Abstract
The treatment of chronic bone and joint infections is characterized by obstinate persistency of the causing microorganisms and resulting long term disability to patients, associated with remarkable costs for the health care system. Difficulties derive from biofilm formed on dead bone and eventual implants, with resistance against immunological defences and antimicrobial substances. Biofilm embedded bacteria require up to 1000 times the antibiotic concentration of planktonic bacteria for elimination. Systemic antibiotic treatment alone cannot provide the concentrations required and surgical intervention is always prerequisite for potentially providing a cure. A second issue is that osseous defects are almost always present after surgical debridement, and it is difficult to address their reconstruction. One option is to use bone grafts, either from the patient´s own body or from foreign donors (allografts). Grafts are usually unvascularized and are prone to colonization with bacteria. Loading of allografts with antibiotics may not only protect grafts from bacterial adhesion but, using appropriate processing methods, may also provide high local antibiotic concentrations that may eliminate remaining sessile pathogens. For efficient action as antibiotic carriers, the release of antibiotics should be above the minimum biofilm eradication concentration (MBEC) for a prolonged period of time. Cleaning the bone from bone marrow opens a large reservoir for storage of antimicrobial substances that, after implantation, may be released to the surrounding in a sustained mode, possibly eliminating remaining biofilm remnants. Removal of bone marrow, leaving a pure matrix, provides increased safety and improved revascularization of the graft. Local provision of antibiotic concentrations above the MBEC may enable simultaneous internal fixation with osteosynthetic material and single stage exchange of infected endoprostheses, resulting in shorter hospital stays with reduced pain and faster rehabilitation of patients.
Keywords: bone, allograft, infection, antibiotic carrier, biofilm, reconstruction, one stage treatment, quality of life.
Introduction
Bacterial contamination of bone and orthopaedic implants may occur via the blood stream, through open wounds (fractures, ulcers) or surgical procedures, especially when foreign material is implanted as in joint replacement or osteosynthesis. Damage to tissue leads to a decrease in blood supply and depression of immune responses, which can cause the formation of necrotic tissue and favour bacterial invasion. Bacteria, mostly Staphylococci, are then able to attach to dead material. Clinical signs of infection persisting for longer than 10 days are associated with the development of necrotic bone and leads to chronic osteomyelitis (COM). Prosthetic joint infection (PJI) provides another challenge that is considered one of the most feared threats in orthopaedic surgery.
Bacteria that cause these infections live in well-developed, surface-associated communities called biofilms. Biofilms form immediately after attachment to a surface and are considered mature within days to weeks. These organisms are enclosed in a slimy matrix and have changed their lifestyle so they are protected from host defenses and antibiotics. Systemic antibiotic therapy can control acute phases but reachable concentrations are too low to resolve the basic biofilm nidus of the infection. Now that it has been realized that these orthopaedic infections are caused by well-organized biofilm-forming bacterial pathogens, new technologies that deliver high concentrations of antibiotics locally and "on demand" are required 1. Longer standing infections require removal of implants and all dead material physically before the infection can be resolved, but there is ongoing debate on how to manage this, especially whether re-implantation of a new implant may be performed in one stage or multiple stages 2. Osseous defects caused by osteolysis, sequestration and surgical interventions are common in all respective indications and need to be addressed, both providing dead space management and to restore function of the affected limb. The purpose of this review paper is to detail the use of allograft bone as an antibiotic carrier when treating orthopaedic infections.
Bone Defects
After removal of infected implants and radical sequestrectomy, bony defects always will be present to some extent. There are several options to address this issue; most require multiple stage operations, leading to a prolonged treatment and impairment to patients 3,4,5,6,7,8. All of them require multiple interventions over several months or even years with a high rate of complications, and place physical, financial and psychological pressure on the patient as illustrated by the voluntary amputation rate of 1.6% 9.
Allograft bone is widely used for reconstruction of bony defects and performs favourably during two-stage revisions of total joint replacement 10. However, unvascularized bone grafts are at increased risk to become contaminated and need antimicrobial protection.
Biofilm
Most treatment failures in orthopaedic infections derive from the traditional conceptions of dealing with freely floating planktonic bacteria. William Costerton 11 showed that pathogens may change from familiar planktonic forms into phenotypically different sessile forms after adhesion to poorly vascularized surfaces, forming the organized community of a biofilm. Biofilm embedded bacteria require much higher concentrations of antibiotics for elimination than their planktonic forms 12. Several reasons for that specific behaviour have been elucidated, as antimicrobial molecules must diffuse through the biofilm matrix in order to inactivate the encased cells. The extracellular polymeric substances constituting this matrix present a diffusional barrier for these molecules by influencing either the rate of transport of the molecule to the biofilm interior or the reaction of antimicrobial material with matrix material. Conditions that elicit decreased growth, such as nutrient limitation or presence of toxic substances (antibiotics), favour the formation of biofilms.
Anthony Gristina has shown that the biofilm concept fully applies for Chronic osteomyelitis (COM) 13. In COM and PJI, our most obstinate opponents are not the familiar planktonic pathogens but their phenotypically different sessile forms embedded in an extracellular matrix, the glycocalix 1,14. The surface of unvascularized bone and eventual implants may act as a substratum for the attachment of bacteria and formation of biofilms. Debridement may remove the predominant number of bacteria, but even after a perfect debridement, some colonies can detach from the biofilm during manipulation and remain. The colonies may be able to re-colonize niches with poorly vascularized surfaces and freshly implanted devices, causing recurrence after an indefinite period of time. This has been the reason for avoiding simultaneous insertion of alloplastic material at the freshly debrided site and for using external fixators or spacers for temporary stabilization.
To increase concentrations of antibiotics at the infection site it has been suggested to deliver antibiotics through a local drug releasing system. Buchholz et al. were the first to mix antibiotics and PMMA for creating a local carrier 15. From these findings, Klemm et al. developed techniques using antibiotic loaded bone cement in form of beads to be placed into debrided bone defects 16. However, it meanwhile has become clear that antibiotic concentrations produced by antibiotic loaded cement may kill planktonic bacteria but are not effective in eliminating remaining biofilm clusters. Between 90% and 95% of the antibiotic remains trapped in the cement, and the amounts released from the surface create only moderate concentrations within the first hours after implantation. This may make antibiotic loaded cement ineffective as an anti-biofilm tool. 90% of implanted bead chains and 50% of spacers are covered with biofilms at removal 17,18, often associated with the induction of resistance even in planktonic forms 19,20. Small colony variants (SCVs) require up to 100 fold of the minimum inhibiting concentrations (MIC), and biofilm embedded pathogens require up to 1000 fold MIC for elimination21. These levels are usually unattainable through systemic antibiotic therapy as well as for antibiotics released from PMMA 22. PMMA usually acts as a temporary spacer that needs to be removed in a further procedure. This disadvantage is avoided by using a bioresorbable material as a carrier. Ceramics may be supplemented with antibiotics as local carriers, as described in the respective paper by Ferguson et al. They provide the advantage of being resorbable and avoiding the necessity of removal in a second procedure. However, antibiotic concentrations still rarely reach MBEC and inflammatory reactions associated with prolonged wound drainage have been described frequently. Defects greater than 2cm are probably not suitable for the use of ceramic void fillers due to uncertainty about bone formation and non-union risk. Their usage is recommended only in well-defined indications.
Bone is the best substitute for bone
Bone grafting has been used in COM over many decades23 whereas their use in PJI has been very restricted out of fear of re-infection. Autologous bone (i.e. bone from the same patient as donor) has been considered preferable for reconstruction, but is available in limited amounts, additional surgery is required, and there is a risk of colonization of harvested bone. Allograft bone is available in unlimited amounts, but since it is not vascularized, it may be a substrate for bacterial growth either. To lower this risk, the addition of antibiotics has been suggested by few authors. Witso et al. used netilmicin-loaded allografts for reconstruction in revision hip or knee surgery and found no adverse effects 24. Buttaro et al 25 favourably used vancomycin-supplemented cancellous grafts for reconstruction after infected revision total hip replacement (THR) without systemic effects 26. These authors used otherwise unprocessed fresh frozen allografts, admixing antibiotics either in immersion or in powder form that are only efficacious with morsellized fragments of cancellous bone. Michalak et al 27 and Khoo et al 28 impregnated segmental allografts with gentamicin and flucloxacillin, respectively, using iontophoresis for more thorough impregnation. All reached concentrations likely to protect the grafts against bacterial colonization, but the levels were still insufficient for eliminating biofilms that might be left after surgical debridement.
Unprocessed bone is filled with bone marrow that compromises access to the interior of the bone (Fig.1, A, B). Most admixed antibiotics will remain on the outer surface of bone and are likely to be resorbed very quickly. Cleaning the marrow from bone allows uncompromised access into the interior voids, and vastly increases the available surface for adhesion of substances. Consequently, the storage capability for antibiotics may be increased dramatically, as we have showed in our early studies 29.
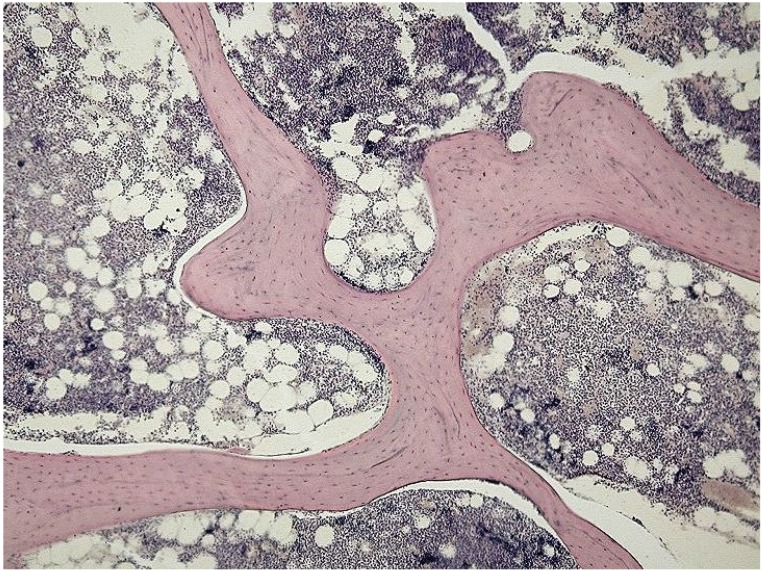
Figure 1.
A (upper figure): Unprocessed bone is filled with marrow that blocks the access to deeper layers of the available matrix surface. From: https://en.wikipedia.org/wiki/File:Spongy_bone_-_trabecules.jpg#filelinks Courtesy: Department of Histology, Jagiellonian University Medical College. B (lower figure): Bone marrow is containing immunogeneic cells and fat, eliciting undesired inflammatory reactions. Eventual pathogens may be present exclusively in the marrow, and not inside the bony matrix. From: https://commons.wikimedia.org/wiki/File%3AGray72-en.svg.
One of the predisposing factors for continuation of infection is inflammation. Inflammation also causes bone resorption by activating osteoclasts 30. When filling a previously infected site, it therefore seems favourable to avoid inflammation as much as possible. Unprocessed bone grafts contain fat that is known to elicit inflammatory reactions, and dead cells likely to elicit an immunological response with enhanced risk of bacterial growth. However, when removing all marrow and soft tissue, all that is left is a pure biological scaffold consisting of collagen and enclosed minerals 31. These parts are identical within all species and are unlikely to cause an immune response 32. Highly purified bone matrix of the same species seems to show the highest biocompatibility of all available materials; it additionally grants the advantages of some load bearing capability and possibility of accelerated incorporation into the host organism through uncompromised osteoconduction 33.
Allografts: Are they safe?
Although reports on undesired events with the use of bone grafts are extremely rare, there are still concerns with the safety of allograft tissue. Concerns mainly date back to the 1990s when transmission of human immunodeficiency virus (HIV) through organs and tissue from an infected but seronegative donor was reported. All recipients of organs and all recipients of unprocessed fresh-frozen bone were infected with HIV-1. However, all recipients of processed or acellular tissues tested negative for HIV-1 antibody 34. Since the introduction of strict screening protocols according to modern tests (such as polymerase chain reaction) and international regulations, no more viral infections have been found 35. In addition, processing methods have been developed that provide validated virus inactivation 36,37. Another issue may be increased bacterial infection after bone grafting. Lord et al. found a 11,7% infection rate after massive allografts in oncological patients38. The same group found an infection rate of 5% after revision THR with the use of fresh frozen allografts 39. This rate markedly increases up to 14% when the allografts are used in the second stage of a two-stage procedure after infected THR 10. It is difficult to differentiate whether the infections originated from remaining bacteria from previous surgery, new contamination during complicated surgery or contamination of the allografts themselves. Fresh frozen femoral heads always bear the risk of undetected bacterial contamination that cannot be detected by routine culturing. Swabs from the surfaces of excised bone are unreliable 40, especially when antibiotics were given prior to harvesting, as is common practice in THR. Gamma irradiation of allografts 41 or other sterilization methods therefore are recommended for increasing safety. However, biological properties of allografts may be altered by the respective sterilization methods and may compromise incorporaton into the host.
Bicompatibility
Removal of bone marrow not only increases safety of allograft bone; it also favours incorporation of the grafted material into the host organism. Aspenberg et al. demonstrated that lipid extracted bone grafts enhanced bank bone incorporation in rabbits, 42 whereas the improvement may be due to the decrease of immunological or inflammatory reactions 43. Another reason for improved incorporation may be found in the uncompromised ingrowth of reparative host tissue into the “empty” lacunae of cancellous bone. The most common approach for cleaning bone from marrow is defatting with ether, chloroform or other chemicals. However, it has become clear that incorporation of bone substitutes is dependent on multiple factors 44 which can hardly be met by synthetic substitutes. Porosity and other mechanical features have a major influence on bone growth which cannot be met by synthetics. Growth factors inside the natural bone matrix may play a significant role in incorporation and may be compromised by aggressive chemical reactions. It therefore appears favourable to use a processing method that leaves the natural composition of the matrix as physiological as possible. One processing technology takes advantage of using super-critical CO2 (scCO2), which currently seems the most gentle and effective technology of cleaning bone allografts. CO2 is absolutely inert and such cannot react with proteins like growth factors inside the bone matrix. When it is put under a pressure of 250 bar with a temperature of 50°C, it transforms into supercritical state. Supercritical means that CO2 has the characteristics of both a gas and a fluid. In this state, CO2 has the ability of high penetration throughout the whole bone (like a gas) combined with high potential of solving lipids and removing associated cellular components (like a solvent fluid) 45. Using this technology, lipids and cells are eliminated while the collagen matrix consisting of collagen and minerals is maintained. Osteoinductive proteins are not to be altered and the native composition of the matrix may promote uncompromised osteoconduction. By removing fat and cellular components, antigens are removed and immunological reactions are avoided.46 The virus-inactivating effect of the scCO2 process has been validated several times. 37
Consequences for an effective therapy
An infected operative site cannot be sterilized by debridement alone. Debridement will remove the predominant amount of bioburden, but even the most careful cleaning cannot prevent residual small bacterial colonies from being displaced to new niches in the debrided site. Antibiotic concentrations reached by systemic antibiosis or local therapy with established antibiotic carriers may eradicate planktonic residues, but may not be effective for eliminating micro-clusters disrupted from biofilms that may result in infection recurrence. It is commonly accepted that whatever filler is used, it needs to be protected against recolonization with remaining bacteria. Dead space management after debridement may be performed with antibiotic loaded cement, spacers or bead chains. It should be kept in mind that these are not anti-biofilm tools; their antibiotic content provides short lived prophylactic aid against colonization with planktonic bacteria but is not capable of sterilizing sites contaminated with sessile bacteria and provide no protection against biofilm colonization 47-50.
For eliminating residual biofilm fragments, local carriers are necessary to provide sufficiently high local antibiotic concentrations for prolonged periods of time 51,52, For mature biofilms of S.aureus, Post et al. showed that after 28 days under static conditions, the S.aureus biofilm was completely eradicated at 200 mg/L vancomycin and higher concentrations, but not under 100 mg/L.53. Fragments of biofilms are more vulnerable to antibiotics compared to intact biofilm systems, 54,55 but their elimination still requires concentrations exceeding the levels provided by systemic or conventional local antibiotic therapy.
Local antibiotic delivery
The high antibiotic concentrations needed for biofilm elimination are only feasible by local application. In evaluating novel systems, antibiotics must pass several tests qualifying them for that purpose. Few antibiotics have been identified to meet those criteria, and the most widely used ones include glykopeptides and aminoglycosides. The majority of the pathogens involved in bone infection are gram-positive and susceptible to vancomycin, while most gram-negative organisms are susceptible to tobramycin. Both show inferior tissue penetration compared with other antibiotics, which is a disadvantage to administering them systemically. In local application, the disadvantage turns into an advantage, since there is very slow resorption and penetration from the bone into the vascular system. Vancomycin and tobramycin show the least cytotoxic effect of all commonly used antibiotics 56 and are not likely to cause systemic side effects after local application 26. It therefore may be suggested that local application of antibiotics with similar properties as vancomycin together with an appropriate carrier may be a valuable tool against orthopaedic infections. The carrier should provide for high initial levels to penetrate remaining biofilm fragments rapidly and consequently keep the concentrations above critical levels (which may be estimated between 200 and 500 mg/l for vancomycin) for a minimum of 72 hours.
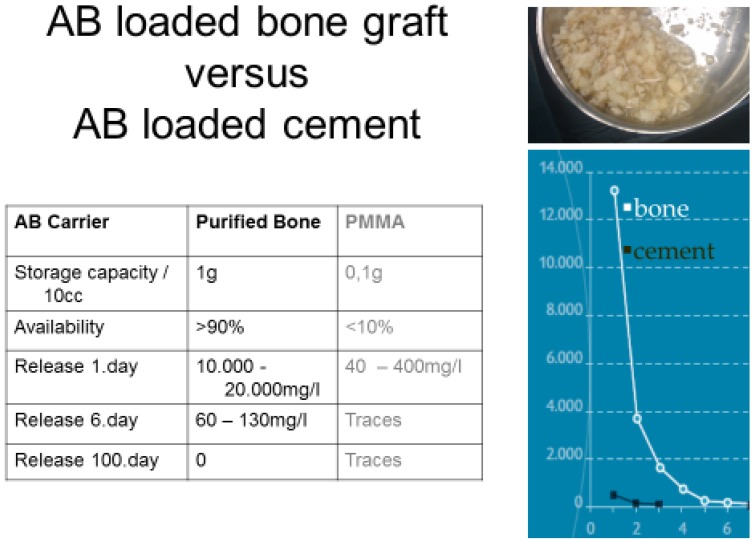
When mixing bone grafts with antibiotics their storage capability for antibiotics vastly exceeds those of PMMA 25,57,58. When loading highly purified cancellous bone, local concentrations of vancomycin can go up to 20,000mg/l (Fig.3) and up to 13.000mg/l with tobramycin 29. With this kind of impregnation, the whole amount of loaded antibiotic is available for antimicrobial activity and the activity remains far beyond the susceptibility of relevant pathogens for weeks. These capacities make them attractive for reconstruction of contaminated sites and act as a powerful local carrier at the same time. As the provided antibiotic concentrations may decontaminate the surrounding, implants may be used simultaneously.
Figure 3.
Loading of carriers with antibiotics. Purified bone may store 10x the amount of vancomycin compared with cement. Almost the whole amount is available, leading to markedly elevated local concentrations and a prolonged biofilm-active release.
Osteomycin™
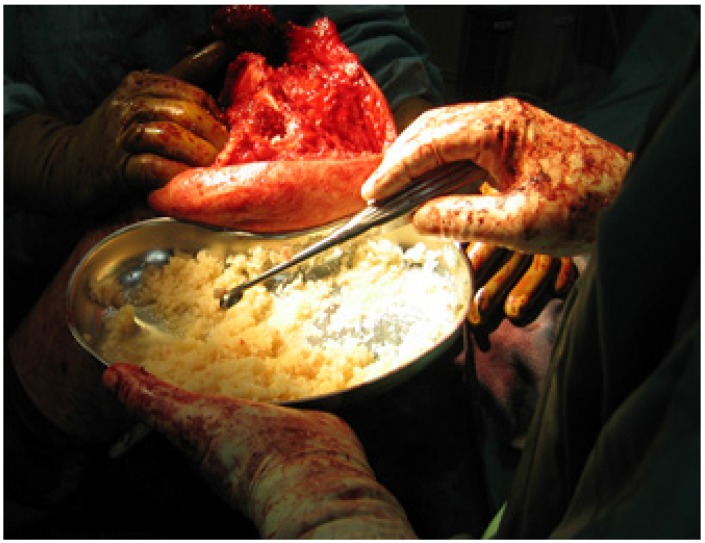
The European Cell and Tissue Bank (ECTB), a non-profit tissue establishment according to European Union (EU)-directives strives for providing safe allografts for reconstruction of osseous defects in contaminated sites. Donors of bone (e.g. femoral heads) are extensively screened by medical history and laboratory tests including PCR for detection of viral DNA. Cancellous parts of bone are morsellized to pieces of 1 to 10mm diameter, cleaned and processed using supercritical CO2 with validated virus-inactivating effect. Lipids and cellular components are removed completely, leaving a pure scaffold of bone matrix. Sterility is performed by gamma irradiation in a dry state. As collagen, minerals and osteoinductive proteins remain mainly unaltered, mechanical and biological properties are only slightly modified. The purified matrix is then impregnated with high loads of vancomycin or tobramycin, respectively. The standardized impregnation technique provides a uniform concentration of 1g vancomycin or 0.4g tobramycin, respectively, in 10cc of bone29. Due to the proprietary impregnation technique, antibiotics are deposited throughout the whole graft, mainly in the lacunae of the spongy matrix. The antibiotic bone compound finally is lyophilized, providing storage life up to 2 years under room temperature. After rehydration, OSTEOmycin™ reconstitutes to a mouldable mass ready for impaction grafting (Fig.4). After implantation, it elutes vancomycin or tobramycin in a sustained way, providing extremely high local concentrations with a logarithmic decrease over the following weeks. (Fig.3). Release of the antibiotic is completed after 4 weeks, and such is unlikely to create resistances or initiate the formation of SCVs. Remodelling of the grafted bone follows the patterns of “creeping substitution” (Fig. 5).
Figure 4.
Antibiotic impregnated cancellous bone (Osteomyin™). Granules with a median size of 5mm in the re-hydrated condition provide a mouldable mass suitable for impaction grafting.
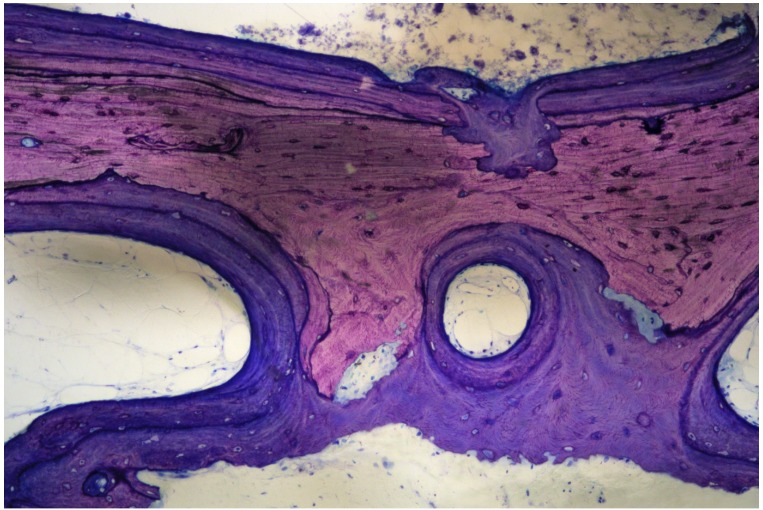
Figure 5.
Biopsy of grafted OsteomycinTM, 6 months after removal of an infected dental implant and simultaneous implantation into the maxilla. “Creeping substitution” with osteoclasts resorbing the graft and osteoblasts depositing new bone simultaneously. There are no signs of inflammation.
Treatment Protocol
Treatment of bone infection is a highly specialized discipline and should be reserved for experienced surgeons in a dedicated centre. A clear algorithm should be followed that should respect the present scientific knowledge on biofilm behaviour and bone healing. In order to eradicate biofilm mediated infections, basic requirements need to be followed (the 5 D's):
Detect: Localize the infection as accurately as possible.
Debride: Drastically reduce the number of organisms and their means of livelihood by debriding all identified avital material as radically as possible;
Disrupt: Disturb the living community of residual biofilm colonies by mechanically disrupting their established structures as thoroughly as possible.
Dead space management: Obliterate possible colonization grounds by filling dead space with inaccessible biocompatible material as completely as possible;
Decontaminate: Eliminate sessile bacteria inside remaining fragments using antimicrobial substances in concentrations as high and as consistently as possible.
In addition, reconstruction of defects is favourable and often necessary to restore the function of the affected limb. As ABC simultaneously provides for reconstruction of defects and delivery of antibiotics, single stage supply appears feasible. Unstable fractures or pseudoarthroses may be stabilized by internal fixation, implants for re-implantation may be placed without the use of cement. Wound closure may be performed primarily; in cases with compromised soft tissue skin closure may require coverage with muscular or musculocutaneous flaps.
General surgical principles in Osteitis Centre Vienna
Access to infected parts follows predetermined pathways. Sinus tracts and / or scars from former surgical procedures always are excised. The soft tissue on the way is carefully examined and debrided immediately as soon as infectious tissue is identified.
Metallic implants, cement and sequesters must be removed completely. Implants and necrotic bone fragments usually are sent to sonication to better identify organisms. Tissue specimen from at least 4 different locations are sent for culturing.
Sclerotic bone should not be sacrificed as long as it is vascularized. Even poorly vascularized parts may serve an important role for stability. Since bacteria are only attached to the surface, cautious abrasion is sufficient for elimination of biofilms. Abrasion of surfaces is performed using a high speed burr with different sizes of drills. (Fig. 5)
Every debridement is followed by extensive lavage using several litres of pulsed saline. After completion of cleaning, gloves, gowns and instruments are changed and the second stage commences as “clean surgery”. Remaining cavities and defects are filled with highly purified ABC (Fig.6). To address the problem of potentially undetected polymicrobial colonization, it seems favourable to reserve monotherapy to cases with strong evidence of monomicrobial gram-positive infections, i.e. acute onset of symptoms with typical clinical appearance (fever, pus) and unambiguous culture. Chronic infections with prior infection related surgery or negative cultures should be considered polymicrobial. These patients should be treated with a combination of two or more antibiotics, whereas combinations of vancomycin with tobramycin seem to be favourable, taking advantage of the synergistic activity of the two antibiotics 59,60. This combined approach should cover most of the relevant pathogens. In the case of gram-positive infections, vancomycin impregnated bone (Osteomycin™ V) may be used, and in gram-negative infections, vancomycin impregnated bone may be combined with tobramycin impregnated graft (Osteomycin™ T). Filling is performed stepwise using an impaction grafting technique 61-63. Uncovered surfaces may be sealed with fibrin glue for preventing dislocation. Stabilization is performed with internal fixation, as in aseptic surgery (Fig.6, Fig.7). In hips uncemented implants are used for reconstruction (Fig.8), in knees implants are connected with uncemented stems, cement is only used at articular surfaces.
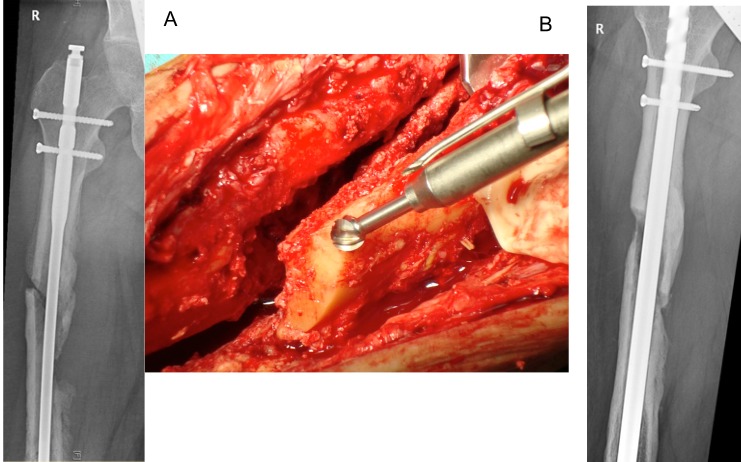
Figure 6.
A 24 year old male who was in a car accident with femur fracture treated with intramedullary nailing. He sustained a postoperative infection, with 3 revisions and exchange of the femoral nail. The patient presented with fever, persistent fistulation, and positive cultures (methicillin sensitive S.aureus and methicillin resistant S.epidermidis). A) Preop. X-Ray. The presence of a loose nail with unstable pseudarthrosis and major osseous defect at the non-union and around the distal locking screws (Cierny-Mader stage 4). The septic non-union was exposed and debrided. A high speed burr with stepless rotation up to 60.000rpm and integrated irrigation/cooling system provides abrasion of scleroses down to slightly bleeding bone (“Paprika-sign”), using drills of variable sizes. B) Postoperative. An exchange of the intramedullary nail was performed with rigid fixation by locking screws proximally and distally, with defects filled with antibiotic-bone-compound ABC.
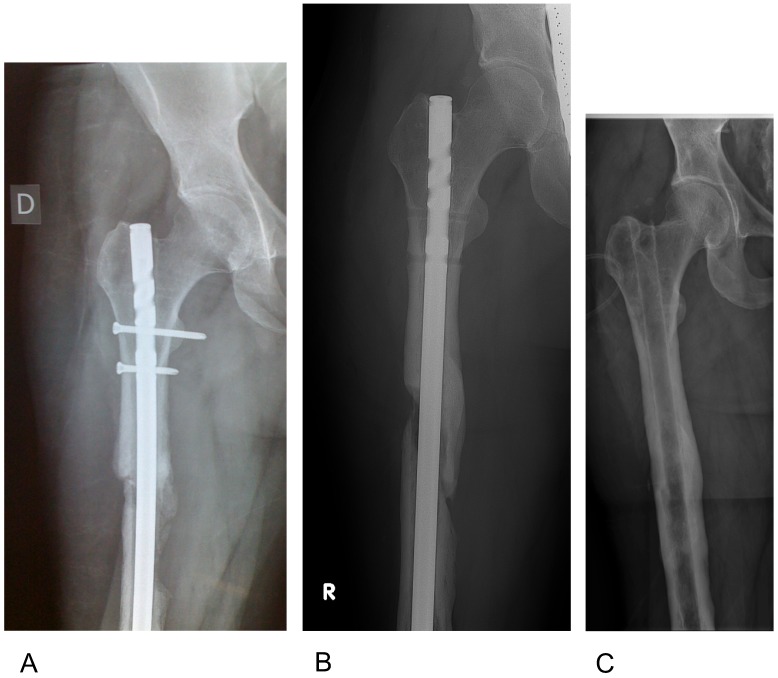
Figure 7.
Radiographical follow up of the case in Fig 6. A) 6 weeks postop: Fully weight bearing, no sign of infection. B) 1 yr postop. Dynamization was performed by removing the proximal interlocking screws; the patient is fully weight bearing with no signs of infection. C) 7yrs postop: Hardware removal. Complete union, defects restored. The patient returned to sports with no signs of infection.
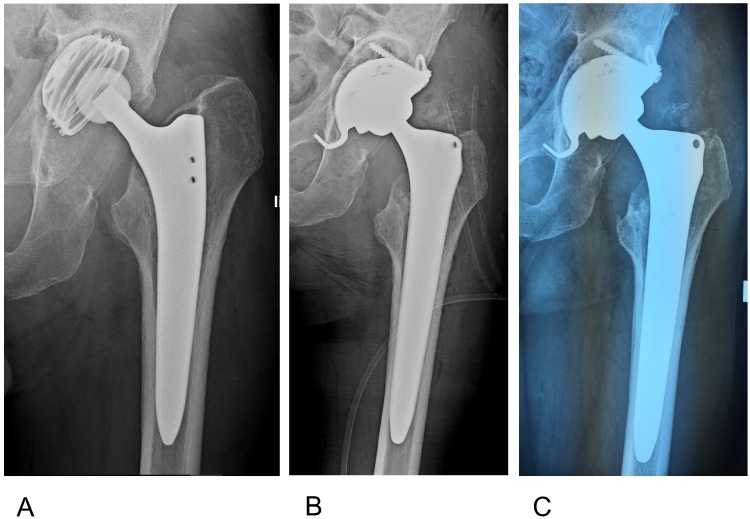
Figure 8.
A 66 year old male who sustained a femoral neck fracture treated with uncemented THR. Postoperatively he complained of unspecific pain with only slightly elevated infection markers. A) 3 years later loosening of the acetabular component was diagnosed with marked osseous defect periacetabular and signs of osteolysis around the proximal part of the stem. B) One stage exchange with uncemented components. The defects were filled with antibiotic impregnated bone Osteomycin V TM. Sonification of explanted material revealed growth of 2 strains of S.epidermidis (MSSE) and Propionibact. sp. Hospital stay was one week, with Cefuroxim intravenously, followed by 6 weeks Amoxicillin/Clavulanic acid + Rifampicin orally. C) 6 months postop: The patient is painfree with no sign of infection and unlimited mobility. There is partial remodelling of the allograft material with no sign of loosening of implants.
Complete soft tissue coverage is essential for success, and may require muscular or fasciocutanous flaps for closure. In these cases, plastic surgery may become necessary immediately following reconstruction. Drains are placed before wound closure, and are left without suction for 24hours, with suction for 1-3 additional days.
Postoperative antibiotic treatment follows the results of preoperative cultures. In cases of culture negative infection, we routinely administer a second generation cephalosporin until the results of intraoperative cultures become available. Flaps usually require one week of bed rest, while all other cases are mobilized immediately with at least partial weight bearing. C-M stage 1 and 2 patients are usually capable of full weight bearing within a few days. C-M stage 3 and 4 patients may require up to 6 weeks of partial weight bearing. After revision THR and TKR 6 weeks of partial weight bearing are recommended.
Conclusion
Appropriately processed allograft bone provides potential as an excellent carrier for antibiotics. Its use is safe and efficient in the restoration of osseous defects. Osteomyelitic lesions and infected implants may be successfully treated using thorough debridement in conjunction with antibiotic impregnated allograft bone, which provides dead space management and reconstruction of deficient areas of bone at the same time. As long as the local antibiotic levels are higher than the dosage required for eliminating biofilm embedded bacteria contamination of alloplastic material should not be expected. Using antibiotic impregnated grafts, the treatment of infection, reconstruction, internal stabilization and / or re-implantation may be performed within a single operation as under non-septic conditions. Re-infection may occur in complex cases where secluded infection foci are not detected during debridement. Eventual re-revisions are markedly less demanding when missed foci are detected early. Care should be taken to provide good soft tissue coverage, using muscle flaps in situations with poor tissue coverage. The choice of stabilizing or prosthetic material should be considered carefully.
Although the results of the new protocol seem very promising, we can never be fully certain that bone infection is cured. Assuming that recurrence may occur within an unknown period of time, it should be the responsibility of the surgeon to provide for a treatment to keep the risk of reinfection for the patient to an absolute minimum. Frequent surgical interventions with long hospital stays, correlating with increased pain and / or reduced mobility should be avoided as much as possible. Antibiotic impregnated allograft bone seems to be an advantageous adjunct in reaching the goals of minimizing the number of treatments while providing effective infection treatment and restoring patient function.
Figure 2.
Highly purified bone offers the advantages of 1. Increased safety, 2. Accelerated incorporation, and 3. Enormous reservoir / surface available for storage and adhesion of antimicrobial substances. Stored antibiotics are able to diffuse through the open canaliculi, providing controlled release over weeks after implantation.
References
- 1.Costerton JW. Biofilm theory can guide the treatment of device-related orthopaedic infections. Clin Orthop Relat Res. 2005:7–11. doi: 10.1097/00003086-200508000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Lichstein P, Gehrke T, Lombardi A. et al. One-stage vs two-stage exchange. J Arthroplasty. 2014;29:108–11. doi: 10.1016/j.arth.2013.09.048. [DOI] [PubMed] [Google Scholar]
- 3.Masquelet AC, Fitoussi F, Begue T, Muller GP. [Reconstruction of the long bones by the induced membrane and spongy autograft] Ann Chir Plast Esthet. 2000;45:346–53. [PubMed] [Google Scholar]
- 4.Christou C, Oliver RA, Yu Y, Walsh WR. The Masquelet technique for membrane induction and the healing of ovine critical sized segmental defects. PLoS One. 2014;9:e114122. doi: 10.1371/journal.pone.0114122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karger C, Kishi T, Schneider L, Fitoussi F, Masquelet AC. Treatment of posttraumatic bone defects by the induced membrane technique. Orthop Traumatol Surg Res. 2012;98:97–102. doi: 10.1016/j.otsr.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 6.Ilizarov GA, Lediaev VI. [Replacement of defects of long tubular bones by means of one of their fragments] Vestn Khir Im I I Grek. 1969;102:77–84. [PubMed] [Google Scholar]
- 7.Arora S, Batra S, Gupta V, Goyal A. Distraction osteogenesis using a monolateral external fixator for infected non-union of the femur with bone loss. Journal of orthopaedic surgery. 2012;20:185–90. doi: 10.1177/230949901202000210. [DOI] [PubMed] [Google Scholar]
- 8.Low CK, Pho RW, Kour AK, Satku K, Kumar VP. Infection of vascularized fibular grafts. Clin Orthop. 1996:163–72. doi: 10.1097/00003086-199602000-00022. [DOI] [PubMed] [Google Scholar]
- 9.Papakostidis C, Bhandari M, Giannoudis PV. Distraction osteogenesis in the treatment of long bone defects of the lower limbs: effectiveness, complications and clinical results; a systematic review and meta-analysis. Bone Joint J. 2013;95B:1673–80. doi: 10.1302/0301-620X.95B12.32385. [DOI] [PubMed] [Google Scholar]
- 10.Ammon P, Stockley I. Allograft bone in two-stage revision of the hip for infection. Is it safe? J Bone Joint Surg Br. 2004;86:962–5. doi: 10.1302/0301-620x.86b7.14292. [DOI] [PubMed] [Google Scholar]
- 11.Costerton JW, Geesey GG, Cheng KJ. How bacteria stick. Scientific American. 1978;238:86–95. doi: 10.1038/scientificamerican0178-86. [DOI] [PubMed] [Google Scholar]
- 12.Holmberg A, Rasmussen M. Mature biofilms of Enterococcus faecalis and Enterococcus faecium are highly resistant to antibiotics. Diagnostic Microbiology and Infectious Disease. 2016;84:19–21. doi: 10.1016/j.diagmicrobio.2015.09.012. [DOI] [PubMed] [Google Scholar]
- 13.Gristina AG, Oga M, Webb LX, Hobgood CD. Adherent bacterial colonization in the pathogenesis of osteomyelitis. Science. 1985;228:990–3. doi: 10.1126/science.4001933. [DOI] [PubMed] [Google Scholar]
- 14.Gristina AG, Costerton JW. Bacterial adherence to biomaterials and tissue. The significance of its role in clinical sepsis. J Bone Joint Surg Am. 1985;67:264–73. [PubMed] [Google Scholar]
- 15.Buchholz HW, Engelbrecht H. [Depot effects of various antibiotics mixed with Palacos resins] Chirurg. 1970;41:511–5. [PubMed] [Google Scholar]
- 16.Klemm K. [Gentamicin-PMMA-beads in treating bone and soft tissue infections (author's transl)] Zentralbl Chir. 1979;104:934–42. [PubMed] [Google Scholar]
- 17.Neut D, van De Belt H, Stokroos I, van Horn JR, van Der Mei HC, Busscher HJ. Biomaterial-associated infection of gentamicin-loaded PMMA beads in orthopaedic revision surgery. J Antimicrob Chemother. 2001;47:885–91. doi: 10.1093/jac/47.6.885. [DOI] [PubMed] [Google Scholar]
- 18.Nelson CL, Jones RB, Wingert NC, Foltzer M, Bowen TR. Sonication of antibiotic spacers predicts failure during two-stage revision for prosthetic knee and hip infections. Clin Orthop Relat Res. 2014;472:2208–14. doi: 10.1007/s11999-014-3571-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tunney MM, Ramage G, Patrick S, Nixon JR, Murphy PG, Gorman SP. Antimicrobial susceptibility of bacteria isolated from orthopedic implants following revision hip surgery. Antimicrob Agents Chemother. 1998;42:3002–5. doi: 10.1128/aac.42.11.3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang CC, Merritt K. Microbial adherence on poly(methyl methacrylate) (PMMA) surfaces. J Biomed Mater Res. 1992;26:197–207. doi: 10.1002/jbm.820260206. [DOI] [PubMed] [Google Scholar]
- 21.Saginur R, Stdenis M, Ferris W. et al. Multiple combination bactericidal testing of staphylococcal biofilms from implant-associated infections. Antimicrob Agents Chemother. 2006;50:55–61. doi: 10.1128/AAC.50.1.55-61.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van de Belt H, Neut D, Schenk W, van Horn JR, van der Mei HC, Busscher HJ. Infection of orthopedic implants and the use of antibiotic-loaded bone cements. A review. Acta Orthop Scand. 2001;72:557–71. doi: 10.1080/000164701317268978. [DOI] [PubMed] [Google Scholar]
- 23.Hazlett JW. THE USE OF CANCELLOUS BONE GRAFTS IN THE TREATMENT OF SUBACUTE AND CHRONIC OSTEOMYELITIS. J Bone Joint Surg Br. 1954;36B:584–90. doi: 10.1302/0301-620X.36B4.584. [DOI] [PubMed] [Google Scholar]
- 24.Witso E, Persen L, Benum P, Aamodt A, Husby OS, Bergh K. High local concentrations without systemic adverse effects after impaction of netilmicin-impregnated bone. Acta Orthop Scand. 2004;75:339–46. doi: 10.1080/00016470410001295. [DOI] [PubMed] [Google Scholar]
- 25.Buttaro MA, Pusso R, Piccaluga F. Vancomycin-supplemented impacted bone allografts in infected hip arthroplasty. Two-stage revision results. J Bone Joint Surg Br. 2005;87:314–9. doi: 10.1302/0301-620x.87b3.14788. [DOI] [PubMed] [Google Scholar]
- 26.Buttaro MA, Gimenez MI, Greco G, Barcan L, Piccaluga F. High active local levels of vancomycin without nephrotoxicity released from impacted bone allografts in 20 revision hip arthroplasties. Acta Orthop. 2005;76:336–40. [PubMed] [Google Scholar]
- 27.Michalak KA, Khoo PPC, Yates PJ, Day RE, Wood DJ. Iontophoresed segmental allografts in revision arthroplasty for infection. J Bone Joint Surg Br. 2006;88B:1430–7. doi: 10.1302/0301-620X.88B11.18335. [DOI] [PubMed] [Google Scholar]
- 28.Khoo PPC, Michalak KA, Yates PJ, Megson SM, Day RE, Wood DJ. Iontophoresis of antibiotics into segmental allografts. J Bone Joint Surg Br. 2006;88B:1149–57. doi: 10.1302/0301-620X.88B9.17500. [DOI] [PubMed] [Google Scholar]
- 29.Winkler H, Janata O, Berger C, Wein W, Georgopoulos A. In vitro release of vancomycin and tobramycin from impregnated human and bovine bone grafts. J Antimicrob Chemother. 2000;46:423–8. doi: 10.1093/jac/46.3.423. [DOI] [PubMed] [Google Scholar]
- 30.Gruber R, Stadlinger B, Terheyden H. Cell-to-cell communication in guided bone regeneration: molecular and cellular mechanisms. Clin Oral Implants Res; 2016. [DOI] [PubMed] [Google Scholar]
- 31.Katz J, Mukherjee N, Cobb RR, Bursac P, York-Ely A. Incorporation and immunogenicity of cleaned bovine bone in a sheep model. J Biomater Appl. 2009;24:159–74. doi: 10.1177/0885328208095174. [DOI] [PubMed] [Google Scholar]
- 32.Friedlaender GE. Bone allografts: the biological consequences of immunological events. J Bone Joint Surg Am. 1991;73:1119–22. [PubMed] [Google Scholar]
- 33.Thoren K, Aspenberg P. Increased bone ingrowth distance into lipid-extracted bank bone at 6 weeks. A titanium chamber study in allogeneic and syngeneic rats. Arch Orthop Trauma Surg. 1995;114:167–71. doi: 10.1007/BF00443391. [DOI] [PubMed] [Google Scholar]
- 34.Simonds RJ, Holmberg SD, Hurwitz RL. et al. Transmission of human immunodeficiency virus type 1 from a seronegative organ and tissue donor. N Engl J Med. 1992;326:726–32. doi: 10.1056/NEJM199203123261102. [DOI] [PubMed] [Google Scholar]
- 35.Ng VY. Risk of disease transmission with bone allograft. Orthopedics. 2012;35:679–81. doi: 10.3928/01477447-20120725-04. [DOI] [PubMed] [Google Scholar]
- 36.Pruss A, Hansen A, Kao M. et al. Comparison of the efficacy of virus inactivation methods in allogeneic avital bone tissue transplants. Cell Tissue Bank. 2001;2:201–15. doi: 10.1023/A:1021164111246. [DOI] [PubMed] [Google Scholar]
- 37.Fages J, Poirier B, Barbier Y. et al. Viral inactivation of human bone tissue using supercritical fluid extraction. Asaio J. 1998;44:289–93. doi: 10.1097/00002480-199807000-00009. [DOI] [PubMed] [Google Scholar]
- 38.Lord CF, Gebhardt MC, Tomford WW, Mankin HJ. Infection in bone allografts. Incidence, nature, and treatment. The Journal of bone and joint surgery American volume. 1988;70:369–76. [PubMed] [Google Scholar]
- 39.Tomford WW, Thongphasuk J, Mankin HJ, Ferraro MJ. Frozen musculoskeletal allografts. A study of the clinical incidence and causes of infection associated with their use. The Journal of bone and joint surgery American volume. 1990;72:1137–43. [PubMed] [Google Scholar]
- 40.Dennis JA, Martinez OV, Landy DC. et al. A comparison of two microbial detection methods used in aseptic processing of musculoskeletal allograft tissues. Cell Tissue Bank. 2011;12:45–50. doi: 10.1007/s10561-009-9158-8. [DOI] [PubMed] [Google Scholar]
- 41.Singh R, Singh D, Singh A. Radiation sterilization of tissue allografts: A review. World journal of radiology. 2016;8:355–69. doi: 10.4329/wjr.v8.i4.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aspenberg P, Thoren K. Lipid extraction enhances bank bone incorporation. An experiment in rabbits. Acta Orthop Scand. 1990;61:546–8. doi: 10.3109/17453679008993579. [DOI] [PubMed] [Google Scholar]
- 43.Thoren K, Aspenberg P, Thorngren KG. Lipid extracted bank bone. Bone conductive and mechanical properties. Clin Orthop Relat Res. 1995:232–46. [PubMed] [Google Scholar]
- 44.Porter JR, Ruckh TT, Popat KC. Bone tissue engineering: A review in bone biomimetics and drug delivery strategies. Biotechnology Progress. 2009;25:1539–60. doi: 10.1002/btpr.246. [DOI] [PubMed] [Google Scholar]
- 45.Fages J, Marty A, Delga C, Condoret JS, Combes D, Frayssinet P. Use of supercritical CO2 for bone delipidation. Biomaterials. 1994;15:650–6. doi: 10.1016/0142-9612(94)90162-7. [DOI] [PubMed] [Google Scholar]
- 46.Frayssinet P, Rouquet N, Mathon D, Autefage A, Fages J. Histological integration of allogeneic cancellous bone tissue treated by supercritical CO2 implanted in sheep bones. Biomaterials. 1998;19:2247–53. doi: 10.1016/s0142-9612(98)00124-0. [DOI] [PubMed] [Google Scholar]
- 47.Greene N, Holtom PD, Warren CA. et al. In vitro elution of tobramycin and vancomycin polymethylmethacrylate beads and spacers from Simplex and Palacos. Am J Orthop. 1998;27:201–5. [PubMed] [Google Scholar]
- 48.Masri B, Duncan C, Beauchamp C. Long-term elution of antibiotics from bone-cement: an in vivo study using the prosthesis of antibiotic-loaded acrylic cement (PROSTALAC) system. J Arthroplasty. 1998;13:331–8. doi: 10.1016/s0883-5403(98)90179-6. [DOI] [PubMed] [Google Scholar]
- 49.Bertazzoni Minelli E, Benini A, Magnan B, Bartolozzi P. Release of gentamicin and vancomycin from temporary human hip spacers in two-stage revision of infected arthroplasty. J Antimicrob Chemother. 2004;53:329–34. doi: 10.1093/jac/dkh032. [DOI] [PubMed] [Google Scholar]
- 50.Walenkamp GH. Gentamicin PMMA beads and other local antibiotic carriers in two-stage revision of total knee infection: a review. J Chemother. 2001;13:66–72. doi: 10.1179/joc.2001.13.Supplement-2.66. [DOI] [PubMed] [Google Scholar]
- 51.Ehrlich GDD, Culture Negative Orthopedic Biofilm Infections. Heidelberg, New York: Springer Berlin Heidelberg; 2012. [Google Scholar]
- 52.Smith AW. Biofilms and antibiotic therapy: is there a role for combating bacterial resistance by the use of novel drug delivery systems? Adv Drug Deliv Rev. 2005;57:1539–50. doi: 10.1016/j.addr.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 53.Post V, Wahl P, Richards RG, Moriarty TF. Vancomycin displays time dependent eradication of mature Staphylococcus aureus biofilms. J Orthop Res; 2016. [DOI] [PubMed] [Google Scholar]
- 54.El-Azizi M, Rao S, Kanchanapoom T, Khardori N. In vitro activity of vancomycin, quinupristin/dalfopristin, and linezolid against intact and disrupted biofilms of staphylococci. Ann Clin Microbiol Antimicrob. 2005;4:2. doi: 10.1186/1476-0711-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fux CA, Wilson S, Stoodley P. Detachment characteristics and oxacillin resistance of Staphyloccocus aureus biofilm emboli in an in vitro catheter infection model. J Bacteriol. 2004;186:4486–91. doi: 10.1128/JB.186.14.4486-4491.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Edin ML, Miclau T, Lester GE, Lindsey RW, Dahners LE. Effect of cefazolin and vancomycin on osteoblasts in vitro. Clin Orthop Relat Res. 1996:245–51. [PubMed] [Google Scholar]
- 57.Witso E, Persen L, Loseth K, Benum P, Bergh K. Cancellous bone as an antibiotic carrier. Acta Orthop Scand. 2000;71:80–4. doi: 10.1080/00016470052943955. [DOI] [PubMed] [Google Scholar]
- 58.Witso E, Persen L, Loseth K, Bergh K. Adsorption and release of antibiotics from morselized cancellous bone. In vitro studies of 8 antibiotics. Acta Orthop Scand. 1999;70:298–304. doi: 10.3109/17453679908997812. [DOI] [PubMed] [Google Scholar]
- 59.Watanakunakorn C, Tisone JC. Synergism between vancomycin and gentamicin or tobramycin for methicillin-susceptible and methicillin-resistant Staphylococcus aureus strains. Antimicrob Agents Chemother. 1982;22:903–5. doi: 10.1128/aac.22.5.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gonzalez Della Valle A, Bostrom M, Brause B, Harney C, Salvati EA. Effective bactericidal activity of tobramycin and vancomycin eluted from acrylic bone cement. Acta Orthop Scand. 2001;72:237–40. doi: 10.1080/00016470152846547. [DOI] [PubMed] [Google Scholar]
- 61.Schreurs BW, Slooff TJ, Gardeniers JW, Buma P. Acetabular reconstruction with bone impaction grafting and a cemented cup: 20 years' experience. Clin Orthop Relat Res. 2001:202–15. doi: 10.1097/00003086-200112000-00023. [DOI] [PubMed] [Google Scholar]
- 62.Bradley GW. Revision total knee arthroplasty by impaction bone grafting. Clin Orthop Relat Res. 2000:113–8. doi: 10.1097/00003086-200002000-00013. [DOI] [PubMed] [Google Scholar]
- 63.Winkler H, Haiden P. Treatment of Chronic Bone Infection. Operative Techniques in Orthopaedics. 2016;26:2–11. [Google Scholar]