be fewer wound healing problems in the Cerament G group, with the complications of infection recurrence and fracture being two times less likely compared to Osteoset T 66.
Outcomes in the use of ceramic antibiotic carriers
The current studies that investigate the outcomes of chronic osteomyelitis in humans treated with commercially available antibiotic carriers are presented in Table 5. Only studies investigating long bone osteomyelitis and including more than two cases were included in this analysis.
Table 5.
Papers looking at outcome of long bone osteomyelitis in humans with ceramic antibiotic carriers.
| Paper | Material (Contents) | Number treated | Mean Age | Male: female | Mean follow up (yrs) | Infection recurrence | Fracture | Bone filling | Wound leak |
|---|---|---|---|---|---|---|---|---|---|
| McKee et al. 2002 (16) | Osteoset T (Calcium sulphate Tobramycin) |
25 | 44.1 | 15/10 | 2.3 (1.7-3.2) |
2/25 (8%) |
3/25 (12%) |
9 required bone graft | 8/25 (32%) |
| Gitelis et al. 2002 (17) | Osteoset T (Calcium sulphate Tobramycin |
6 | 50.0 (26-85) |
3/3 | 2.3 (1.5-3.3) |
0/6 (0%) |
0/6 (0%) |
91% bone ingrowth at final follow up | No significant drainage |
| Chang et al. 2007 (18) | Osteoset T (Calcium sulphate Tobramycin) |
25 | 39.8 (18-69) |
Unknown | Unknown | 5/25 (20%) |
Unknown | 40% bone ingrowth at 1 year | Unknown |
| McKee et al. 2010 (19) | Osteoset T (Calcium sulphate Tobramycin) |
14 | 44.1 | 11/4 | 3.2 (2.2-5) |
2/14 (14%) |
2/14 (14%) |
Not clear (Mean void consolidation time 6 months) |
3/14 (21%) |
| Fleiter et al. 2014 (9) | Herafill G (Calcium sulphate Calcium carbonate Gentamicin) |
20 | 51.1 (24-79) |
16/4 | Not stated (Only 1/20 patients had long term follow up) | 4/20 (20%) |
Unknown | Unknown | Unknown (used drains) |
| Ferguson et al. 2014 (21) | Osteoset T (Calcium sulphate Tobramycin) |
193 | 46.1 (16-82) |
150/43 | 3.7 (1.3-7.1) |
18/193 (9%) |
9/193 (4.7%) |
36.6% no filling 59.0% partial filling 4.4% complete filling |
30/193 (15.5%) |
| Humm et al. 2014 (20) | Osteoset T (Calcium sulphate Tobramycin) |
21 | 49 (26-88) |
18/3 | 1.3 (0.5-2.1) |
1/20 (5%) |
Unknown | Unknown | 7/21 (33.3%) |
| Romanò et al. 2014 (22) | PerOssal (Calcium sulphate Hydroxyapatite Antibiotic targeted to organism) |
27 | 47 (24-74) |
16/11 | 1.8 (1-3) |
3/27 (11.1%) |
0/27 (0%) |
Partial incorporation | 8/27 (29.6%) |
| Romanò et al. 2014 (22) | Calcium deficient hydroxyapatite (Bovine collagen Teicoplanin) |
22 | 45 (23-77) |
14/8 | 1.8 (1-3) |
3/22 (13.6%) |
0/22 (0%) |
Partial incorporation | 6/22 (27.3%) |
| McNally et al. 2016 (65) | Cerament G (Calcium sulphate Hydroxyapatite Gentamicin) |
100 | 51.6 (23-88) |
65/35 | 1.6 (1-2.8) |
4/100 (4%) |
3/100 (3%) |
Unknown | 6/100 (6%) |
Infection recurrence
Infection recurrence rates were reported to be between 0-20% in these series, although the only series with 100% infection clearance contained only six cases 17. Chang et al. also included a control group of patients treated with debridement alone 18. The recurrence rate in this group was 40%, compared to 20% in the group where Osteoset T was used. McKee et al. compared Osteoset T to a control where antibiotic-loaded PMMA was used. There was no difference in infection recurrence rates between the two groups (14%) 19.
The only study investigating a commercially available biphasic cement showed a lower infection recurrence rate of 4% 65. This large study included patients with poor Cierny & Mader (C-M) hosts and Type III and IV chronic osteomyelitis, infected non-union and concomitant septic arthritis. Eighty percent of the cases had significant co-morbidities (C-M Class B hosts) that would normally predict a high recurrence rate.
Bone healing
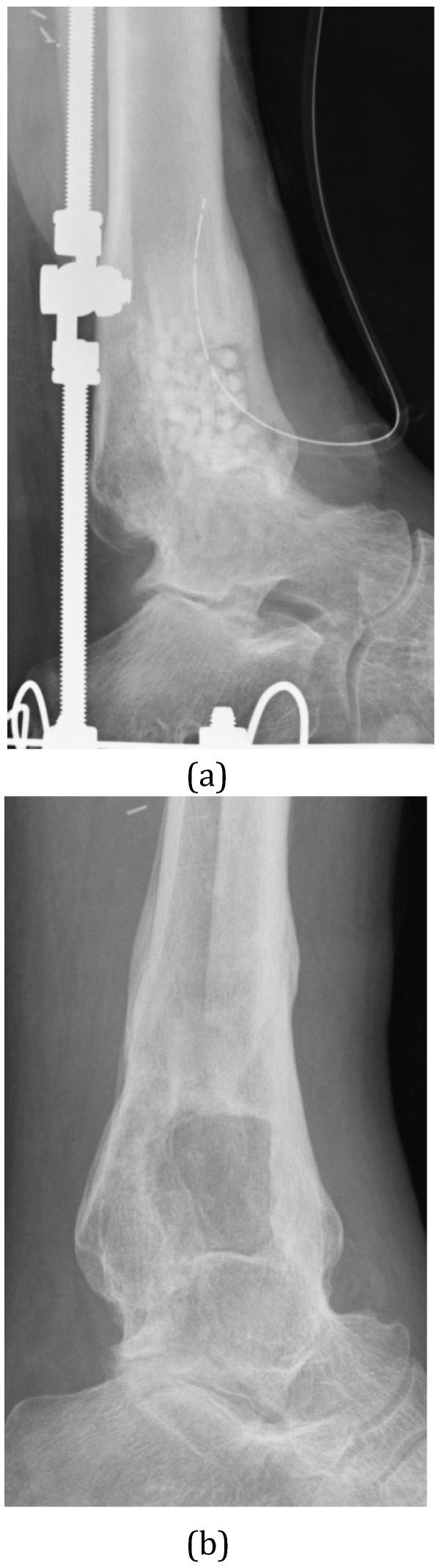
The degree of bone healing seen after removal of dead bone is important because there is a risk of subsequent fracture if the bone is unable to repair sufficiently. The use of calcium sulphate as a bone void filler has been associated with a fracture rate of between 5-14% 16,19,21 and may not allow adequate bone healing after excision (Figure 3). To minimize the risk of subsequent fracture following debridement surgery, it is important to plan the best approach to the sequestrum. Ideally, if a good bar of involucrum or healthy bone exists, then the surgical approach should try to preserve this. There is a greater risk of fracture if there is a larger defect, particularly if it involves the cortex. In theory, a filler with greater mechanical strength might offer a reduced risk of subsequent fracture when treating cortical defects. McNally et al. demonstrated a low fracture rate of 3% in their series using Cerament G 65.
Figure 3.
(A) This infected ankle fusion was treated by radical excision, Ilizarov stabilisation and filling of the cortico-medullary defect with Calcium Sulphate pellets containing Tobramycin. (B) At one year after surgery, the patient is infection-free and the biocomposite has dissolved. There is almost no new bone formation within the resection defect.
Problems with Bioceramic carriers
Wound healing
Inflammatory reactions have been described with the use of calcium sulphate void fillers 40,67,68. It is postulated that with the rapid resorption seen with calcium sulphate, there is release of a calcium-rich fluid which can incite inflammation 33. Several series have commented on a high wound leakage rate when using calcium sulphate antibiotic carriers with rates of 32% 16, 23% 67, and 15% 21 in three difference series. In the last series, early wound leakage was not predictive of infection recurrence and the advice was that early wound ooze can be managed expectantly as the vast majority of cases resolve without intervention. Wound ooze seemed to be a smaller issue with the use of the biphasic cement carrier Cerament G, with McNally et al. demonstrating a wound leak rate of 6% in their series of 100 cases 65. This may reflect the smaller volume of calcium sulphate present and the larger percentage of less soluble hydroxyapatite.
Safety
Antibiotic toxicity
There have been two reports of systemic toxicity from local antibiotic carriers; in one series with the use of gentamicin impregnated sponges in infected total hip arthroplasty 69 and in another study using demineralized bone matrix containing gentamicin 70. However, no published series investigating ceramic antibiotic carriers have thus far demonstrated systemic toxicity. Systemic antibiotic levels in rabbit 71,72 and canine 8 models were low, and were only detectable during the first day after implantation of calcium sulphate antibiotic carriers and remained undetectable thereafter. A study investigating the release profile of gentamicin from a calcium sulphate carrier in 20 patients with osteomyelitis found that urinary and serum antibiotic levels remained low and reached a peak of only 0.2μg/mL (range 0.05-0.6μg/mL) at 24 hours 9. The peak level which is associated with gentamicin toxicity is >12 µg/mL.
In our study of serum levels of gentamicin in 32 patients with implantation of Cerament G into fracture, tumour and osteomyelitis bone defects, all patient had very low serum levels (below 1µg/mL) in the first 5 days after surgery 73.
Hypercalcaemia
In one case series, the use of a calcium sulphate carrier (Stimulan) containing vancomycin and gentamicin for the treatment of periprosthetic injection in total hip replacement resulted in transient hypercalcemia in 3 out of 15 patients 71. One patient required treatment with intravenous fluids. Another single case report showed a transient hypercalcemia with the use of calcium sulphate (Osteoset) containing a mixture of vancomycin and tobramycin in a patient undergoing revision hip arthroplasty for infection 74. The patient developed delirium and serum calcium levels remained high for 7 days, necessitating treatment with intravenous saline and a single dose of Calcitonin 200IU on day seven. There is currently no other evidence of hypercalcemia occurring in the treatment of chronic osteomyelitis in any other series. Hypercalcemia was also investigated in a calcium sulphate animal study, and it was not detected 8.
Composite Carriers containing growth factors
Some research has looked at combining the osteoconductive properties of composite bioceramics with the osteoinductive properties of BMP-2. Although the osteoblast effect and osteoinductive potential of BMP's are well established, there exists a simultaneous osteoclast induction, causing premature resorption of the newly formed callus 75,76. As a result, Raina et al. have looked at a composite bioceramic containing a combination of hydroxyapatite and calcium sulphate in alpha hemihydrate and dehydrate forms along with BMP-2 and the bisphosphanate zoledronic acid 77. In a rat model, the mineralised volume of bone recovered was significantly higher when the biphasic material was combined with both BMP-2 and zoledronic acid as compared to BMP-2 alone or on its own. In another animal study, Schlickewei et al. investigated the combination of the bisphosphanate alendronic acid with an injectable calcium phosphate cement in a rabbit tibia model 78. Twelve weeks following implantation, they reported no difference in new bone formation between the calcium phosphate cement containing alendronic acid and the calcium phosphate cement alone.
Tissue engineering involves the addition of living cells to a graft substitute with the aim of producing an osteogenic material. Bone marrow aspirate can be centrifuged and the mesenchymal cells taken and added to graft material. This can be achieved by using bone marrow aspirates or by culturing stem cells to add to the graft 79. Some researches have achieved 50 population doublings of cultured bone marrow cells, theoretically achieving better osteogenic potential for grafts to which they are added 79,80. At present, the major limiting step seems to be achieving vascularisation of the graft to maintain the viability of these bone-forming cells following implantation in grafts large enough for clinical applications 80. There are no clinical data on the use of combinations of tissue engineering techniques and bioceramics with antibiotics in the management of bone infections.
Oxford Protocol for dead space management with ceramic biocomposite antibiotic carriers
Based on the available clinical studies, it is difficult to give clear guidance on the use of ceramic biocomposites in specific patients. There are few comparative studies or randomized trials. It is important to define the type of bone defect which must be managed and the requirement for bone formation. The Cierny-Mader classification can help in determining the preferred surgical options for dead space management.
In Type I, with a purely medullary defect, there is a requirement for high antibiotic elution but new bone formation is less important, as there is little compromise of the cortex. Calcium sulphate pellets containing antibiotic are easy to use in this indication. The medullary sequestrum can be removed by using a powered reamer of gradually increasing size under image intensifier guidance. The pellets can be passed into the medullary canal via the reamer entry point, using a plastic tube in the medullary canal. The calcium sulphate will degrade relatively quickly, releasing high levels of the antibiotic. Concerns about limited mechanical support or osteoconduction potential offered by the calcium sulphate are not relevant in this situation because the outer cortex remains intact. It may be preferable to maintain the patency of the medullary canal in case future instrumentation is required. Furthermore, the issues surrounding wound leakage with calcium sulphate are of less relevance because the pellets are contained within the medullary cavity.
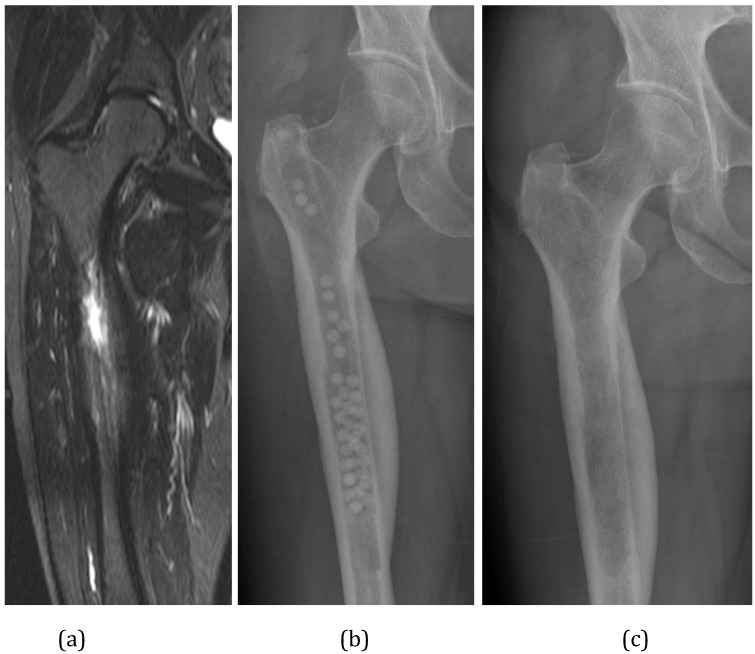
This approach has been shown to be highly effective in medullary (C-M Type I) osteomyelitis 19, 21 (Figure 4).
Figure 4.
(A) This diabetic patient presented with a haematogenous infection of the medullary canal of the upper femur (Cierny & Mader Type I). The MRI shows the extensive medullary oedema, intramedullary abscess and cortical involucrum. (B) The infection was excised by medullary reaming and the dead space filled with Calcium Sulphate pellets with gentamicin. (C) At 4 months after surgery, the pellets have dissolved.
In Type II lesions, the sequestrum is purely cortical. Following complete removal of the affected bone, the priority is to ensure that the area is covered with a healthy, well-vascularized soft tissue envelope. If possible, this can be achieved by direct closure, or if not, with a local or free muscle flap. These lesions do not require antibiotic carriers if the soft tissues are able to cover the excision site. Full excision of the cortical lesion should ensure adequate systemic delivery of antibiotic through the normal medullary bone and overlying soft-tissues.
In Type III lesions, there is a cortico-medullary lesion with an intact pillar of healthy cortical bone crossing the infected zone. In these lesions, pre-operative planning should include cross sectional imaging so that the surgical approach to the infected bone can be made without compromising the unaffected bone integrity. The dead space created by excision will be a cavitary defect. In this situation, a bioceramic with good void filling and initial mechanical stability is preferred (Figure 5). Injectable void filler can fill all areas of the defect, eliminating all dead space and providing maximal structural support. It has the added advantage that the exposed bone can be coated with a carrier, which delivers antibiotics directly to the surface at high concentrations. Composite carriers can provide a scaffold for new bone formation as their constituents undergo phased resorption. They have been shown to allow osteoconduction, potentially enhancing defect filling with living bone 48. This can avoid secondary bone grafting in many cases 65. As with calcium sulphate carriers, biocomposites can produce wound leakage of material during the dissolution phase. Good vascularized soft tissue closure is important, and therefore flap coverage may be required over the tibia.
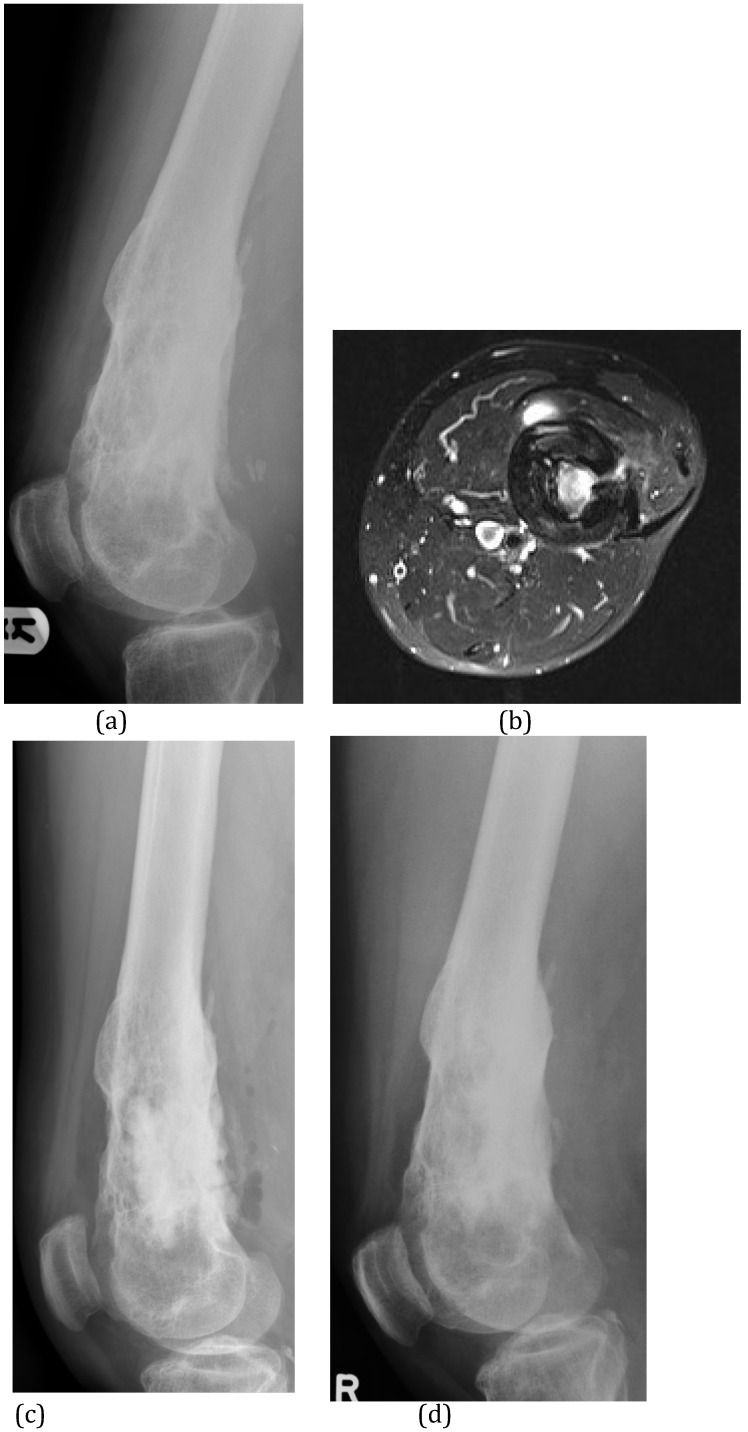
Figure 5.
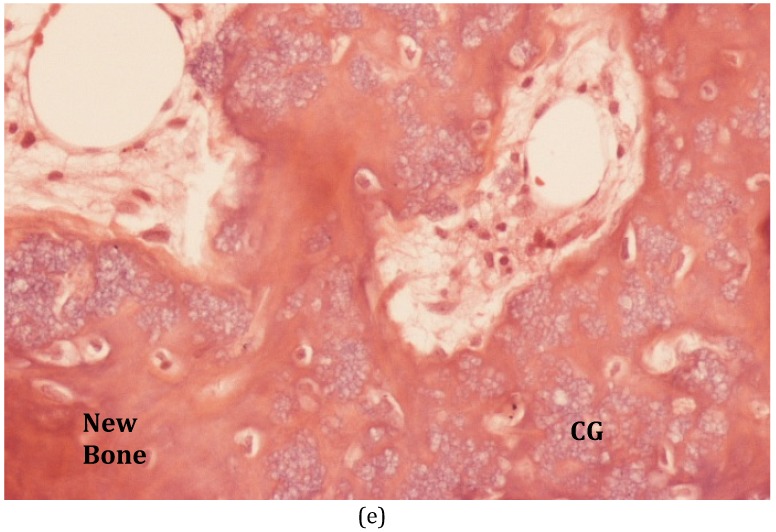
(A) This patient suffered C-M Type III osteomyelitis of the distal femur after an open fracture. (B) The MRI shows the extensive medullary sequestration, the lateral cortical opening (cloaca) and anterior abscess. (C) At operation, the infected bone was excised and the cavitary defect filled with 30mls of calcium sulphate/hydroxyapatite biocomposite with gentamicin (Cerament™G). (D) Six months after implantation, the biocomposite has undergone major remodeling. (E) Bone biopsy (Haematoxylin and Eosin stained microscopy) shows widespread new bone formation within and on the surface of the material (CG = Cerament ™G).
In Cierny-Mader Type IV lesions with segmental involvement, the role for ceramic biocomposites is not well defined. There is currently little published data on the results of the use of bioabsorbable antibiotic carriers in non-unions and segmental defects. The evidence around bone formation with calcium sulphate alone would suggest that it is not sufficient to allow complete bone healing of a segmental defect after excision for infection 21, 27.
In a single study including 10 cases with infected non-unions, small segmental defects of up to 1cm were treated with single stage surgery, filling the bone defect with calcium sulphate/hydroxyapatite with gentamicin (Cerament G). Eight of the 10 cases healed with a single operation and all 10 remained infection-free at final review 65.
In our protocol, defects up to 2cm in the lower limb are managed with acute shortening for bone contact with internal or external fixation. When internal fixation is used, we coat the implant with Cerament G to reduce colonization of the implant surface (Figure 6). Defects above 2cm in the lower limb are probably not suitable for the use of ceramic void fillers due to uncertainty about formation of bone and non-union risk. However, if a staged treatment is planned, it is possible to manage the segmental infection first, using an antibiotic loaded biocomposite, followed by secondary bone reconstruction after eradication of infection.
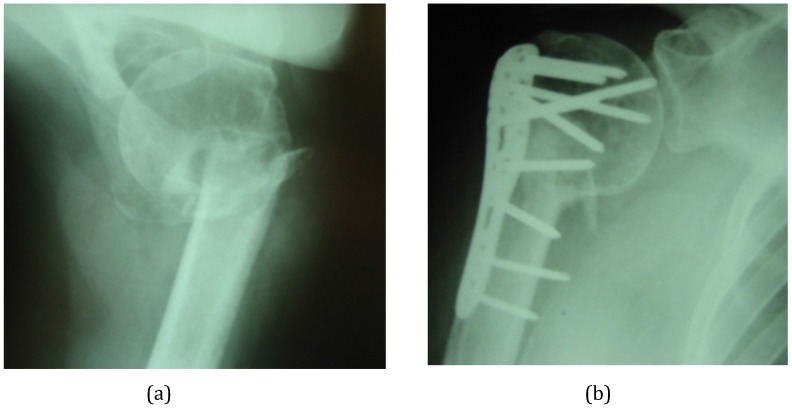
Figure 6.
(A) This infected non-union of the proximal humerus occurred after attempted minimal internal fixation with Kirschner wires. The non-union has been excised and internally fixed in a single procedure. (B) The small bone defects around the non-union, the empty holes in the plate and the central medullary space have been filled with calcium sulphate/hydroxyapatite biocomposite with gentamicin (Cerament™G).
For tibial or femoral defects between 2-5cm, we prefer acute compression at the fracture site with relengthening through a corticotomy at another level using a circular or monolateral frame (bifocal compression-distraction). Defects above 5 cm will usually require a bone transport technique.
In the upper limb, humeral segmental infections can often be managed with shortening for bone contact. For large defects (>3cm), particularly in the forearm, we prefer the use of free vascularized fibular grafts with external fixation. When internal fixation is needed, coating with an antibiotic carrier is a useful adjunct.
Conclusion
Local release of antibiotic has advantages in the treatment of chronic osteomyelitis and infected fractures. The adequacy of surgical debridement is still key to successful clearance of infection, but local antibiotic carriers seem to afford greater success rates by targeting the residual organisms present after debridement.
A dissolving ceramic antibiotic carrier raises the possibility of single stage surgery with definitive closure and avoids the need for subsequent surgery for spacer removal.
Although calcium sulphate pellets have been shown to be effective in treating local infection, newer polyphasic carriers are now available which may support greater osseous repair and reduce the risk of further fracture or the need for secondary reconstructive surgery. The use of ceramic biocomposites to deliver antibiotics together with BMPs, bisphosphonates, growth factors or living cells is under investigation and merits further study. Planning surgery based on the Cierny-Mader classification helps to guide the most appropriate treatment strategy and antibiotic carrier selection, although there is still no clear evidence to guide the management of Grade IV segmental infections.
References
- 1.Trampuz A, Zimmerli DW. Antimicrobial agents in orthopaedic surgery. Drugs. 2006;66(8):1089–106. doi: 10.2165/00003495-200666080-00005. [DOI] [PubMed] [Google Scholar]
- 2.Brady RA, Leid JG, Calhoun JH, Costerton JW, Shirtliff ME. Osteomyelitis and the role of biofilms in chronic infection. Federation of European Microbiological Societies Immunology & Medical Microbiology. 2008;52(1):13–22. doi: 10.1111/j.1574-695X.2007.00357.x. [DOI] [PubMed] [Google Scholar]
- 3.Cierny G, Mader JT, Penninck JJ. The classic: A clinical staging system for adult osteomyelitis. Clinical Orthopaedics & Related Research. 2003;414:7–24. doi: 10.1097/01.blo.0000088564.81746.62. [DOI] [PubMed] [Google Scholar]
- 4.McNally M, Nagarajah K. Osteomyelitis. Orthopaedics and Trauma. 2010;24(6):416–29. [Google Scholar]
- 5.Mayberry-Carson KJ, Tober-Meyer B, Smith JK, Lambe DW, Costerton JW. Bacterial adherence and glycocalyx formation in osteomyelitis experimentally induced with staphylococcus aureus. Infection and Immunity. 1984;43(3):825–33. doi: 10.1128/iai.43.3.825-833.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walenkamp GH, Vree TOMB, Van Rens TJ. Gentamicin-PMMA beads: Pharmacokinetic and nephrotoxicological study. Clinical Orthopaedics & Related Research. 1986;205:171–83. [PubMed] [Google Scholar]
- 7.Stewart PS, Costerton JW. Antibiotic resistance of bacteria in biofilms. The Lancet. 2001;358(9276):135–8. doi: 10.1016/s0140-6736(01)05321-1. [DOI] [PubMed] [Google Scholar]
- 8.Turner TM, Urban RM, Hall DJ, Chye PC, Segreti J, Gitelis S. Local and systemic levels of tobramycin delivered from calcium sulfate bone graft substitute pellets. Clinical Orthopaedics & Related Research. 2005;437:97–104. doi: 10.1097/01.blo.0000175127.37343.0d. [DOI] [PubMed] [Google Scholar]
- 9.Fleiter N, Walter G, Bösebeck H, Vogt S, Büchner H, Hirschberger W, Hoffmann R. Clinical use and safety of a novel gentamicin-releasing resorbable bone graft substitute in the treatment of osteomyelitis/osteitis. Bone and Joint Research. 2014;3(7):223–9. doi: 10.1302/2046-3758.37.2000301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cornell CN, Tyndall D, Waller S, Lane JM, Brause BD. Treatment of experimental osteomyelitis with antibiotic-impregnated bone graft substitute. Journal of Orthopaedic Research. 1993;11(5):619–26. doi: 10.1002/jor.1100110502. [DOI] [PubMed] [Google Scholar]
- 11.Korkusuz F, Uchida A, Shinto Y, Araki N, Inoue K, Ono K. Experimental implant-related osteomyelitis treated by antibiotic-calcium hydroxyapatite ceramic composites. Bone & Joint Journal. 1993;75(1):111–4. doi: 10.1302/0301-620X.75B1.8380599. [DOI] [PubMed] [Google Scholar]
- 12.Shirtliff ME, Calhoun JH, Mader JT. Experimental osteomyelitis treatment with antibiotic-impregnated hydroxyapatite. Clinical Orthopaedics & Related Research. 2002;401:239–47. doi: 10.1097/00003086-200208000-00027. [DOI] [PubMed] [Google Scholar]
- 13.Thomas DB, Brooks DE, Bice TG, DeJong ES, Lonergan KT, Wenke JC. Tobramycin-impregnated calcium sulfate prevents infection in contaminated wounds. Clinical Orthopaedics & Related Research. 2005;441:366–271. doi: 10.1097/01.blo.0000181144.01306.b0. [DOI] [PubMed] [Google Scholar]
- 14.Wenke JC, Owens BD, Svoboda SJ, Brooks DE. Effectiveness of commercially-available antibiotic-impregnated implants. Journal of Bone & Joint Surgery (Br) Volume. 2006;88(8):1102–4. doi: 10.1302/0301-620X.88B8.17368. [DOI] [PubMed] [Google Scholar]
- 15.Yarboro SR, Baum EJ, Dahners LE. Locally administered antibiotics for prophylaxis against surgical wound infectionan in vivo study. The Journal of Bone & Joint Surgery (Am) 2007;89(5):929–33. doi: 10.2106/JBJS.F.00919. [DOI] [PubMed] [Google Scholar]
- 16.McKee MD, Wild LM, Schemitsch EH, Waddell JP. The use of an antibiotic-impregnated, osteoconductive, bioabsorbable bone substitute in the treatment of infected long bone defects: Early results of a prospective trial. Journal of Orthopaedic Trauma. 2002;16(9):622–7. doi: 10.1097/00005131-200210000-00002. [DOI] [PubMed] [Google Scholar]
- 17.Gitelis S, Brebach GT. The treatment of chronic osteomyelitis with a biodegradable antibiotic-impregnated implant. Journal of Orthopaedic Surgery Hong-Kong. 2002;10(1):53–60. doi: 10.1177/230949900201000110. [DOI] [PubMed] [Google Scholar]
- 18.Chang W, Colangeli M, Colangeli S, Di Bella C, Gozzi E, Donati D. Adult osteomyelitis: Debridement versus debridement plus osteoset T® pellets. Acta Orthopaedica Belgica. 2007;73(2):238–44. [PubMed] [Google Scholar]
- 19.McKee MD, Li-Bland EA, Wild LM, Schemitsch EH. A prospective, randomized clinical trial comparing an antibiotic-impregnated bioabsorbable bone substitute with standard antibiotic-impregnated cement beads in the treatment of chronic osteomyelitis and infected nonunion. Journal of Orthopaedic Trauma. 2010;24(8):483–90. doi: 10.1097/BOT.0b013e3181df91d9. [DOI] [PubMed] [Google Scholar]
- 20.Humm G, Noor S, Bridgeman P, David M, Bose D. Adjuvant treatment of chronic osteomyelitis of the tibia following exogenous trauma using OSTEOSET®-T: A review of 21 patients in a regional trauma centre. Strategies in Trauma and Limb Reconstruction. 2014;9(3):157–61. doi: 10.1007/s11751-014-0206-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferguson JY, Dudareva M, Riley ND, Stubbs D, Atkins BL, McNally MA. The use of a biodegradable antibiotic-loaded calcium sulphate carrier containing tobramycin for the treatment of chronic osteomyelitis a series of 195 cases. Bone & Joint Journal. 2014;96(6):829–36. doi: 10.1302/0301-620X.96B6.32756. [DOI] [PubMed] [Google Scholar]
- 22.Romanò CL, Logoluso N, Meani E, Romanò D, De Vecchi E, Vassena C, Drago L. A comparative study of the use of bioactive glass S53P4 and antibiotic-loaded calcium-based bone substitutes in the treatment of chronic osteomyelitis. Bone & Joint Journal. 2014;96(6):845–50. doi: 10.1302/0301-620X.96B6.33014. [DOI] [PubMed] [Google Scholar]
- 23.Neut D, van de Belt H, van Horn JR, van der Mei HC, Busscher HJ. Residual gentamicin-release from antibiotic-loaded polymethylmethacrylate beads after 5 years of implantation. Biomaterials. 2003;24(10):1829–31. doi: 10.1016/s0142-9612(02)00614-2. [DOI] [PubMed] [Google Scholar]
- 24.Kendall RW, Duncan CP, Smith JA, Ngui-Yen JH. Persistence of bacteria on antibiotic loaded acrylic depots: A reason for caution. Clinical Orthopaedics & Related Research. 1996;329:273–80. doi: 10.1097/00003086-199608000-00034. [DOI] [PubMed] [Google Scholar]
- 25.De Long WG, Einhorn TA, Koval K, McKee M, Smith W, Sanders R, Watson T. Bone grafts and bone graft substitutes in orthopaedic trauma surgery: A critical analysis. Journal of Bone and Joint Surgery. American Volume. 2007;89(3):649–58. doi: 10.2106/JBJS.F.00465. [DOI] [PubMed] [Google Scholar]
- 26.Delloye C, Cornu O, Druez V, Barbier O. Bone allografts. Bone & Joint Journal. 2007;89(5):574–80. doi: 10.1302/0301-620X.89B5.19039. [DOI] [PubMed] [Google Scholar]
- 27.Kurien T, Pearson RG, Scammell BE. Bone graft substitutes currently available in orthopaedic practice the evidence for their use. Bone & Joint Journal. 2013;95(5):583–97. doi: 10.1302/0301-620X.95B5.30286. [DOI] [PubMed] [Google Scholar]
- 28.Urist MR. Bone transplants and implants. In: Urist MR, editor. Fundamental and clinical bone physiology. Lippincott Williams & Wilkins; 1980. [Google Scholar]
- 29.Delloye C. Current situation and future of tissue banking in orthopaedics. European Instructional Course Lectures. 1993;1:161–72. [Google Scholar]
- 30.Dimitriou R, Tsiridis E, Giannoudis PV. Current concepts of molecular aspects of bone healing. Injury. 2005;36(12):1392–404. doi: 10.1016/j.injury.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 31.Daculsi G, Passuti N. Effect of the macroporosity for osseous substitution of calcium phosphate ceramics. Biomaterials. 1990;11:86–7. [PubMed] [Google Scholar]
- 32.Blokhuis TJ, Termaat MF, den Boer FC, Patka P, Bakker FC, Henk JTM. Properties of calcium phosphate ceramics in relation to their in vivo behavior. Journal of Trauma and Acute Care Surgery. 2000;48(1):179–86. doi: 10.1097/00005373-200001000-00037. [DOI] [PubMed] [Google Scholar]
- 33.Campana V, Milano G, Pagano E, Barba M, Cicione C, Salonna G. et al. Bone substitutes in orthopaedic surgery: From basic science to clinical practice. Journal of Materials Science: Materials in Medicine. 2014;25(10):2445–61. doi: 10.1007/s10856-014-5240-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arrington ED, Smith WJ, Chambers HG, Bucknell AL, Davino NA. Complications of iliac crest bone graft harvesting. Clinical Orthopaedics & Related Research. 1996;329:300–9. doi: 10.1097/00003086-199608000-00037. [DOI] [PubMed] [Google Scholar]
- 35.Williams DF. On the mechanisms of biocompatibility. Biomaterials. 2008;29(20):2941–53. doi: 10.1016/j.biomaterials.2008.04.023. [DOI] [PubMed] [Google Scholar]
- 36.Fillingham Y, Jacobs J. Bone grafts and their substitutes. Bone & Joint Journal. 2016;98B(1 Supp A):6–9. doi: 10.1302/0301-620X.98B.36350. [DOI] [PubMed] [Google Scholar]
- 37.Roberts TT, Rosenbaum AJ. Bone grafts, bone substitutes and orthobiologics: The bridge between basic science and clinical advancements in fracture healing. Organogenesis. 2012;8(4):114–24. doi: 10.4161/org.23306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ochsner P, Borens O, Bodler P, Broger I, Maurer T, Nötzli H, Infections of the musculoskeletal system. Basic principles, prevention, diagnosis and treatment. Swiss Orthopaedics and the Swiss Society for Infectious Diseases Expert Group; 2014. [Google Scholar]
- 39.Gitelis S, Piasecki P, Turner T, Haggard W, Charters J, Urban R. Use of a calcium sulfate-based bone graft substitute for benign bone lesions. Orthopedics. 2001;24(2):162–6. doi: 10.3928/0147-7447-20010201-19. [DOI] [PubMed] [Google Scholar]
- 40.Kelly CM, Wilkins RM, Gitelis S, Hartjen C, Watson JT, Kim PT. The use of a surgical grade calcium sulfate as a bone graft substitute: Results of a multicenter trial. Clinical Orthopaedics & Related Research. 2001;382:42–50. doi: 10.1097/00003086-200101000-00008. [DOI] [PubMed] [Google Scholar]
- 41.Coetzee AS. Regeneration of bone in the presence of calcium sulfate. Archives of Otolaryngology Head & Neck Surgery. 1980;106(7):405–9. doi: 10.1001/archotol.1980.00790310029007. [DOI] [PubMed] [Google Scholar]
- 42.Schlickewei CW, Yarar S, Rueger JM. Eluting antibiotic bone graft substitutes for the treatment of osteomyelitis in long bones. A review: Evidence for their use? Journal of Orthopedic Research and Reviews. 2014;6:71–9. [Google Scholar]
- 43.Inzana JA, Schwarz EM, Kates SL, Awad HA. Biomaterials approaches to treating implant-associated osteomyelitis. Biomaterials. 2016;81:58–71. doi: 10.1016/j.biomaterials.2015.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bohner M. Design of ceramic-based cements and putties for bone graft substitution. Eur Cell Mater. 2010;20(1):3–10. doi: 10.22203/ecm.v020a01. [DOI] [PubMed] [Google Scholar]
- 45.Bohner M. Calcium orthophosphates in medicine: From ceramics to calcium phosphate cements. Injury. 2000;31:D37–47. doi: 10.1016/s0020-1383(00)80022-4. [DOI] [PubMed] [Google Scholar]
- 46.Moore WR, Graves SE, Bain GI. Synthetic bone graft substitutes. ANZ Journal of Surgery. 2001;71(6):354–61. [PubMed] [Google Scholar]
- 47.Allison DC, Lindberg AW, Samimi B, Mirzayan R, Menendez LR. A comparison of mineral bone graft substitutes for bone defects. US Oncology & Hematology. 2011;7(1):38–49. [Google Scholar]
- 48.Khan SN, Tomin E, Lane JM. Clinical applications of bone graft substitutes. Orthopedic Clinics. 2000;31(3):389–98. doi: 10.1016/s0030-5898(05)70158-9. [DOI] [PubMed] [Google Scholar]
- 49.Nilsson M, Zheng MH, Tägil M. The composite of hydroxyapatite and calcium sulphate: A review of preclinical evaluation and clinical applications. Expert Review of Medical Devices. 2013;10(5):675–84. doi: 10.1586/17434440.2013.827529. [DOI] [PubMed] [Google Scholar]
- 50.Ginebra M-P, Traykova T, Planell JA. Calcium phosphate cements as bone drug delivery systems: A review. Journal of Controlled Release. 2006;113(2):102–10. doi: 10.1016/j.jconrel.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 51.Hamanishi C, Kitamoto K, Tanaka S, Otsuka M, Kitahashi T. A self-setting TTCP-DCPD apatite cement for release of vancomycin. Journal of Biomedical Materials Research. 1996;33(3):139–43. doi: 10.1002/(SICI)1097-4636(199623)33:3<139::AID-JBM3>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 52.Dorozhkin SV. Bioceramics of calcium orthophosphates. Biomaterials. 2010;31(7):1465–85. doi: 10.1016/j.biomaterials.2009.11.050. [DOI] [PubMed] [Google Scholar]
- 53.Tamimi F, Sheikh Z, Barralet J. Dicalcium phosphate cements: Brushite and monetite. Acta Biomaterialia. 2012;8(2):474–87. doi: 10.1016/j.actbio.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 54.Dorozhkin SV. Self-setting calcium orthophosphate formulations. Journal of Functional Biomaterials. 2013;4(4):209–311. doi: 10.3390/jfb4040209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Takechi M, Miyamoto Y, Ishikawa K, Nagayama M, Kon M, Asaoka K, Suzuki K. Effects of added antibiotics on the basic properties of anti-washout-type fast-setting calcium phosphate cement. Journal of Biomedical Materials Research. 1998;39(2):308–16. doi: 10.1002/(sici)1097-4636(199802)39:2<308::aid-jbm19>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 56.Dorozhkin SV. Biphasic, triphasic and multiphasic calcium orthophosphates. Acta Biomaterialia. 2012;8(3):963–77. doi: 10.1016/j.actbio.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 57.Rauschmann MA, Wichelhaus TA, Stirnal V, Dingeldein E, Zichner L, Schnettler R, Alt V. Nanocrystalline hydroxyapatite and calcium sulphate as biodegradable composite carrier material for local delivery of antibiotics in bone infections. Biomaterials. 2005;26(15):2677–84. doi: 10.1016/j.biomaterials.2004.06.045. [DOI] [PubMed] [Google Scholar]
- 58.Maier GS, Roth KE, Andereya S, Birnbaum K, Niedhart C, Lühmann M. et al. In vitro elution characteristics of gentamicin and vancomycin from synthetic bone graft substitutes. The Open Orthopaedics Journal. 2013;7(1):624–9. doi: 10.2174/1874325001307010624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Webb ND, McCanless JD, Courtney HS, Bumgardner JD, Haggard WO. Daptomycin eluted from calcium sulfate appears effective against staphylococcus. Clinical Orthopaedics & Related Research. 2008;466(6):1383–7. doi: 10.1007/s11999-008-0245-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Scharer BM, Sanicola SM. The in vitro elution characteristics of vancomycin from calcium phosphate-calcium sulfate beads. The Journal of Foot and Ankle Surgery. 2009;48(5):540–2. doi: 10.1053/j.jfas.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 61.Wang Y, Wang X, Li H, Xue D, Shi Z, Qi Y. et al. Assessing the character of the rhbmp-2-and vancomycin-loaded calcium sulphate composites in vitro and in vivo. Archives of Orthopaedic and Trauma Surgery. 2011;131(7):991–1001. doi: 10.1007/s00402-011-1269-6. [DOI] [PubMed] [Google Scholar]
- 62.Kwong FN, Porter RM, Griffin DR, Evans CH. Effect of tobramycin on the osteogenesis of stem cells derived from human bone marrow. Journal of Bone & Joint Surgery, British Volume. 2009;91(Supp I):130–1. [Google Scholar]
- 63.Vester H, Wildemann B, Schmidmaier G, Stöckle U, Lucke M. Gentamycin delivered from a PDLLA coating of metallic implants: In vivo and in vitro characterisation for local prophylaxis of implant-related osteomyelitis. Injury. 2010;41(10):1053–9. doi: 10.1016/j.injury.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 64.Rathbone CR, Cross JD, Brown KV, Murray CK, Wenke JC. Effect of various concentrations of antibiotics on osteogenic cell viability and activity. Journal of Orthopaedic Research. 2011;29(7):1070–4. doi: 10.1002/jor.21343. [DOI] [PubMed] [Google Scholar]
- 65.McNally M, Ferguson J, Diefenbeck M, Lau A, Scarborough M, Ramsden A, Atkins B. Single-stage treatment of chronic osteomyelitis with a new absorbable, gentamicin-loaded, calcium sulphate / hydroxyapatite biocomposite. A prospective series of 100 cases. Bone and Joint Journal. 2016;98B:1289–1296. doi: 10.1302/0301-620X.98B9.38057. [DOI] [PubMed] [Google Scholar]
- 66.McNally M, Ferguson J, Kendall J, Dudareva M, Scarborough M, Stubbs D. A comparative study of three bioabsorbable antibiotic carriers in chronic osteomyelitis: 313 patients with minimum one-year follow-up. Bone & Joint Journal Orthopaedic Proceedings Supplement. 2015;97(Supp 16):21. [Google Scholar]
- 67.Borrelli J, Prickett WD, Ricci WM. Treatment of nonunions and osseous defects with bone graft and calcium sulfate. Clinical Orthopedics and Related Research. 2003;411:245–54. doi: 10.1097/01.blo.0000069893.31220.6f. [DOI] [PubMed] [Google Scholar]
- 68.Nystrom L, Raw R, Buckwalter J, Morcuende JA. Acute intraoperative reactions during the injection of calcium sulfate bone cement for the treatment of unicameral bone cysts: A review of four cases. The Iowa Orthopaedic Journal. 2008;28:81–4. [PMC free article] [PubMed] [Google Scholar]
- 69.Swieringa AJ, Tulp NJ. Toxic serum gentamicin levels after the use of gentamicin-loaded sponges in infected total hip arthroplasty. Acta Orthopaedica. 2005;76(1):75–7. doi: 10.1080/00016470510030355. [DOI] [PubMed] [Google Scholar]
- 70.Urban RM, Turner TM, Hall DJ, Infanger S, Cheema N, Lim TH. Healing of large defects treated with calcium sulfate pellets containing demineralized bone matrix particles. Orthopedics. 2003;26(5):S581–5. doi: 10.3928/0147-7447-20030502-11. [DOI] [PubMed] [Google Scholar]
- 71.Nelson CL, McLaren SG, Skinner RA, Smeltzer MS, Roby Thomas J, Olsen KM. The treatment of experimental osteomyelitis by surgical debridement and the implantation of calcium sulfate tobramycin pellets. Journal of Orthopaedic Research. 2002;20(4):643–7. doi: 10.1016/S0736-0266(01)00133-4. [DOI] [PubMed] [Google Scholar]
- 72.Xie Z, Liu X, Jia W, Zhang C, Huang W, Wang J. Treatment of osteomyelitis and repair of bone defect by degradable bioactive borate glass releasing vancomycin. Journal of Controlled Release. 2009;139(2):118–26. doi: 10.1016/j.jconrel.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 73.Stravinskas M, Horstmann P, Ferguson J, Hettwer W, Nilsson M, Tarasevicius S. et al. Pharmacokinetics of gentamicin eluted from a regenerating bone graft substitute: In vitro and in vivo clinical release. Bone & Joint Research Bone Joint Res. 2016;5:427–435. doi: 10.1302/2046-3758.59.BJR-2016-0108.R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Carlson Jr CR, Markulis E, Thomp-son E, Havill J. A novel case of hyper-calcemia following the use of calcium sulfate beads. Nephrol Open J. 2015;1(1):17–9. [Google Scholar]
- 75.Kaneko H, Arakawa T, Mano H, Kaneda T, Ogasawara A, Nakagawa M. et al. Direct stimulation of osteoclastic bone resorption by bone morphogenetic protein (BMP)-2 and expression of BMP receptors in mature osteoclasts. Bone. 2000;27(4):479–86. doi: 10.1016/s8756-3282(00)00358-6. [DOI] [PubMed] [Google Scholar]
- 76.Giannoudis PV, Kanakaris NK, Einhorn TA. Interaction of bone morphogenetic proteins with cells of the osteoclast lineage: Review of the existing evidence. Osteoporosis International. 2007;18(12):1565–81. doi: 10.1007/s00198-007-0441-x. [DOI] [PubMed] [Google Scholar]
- 77.Raina DB, Isaksson H, Hettwer W, Kumar A, Lidgren L, Tägil M. A biphasic calcium sulphate/hydroxyapatite carrier containing bone morphogenic protein-2 and zoledronic acid generates bone. Scientific Reports. 2016;6:1–13. doi: 10.1038/srep26033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schlickewei CW, Laaff G, Andresen A, Klatte TO, Rueger JM, Ruesing J. et al. Bone augmentation using a new injectable bone graft substitute by combining calcium phosphate and bisphosphonate as compositean animal model. Journal of Orthopaedic Surgery and Research. 2015;10(1):1–13. doi: 10.1186/s13018-015-0263-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rosset P, Deschaseaux F, Layrolle P. Cell therapy for bone repair. Orthopaedics & Traumatology: Surgery & Research. 2014;100(1):S107–12. doi: 10.1016/j.otsr.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 80.Frohlich M, Grayson WL, Wan LQ, Marolt D, Drobnic M, Vunjak-Novakovic G. Tissue engineered bone grafts: Biological requirements, tissue culture and clinical relevance. Current Stem Cell Research & Therapy. 2008;3(4):254–64. doi: 10.2174/157488808786733962. [DOI] [PMC free article] [PubMed] [Google Scholar]