Abstract
The use of antibiotic-loaded cement spacers is an established method in the management of periprosthetic hip and knee joint infections. Despite inconsistencies among published studies, data shows that infection control rates exceed 90% with two-stage exchange arthroplasty. The present work reviews the current literature about antibiotic-loaded cement spacers and concentrates on the indications for spacer implantation, spacer production details, antibiotic impregnation, pharmacokinetic properties, clinical success, mechanical complications, and systemic safety.
Keywords: hip spacer, knee spacer, hip infection, knee infection, antibiotic-loaded bone cement.
Introduction
Over the past four decades, antibiotic-impregnated acrylic bone cement has been established as a valuable tool in the prophylaxis and treatment of orthopaedic infections. Prophylaxis requires low doses of antibiotic(s) in bone cement in order to avoid decreasing the mechanical properties of cement, which is intended for mechanical fixation of prostheses or implants. Generally, low-dose antibiotic-impregnated bone cement is defined as ≤ 1 g antibiotic(s)/40 g polymethylmethacrylate (PMMA). In the treatment of musculoskeletal infections, antibiotic-loaded cement by means of beads or spacers is impregnated with higher doses (usually 4 g antibiotics/40 g PMMA) for infection treatment 1.
Despite numerous prophylactic measures, joint infections still occur in 1-2% of cases after total hip and in 2-3% of cases after total knee replacement 2-3. Such complications can often lead to prolonged and difficult treatment courses and endanger functional outcomes. When establishing a treatment plan, several factors have to be considered: localisation and extent of the infected area, presence of hardware, virulence and antibiotic sensitivity profile of the causative bacterium, time period between primary surgery and infection manifestation (early vs. late infection), and general medical condition of the patient.
During the past two decades, antibiotic-loaded hip and knee spacers have become a popular method of managing such infections with reported success rates of > 90% 2-3. Complications are rather infrequent and consist mostly of mechanical (spacer fracture, dislocation, bone fracture) and those related to the antibiotic impregnation of bone cement (reinfection/infection persistence, systemic side effects such as renal or hepatic failure, allergic reactions).
The aim of the present work is to review the current literature about the use of antibiotic-impregnated cement hip and knee spacers in the management of periprosthetic joint infections (PJI).
Spacer production details
Hip spacers
Hip spacers can be categorized into three different types:
hand-made
standardized, prefabricated, commercially available
standardized, molded, not commercially available
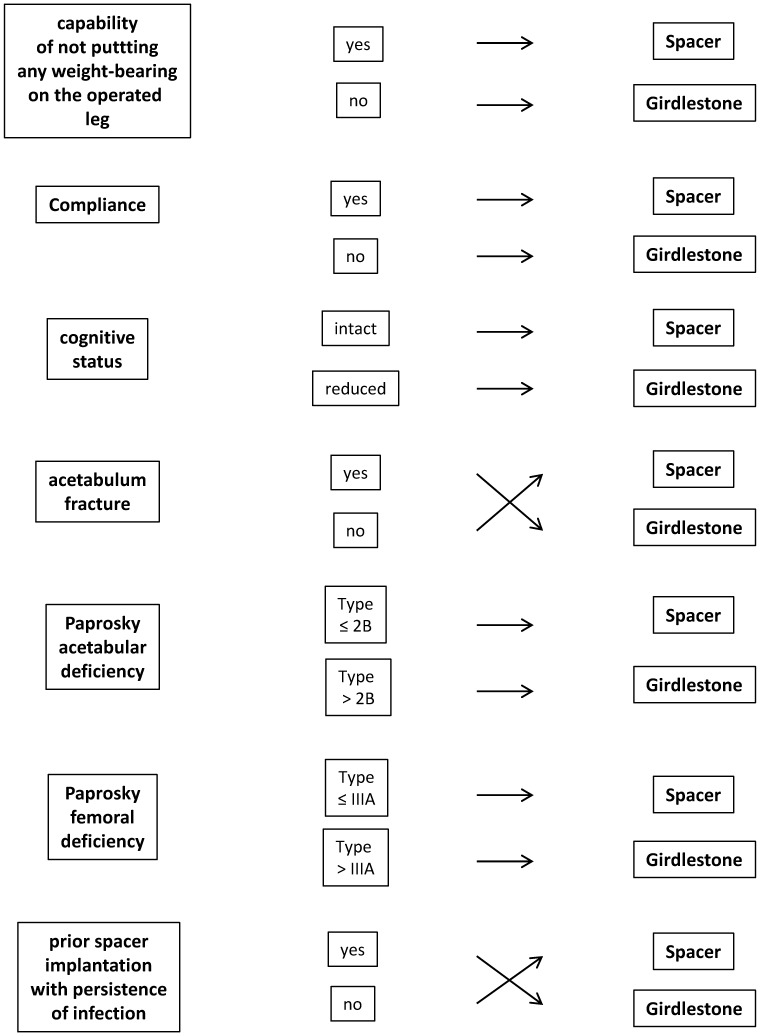
Hand-made spacers are now rare since they do not provide standardized mechanical performance and pharmacokinetic properties, and are associated with a higher risk of spacer dislocations or fractures. A major disadvantage of commercially available, prefabricated spacers lies in the fact that no further antibiotic addition can be made to the cement, in response to the sensitivity profile of the pathogenic organism. Custom-made spacers can be easily produced during surgery and offer the greatest advantages. However, it should be noted that in specific cases, Girdlestone arthroplasty should be taken into consideration as an alternative method (Figure 1).
Figure 1.
Criteria for differentiation between spacer implantation and Girdlestone arthroplasty for the management of late implant-associated hip joint infections.
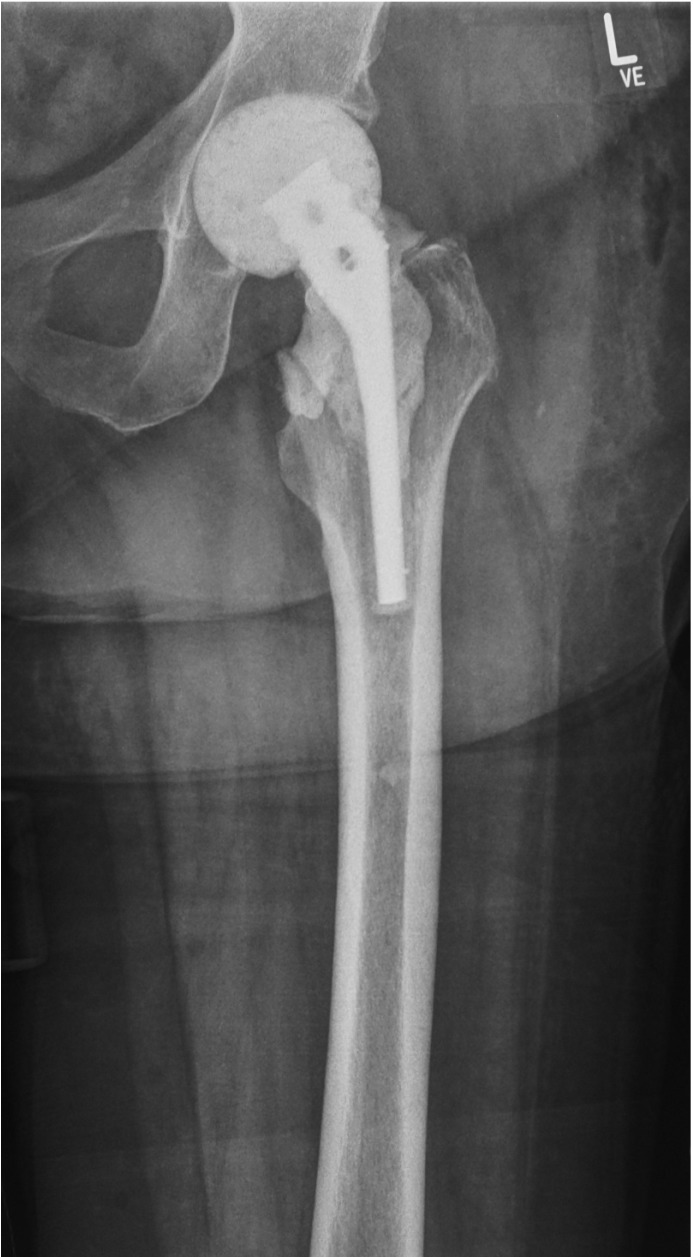
Most hip spacers function as a hemiarthroplasty, whereas only few offer the advantages of a total hip arthroplasty (THA) by additional spacer cup implantation (Figure 2). Unfortunately, to date, no study exists comparing clinical performance and complications among spacers with respect to their articulation and the fixation method. Although proximal cementation of the spacer to the femur might preserve leg length and prevent rotation, no study has demonstrated which one of the two methods is best. Moreover, THA-like spacers may improve the congruence of the joint compared to hemiarthoplasty-like systems; however, no reports have studied whether clinical performance is better with THA-like spacers.
Figure 2.
Anteroposterior X-ray of the left hip joint showing an antibiotic-loaded cement hip spacer with a titanium endoskeleton acting as a hemiarthroplasty.
Knee spacers
Knee spacers can be divided into static and dynamic.
In past years, articulating spacers were used based on the argument that there was better function during the interim period, superior knee motion, improved functional outcomes following reimplantation, and decreased bone loss resulting from spacer migration compared to static spacers 4-7. Systematic literature reviews comparing dynamic to static spacers have provided some contradictory results. Guild et al. reported that articulating spacers had significant increased range of motion, lower reinfection rate, facilitated reimplantation, and developed less bone loss than static spacers 4. Voleti et al. found that both types of spacers demonstrated similar reinfection rates 7. Articulating spacers result in significantly greater range of motion after reimplantation, whereas functional scores were similar in both groups. Rates of wound-related and spacer-related complications were similarly low for both spacer types. Pivec et al. stated that both groups had similar improvement in functional scores, however, articulating spacers had significantly higher range of motion 6. There was no difference in the reinfection rates, complication rates, or reoperation rates between the two groups.
The discrepancy of these some of these statements might be explained by the difference in the publication dates between static and articulating spacers, since many static spacer studies were published in the 1990's 6. Moreover, different lengths of follow-up between both groups might have led to a length-time bias and contributed to differences observed in clinical outcomes, reinfections, and complications 6. Another possible explanation for similar functional scores might be the fact that the range of motion, which was frequently significant different, accounts only for a small proportion of such scores 7.
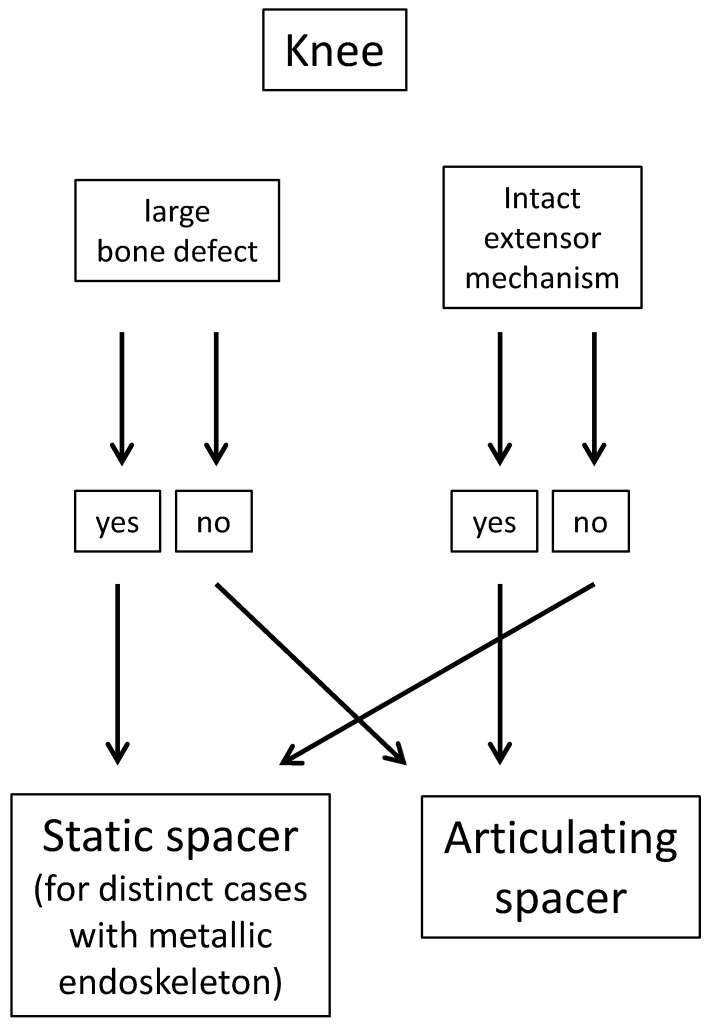
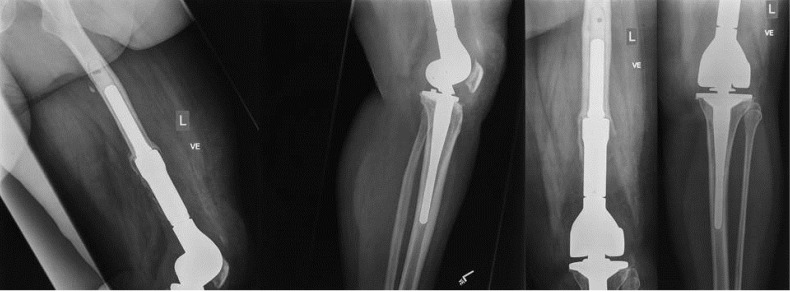
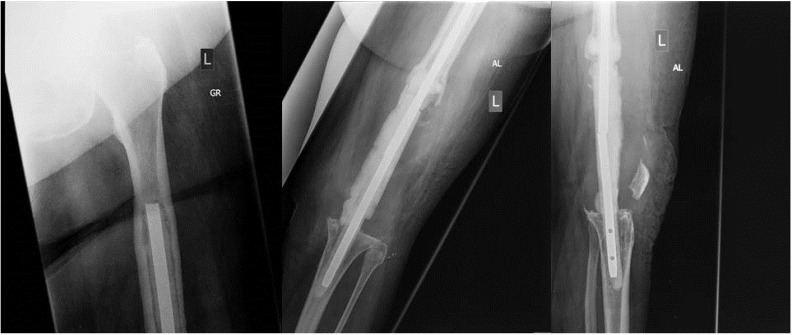
The choice between implantation of a static and an articulating spacer is best made intraoperatively (Figure 3). Based on the amount of bone loss after prosthesis explantation and the status of the extensor mechanism, the orthopaedic surgeon can decide which spacer to implant intraoperatively. With minimal bone loss and an intact extensor mechanism, an articulating spacer might provide a good range of motion between stages. On the other hand, large bone loss and poor quality of the extensor mechanism may facilitate the implantation of a static spacer (Figure 4) or even one with a metallic endoskeleton (Figures 5-6) for preventing postoperative dislocation.
Figure 3.
Criteria for knee spacer implantation.
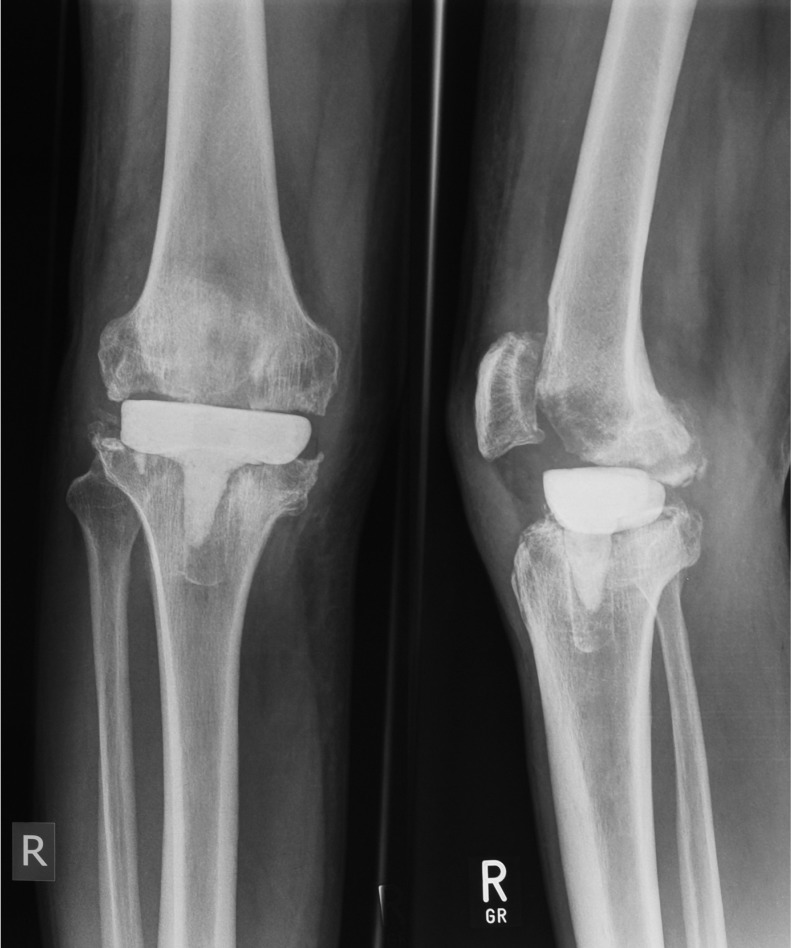
Figure 4.
Anteroposterior and lateral radiographs of a static knee spacer in situ.
Figure 5.
X-rays of the left knee of a 52-year female patient showing a septic loosening of the femoral component two years after implantation of a tumor prosthesis.
Figure 6.
Treatment of the infected tumor prosthesis from Figure 5, consisting of prosthesis explantation and spacer implantation. Due to the large bone defect, an overbridge to maintain leg length and stability was required. Here, an antibiotic-loaded cement-coated nail was implanted with a cement spacer in the leg to solve this problem.
Antibiotic impregnation
An important topic in the use of antibiotic-loaded spacers is the impregnation of the bone cement itself with antibiotics. Not every antibiotic qualifies equally for incorporation into bone cement: desirable characteristics include 1:
its availability in powder form
wide antibacterial spectrum, bactericidal at low concentrations
elution from PMMA in high concentrations for prolonged periods
thermal stability
low or no risk of allergy or delayed hypersensitivity
low influence on the mechanical properties of the cement
low serum protein binding
Aminoglycosides and glycopeptides are known to be the two groups of antibiotics that fulfill most of these criteria. The combination of these antibiotics has the advantage of a wide antimicrobial spectrum with good elution kinetics. Vancomycin is good for treating orthopaedic-related infections since Staphylococci are the most common bacteria causing such infections, and vancomycin possesses an excellent efficacy against these strains, especially resistant strains 1.
Not only is the right antibiotic choice important for adequate local antibiotic therapy, but the amounts of each antibiotic that are incorporated into the cement is important. Depending on the ratio of aminoglycosides and glycopeptides, different synergistic effects between these antibiotic groups should be expected with regard to their elution properties 2, 8. Unfortunately, the ideal amount of antibiotics to be used for spacer impregnation has not been defined, so no recommendations can be made about this (Table 1).
Table 1.
Recommendations for antibiotic impregnation of acrylic bone cement spacers.
| Pathogen organism/Indication | Antibiotic combination (per 40 g pack of PMMA) |
|---|---|
| methicillin-susceptible S. aureus | 0.5 g gentamicin **+ 2 g vancomycin |
| methicillin-resistant S. aureus | 0.5 g gentamicin** + 2 g vancomycin |
| methicillin-susceptible coagulase-negative Staphyloccoci | 0.5 g gentamicin** + 2 g vancomycin |
| methicillin-resistant coagulase-negative Staphyloccoci | 1 g gentamicin + 1 g clindamycin ** + 2 g vancomycin |
| Enterococci | 0.5 g gentamicin** + 2 g vancomycin or 0.5 g gentamicin** + 0.8 g teicoplanin |
| E. coli | 0.5 g gentamicin** + 2 g cefotaxime |
| Ps. aeruginosa | 0.5 g gentamicin** + 2 g cefotaxime or 0.5 g gentamicin** + 2 g meropenem |
| Revision spacer surgery with spacer exchange due to infection persistence | 1 g gentamicin + 1 g clindamycin ** + 2 g vancomycin (for gram-positive organisms) or + 2 g cefotaxime (for gram-negative organisms) |
| unknown | 0.5 g gentamicin** + 2 g vancomycin |
*= these recommendations are made based solely on the personal experience of the author over the past 15 years. **= industrial impregnated.
Pharmacokinetic properties
The ideal spacer should possess high elution kinetics during the early postoperative period, with a further constant release of antibiotic amounts above the minimal inhibitory concentrations (MICs) of the causative organism(s) until the prosthesis reimplantation.
Masri et al. measured the intraarticular antibiotic concentrations in the first days after inserting vancomycin-tobramycin-loaded spacers 9. Peak concentrations on day 1 were 107 µg/ml for tobramycin and 19 µg/ml for vancomycin, determined from wound drainage fluids. These concentrations were 10-30 higher than the MICs of the infecting organisms. An increase of the tobramycin dose enhanced the elution of tobramycin and vancomycin, whereby an increase in the vancomycin concentration lacked such an effect 8. A sufficient elution of antibiotics from PROSTALAC (PROSThesis of Antibiotic-Loaded Acrylic Cement) could be measured over a period of at least 4 months. The duration of the spacer implantation did not have a statistically significant influence on the elution characteristics of both antibiotics.
Isiklar et al. reported of mean concentrations of 57 µg/mL for vancomycin on day 1 from vancomycin-impregnated spacers in the treatment of orthopaedic implant related S. epidermidis infections, also determined from the drainage fluid 10. Hsieh et al. reported on the elution of vancomycin and aztreonam from hip spacers 11. Vancomycin peak concentrations were initially 1.538 µg/ml and fell after 7 days to a mean value of 519 µg/ml. These high concentrations could be attributed either to the high amount of antibiotics incorporated into the cement (4 g vancomycin/4 g aztreonam/40 g bone cement), or aztreonam might have a different influence on the pharmacokinetics of vancomycin than aminoglycosides, so that another synergistic effect might result. Anagnostakos et al. determined the elution of gentamicin and vancomycin from beads and spacers in the drainage fluid using a two-stage protocol in the treatment of infected hip arthroplasties 12. Peak mean concentrations from PMMA beads and spacers were reached for gentamicin (115.70 µg/ml and 21.15 µg/ml, respectively) and vancomycin (80.40 µg/ml and 37.0 µg/ml, respectively) on day 1. The last determined concentrations for the beads group was 3.70 µg/ml for gentamicin and 23.00 µg/ml for vancomycin after 13 days, and 1.85 µg/ml for gentamicin and 6.60 µg/ml for vancomycin after 7 days in the spacer group.
At spacer explantation, Fink et al. determined the amounts of gentamicin, vancomycin, and clindamycin in local tissues 13. All of the tissue samples contained levels of antibiotics that were greater than the MICs previously determined for the pathogens that had caused the periprosthetic infections. Highest measured concentrations were 50.93 µg/g for gentamicin, 177.24 µg/g for vancomycin, and 322.29 µg/g for clindamycin. Similar values for gentamicin have been determined in the study of Mutimer et al. 14.
Some concerns have been expressed with regard to the ideal reimplantation time and whether too early or late spacer removal with prosthesis reimplantation might be associated with an infection persistence or recurrence. Bertazzoni Minelli et al. 15 and Kelm et al. 16 studied the residual antibiotic and antimicrobial properties of explanted spacers in vitro. 0.05-0.4% gentamicin and 0.8-3.3% vancomycin of the initial amount present were released in vitro over a time period of 10 days in the first study 15, indicating that sufficient antibiotic release can persist over several months. Kelm et al. reported similar elution values of gentamicin and vancomycin, and their spacers demonstrated sufficient anitmicrobial properties for at least 14 days in vitro 16.
Clinical success
There exist several factors that can contribute to the emergence of a reinfection or infection persistence. Inadequate debridement of all infected, necrotic, and devitalized tissue, wrong antibiotic choice for impregnation of bone cement, insufficient systemic antibiotic therapy, the virulence of the causative bacterium itself, biofilm production on the surfaces of implant materials, an early prosthesis reimplantation before definitive infection eradication and the general condition of the patient are only some of the most important factors to mention. Moreover, residual cement is significantly associated with a persistence of infection. McDonald et al. have evaluated 81 patients with hip joint infections and treated with a resection arthroplasty 17. The presence of retained cement appeared to be significantly associated with recurrent infection, as 3 of 7 patients who had retained cement had a reinfection, compared to 8 out of 75 from whom the cement had been completely removed. Buttaro et al. treated 10 patients with infection persistence due to residual cement and reported an infection eradication in 8 out of 9 cases after cement removal (one patient declined further surgical treatment) 18.
Although the exact infection eradication rate cannot be easily defined and large inhomogeneities are evident among the published studies, literature reviews on hip 2-3 and knee spacers 4-7 report treatment rates that frequently exceed 90%.
Mechanical complications between stages
With regard to spacer-related complications, several parameters might play a role: spacer production (hand-made vs. standardized), spacer geometry, muscular insufficiency, prior surgical revisions, poor bone and soft-tissue quality (especially the extensor mechanism in the knee), and non-compliance of the patient with regard to partial weight bearing; specifically for hip spacers, the head/neck ratio, acetabular and/or femoral defects, mismatch of spacer head size to the acetabulum size, and the art of femoral fixation.
A review of literature about hip spacers demonstrate that hand-made spacers might dislocate more often than standardized-made ones 2. However, a significant difference could not be assessed due to the heterogeneity of patients and insufficient documentation regarding spacer production and fixation.
Leunig et al. were one of the first who tried to interpret and explain hip spacer implantations 19. The authors recognize that the geometrical form of spacer plays an important role. In spacers that were free of complications, the neck to head-ratio was significantly lower (0.76±0.05) than in those with dislocations (0.96±0.19). A second factor associated with failure was an insufficient deep anchorage in the intramedullary canal, being 22±33 mm in the failure group, while complication-free spacers were on average attached to a depth of 57±41 mm.
Regarding femoral fixation of hip spacers, there exist three methods: i) press-fit, ii) partially or totally cementation, and iii) the “glove “- technique 20. The latter technique has been recently described and provides a stable fixation onto the proximal femur that facilitates spacer explantation since the spacer can be removed at one piece and there is no need for removal of any cement debris compared to other normal cementation techniques. However, it is unclear which of the above mentioned techniques is the most superior one in the prevention of spacer dislocation regarding the femur.
In the literature, dislocation rates after hip spacer implantation may strongly vary depending on the art of the spacer's production as well as the fixation method. Leunig et al. 19 reported dislocations of the hip in 5 of 12 patients after use of hand-formed spacers, whereas Magnan et al. 21 and Duncan et al. 22 noted a rate of 1/10 and 3/13 dislocations after implantation of a standardized hip spacer, respectively. On the other hand, Ries and Jergesen 23, Koo et al. 24, Shin et al. 25 and Takahira et al. 26 could not observe any dislocation during implantation of standardized spacers. In a large collection of 88 spacer implantations, Jung et al. reported a dislocation rate of 17% 27.
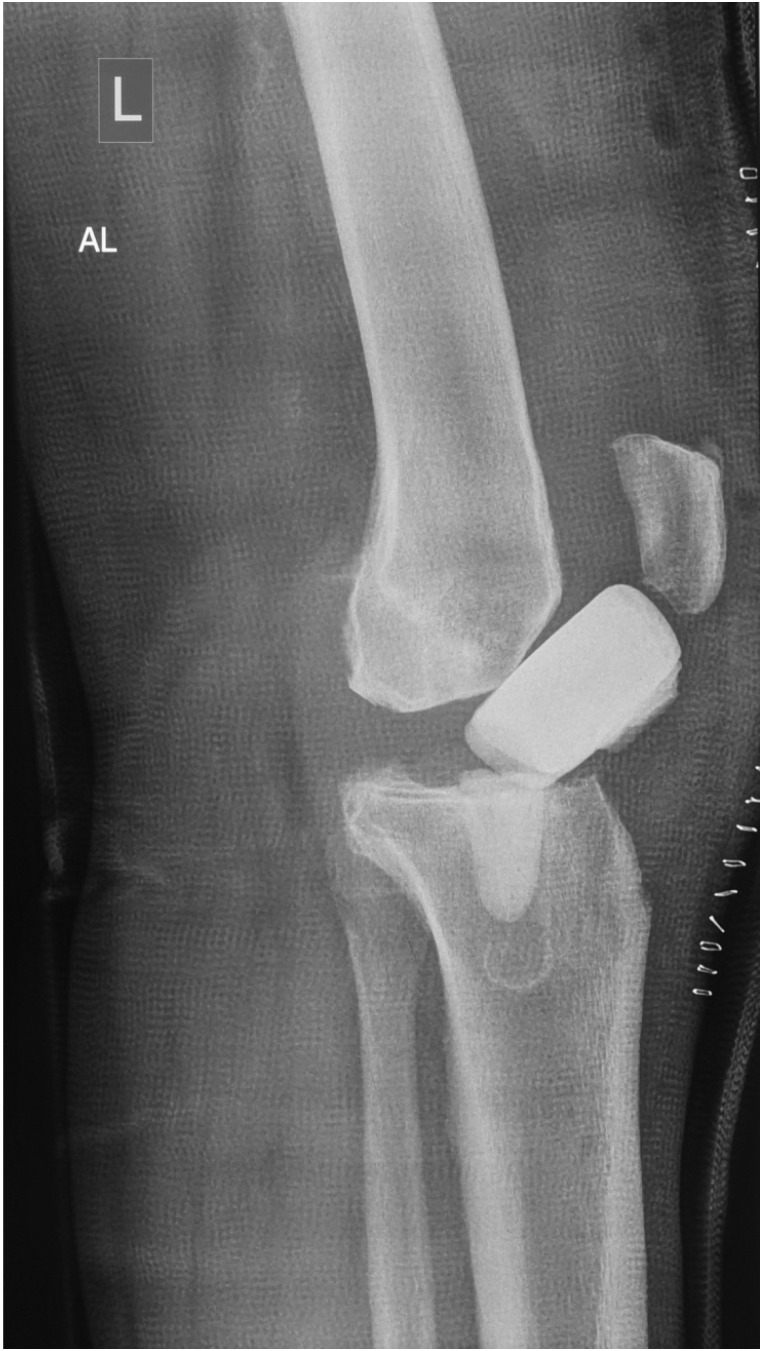
For knee spacers, Struelens et al. retrospectively analyzed spacer-specific problems in a collection of 155 articulating spacer implantations 28. The authors specified six different categories including optimal size and position of the spacer, spacer component tilting, medio-lateral shift of the tibial component in relation to the femoral component, component dislocation, fracture of the spacer (Figure 7), and knee subluxation. Only 67 spacers (43%) were considered optimally sized and positioned. In 24% of the cases, component tilting was found, and in another 21% of the cases, a medio-lateral translation was present relative to each other. A total of 12% showed major spacer complications such as fracture of the spacer, spacer dislocation or knee subluxation.
Figure 7.
Lateral radiographs of a left knee joint showing a fracture with subsequent dislocation of a static spacer one week after implantation.
Although the results of Struelens et al. 28 reported a high complication rate in knee spacers, other studies contradict that. Faschingbauer et al. described only one spacer fracture in a collective of 133 hand-made, static spacers, with a metallic endoskeleton reinforcement 29. Castelli et al. observed mechanical complications in 4% of cases with articulating spacers 30, whereas Kim et al. had no complications using a modified articulating spacer 31. Van Thiel et al. had a spacer fracture rate of 1.7% in a collective of 60 patients 32, and Johnson et al. reported a mechanical complication rate of approximately 12% in a group with articulating spacers versus 0% with static spacers 33.
Systemic safety
Antibiotic-loaded beads and spacers can locally release high antibiotic concentrations which greatly exceed those after systemic administration with no or low systemic toxicity. Salvati et al. investigated urine and serum samples after implantation of gentamicin-loaded cement and beads in 38 and 18 patients, respectively, and observed no toxic effects in these patients at very low gentamicin levels 34. Springer et al. also did not observe any toxic effects from knee spacers even after very high impregnation of antibiotics (3 g vancomycin + 3.6 g gentamicin / 40 g PMMA) in 34 patients, and concluded that drug delivery devices with a high antibiotic/cement-ratio should be regarded safe for clinical use 35.
Despite these reports, an increasing number of cases have been published regarding systemic side effects after the use of bone cement drug delivery systems in past. Van Raaij et al. reported the case of an 83-year-old woman with no history of kidney disease who developed acute renal failure (ARF) after resection of an infected total knee arthroplasty and placement of a gentamicin-impregnated cement spacer (2 g gentamicin / 40 g PMMA) and 7 chains of 30 gentamicin beads (0.945 g gentamicin) 36. Serum gentamicin levels indicated high concentrations that prompted removal of the spacer and subsequent return of normal renal function. Patrick et al. reported two similar cases of ARF in an 82-year-old female 5 months after implantation, and a 79-year-old male patient 6 weeks after implantation of a vancomycin-tobramycin-loaded hip spacer 37. In both cases, the serum tobramycin concentration was elevated, but after spacer explantation, serum creatinine and antibiotic concentrations returned within normal limits. Similar cases have also been described by Curtis et al. 38 and Dovas et al. 39 after use of tobramycin- and gentamicin-vancomycin-impregnated spacers at the site of infected total knee arthroplasties.
Koo et al. reported two cases of hepatic failure and 2 cases of bone marrow depression after hip spacer implantation (1 g gentamicin + 1 g vancomycin + 1 g cefotaxime / 40 g PMMA) out of 22 cases 24. The authors stated that the side effects were resolved after temporary withdrawal of systemic antibiotics, however, it is unknown which systemic antibiotics were used in each case. Isiklar et al. found one case of ARF out of 10 patients after implantation of a vancomycin-loaded hip spacer (2-3 g vancomycin / 40 g PMMA) and intravenous administration of the same antibiotic 10. Cabrita et al. observed 1 case of ARF and 3 cases of allergic reactions out of 33 cases of hip spacer implantation (1 g tobramycin + 1 g vancomycin / 40 g PMMA) 40. Unfortunately, no further details are available about the systemic antibiotics used in these particular cases nor the causes of ARF or allergic reactions.
Wentworth et al. reported 1 case of an allergic (dermatologic) reaction to vancomycin out of 135 cases of hip spacer implantation, whereas no patients had suffered from any renal or hepatic failure 41. Also using the PROSTALAC system, Scharfenberger et al. reported one case of neutropenia after intravenous administration of vancomycin after hip spacer implantation in 28 patients, while no cases of renal of hepatic insufficiency were observed 42.
Despite the abovementioned reports, several points remain unclear regarding to these systemic phenomena. In some cases, renal failure might be attributed to the local and systemic combination of the same or different antibiotic groups with nephrotoxic potential. Interestingly, it seems that the local combination of two potentially nephrotoxic antibiotic groups (aminoglycosides and glycopeptides) alone does not always induce any systemic side effects, but when combined with an intravenous antibiotic which also has a nephrotoxic potential, this may act as a trigger for ARF. Whether these patients have a genetic predisposition towards such an antibiotic treatment and the occurrence of such complications is unknown. Moreover, it is unclear if the age of the patient plays a role in the emergence of ARF. In most cases, elderly patients are more likely to suffer from systemic side effects. Furthermore, no specific explanation exists why in some cases aminoglycosides cause nephrotoxicity, and in other cases, the glycopeptide generates the nephrotoxic effect. The time of ARF manifestation may also vary strongly among reported cases without having any precise explanation for this discrepancy.
Until the exact etiology of renal or hepatic failure is defined, it would be advisable to avoid such combinations (highly antibiotic-loaded cement and systemic antibiotics of the same group) in high-risk (e.g. elderly) patients as long as this is in accordance with the antibiogram of the causative bacterium and does not endanger infection treatment. Careful and frequent monitoring of laboratory parameters is indicated in the detection of antibiotic-induced bone marrow depression and may lead to early adjustment of antibiotic therapy.
Spacer abrasion
The use of articulating spacers raised the question of whether abraded material from spacers could be detected in the synovial membrane at the second stage, which might lead to third-body wear in the new prosthesis. Fink et al. investigated this in 20 cases (16 hip, 4 knee) having been treated with articulating spacers 43. Zirconium dioxide, and traces of chromium and copper were detected in all samples. Cobalt was detectable only in the hip group. Despite the detection of these elements in the synovial membrane, the interpretation of these findings is difficult. Parts of the zirconium and metal particles detected could have originated from the original infected prostheses despite surgical debridement at the second stage. Since there exists no quantitative analysis for measurement of zirconium dioxide, the origin of these particles cannot be definitively stated. Moreover, the detection of chromium and copper might be explained by the fact that normal human tissues naturally contain these trace elements. An alternative option for avoidance of the emergence of abraded material might be the use of static spacers. However, there can still be abraded materials released from static spacers. Last but not least, a major concern is that the abraded material may reduce survival of the reimplanted prosthesis. However, this concern seems to be not substantiated. Hoberg et al. retrospectively investigated the outcome between two-stage revisions for infection and aseptic revisions of the hip joint 44. The survival rates were similar for both groups, with 85.6% at an average of 9.8 years for the aseptic and 82.7% after meanly 10.1 years for the septic group.
Conclusion
Antibiotic-loaded cement spacers are an established method for treating periprosthetic hip and knee joint infections. Literature demonstrates sufficient pharmacokinetic properties after implantation of the spacer and during the second stage. There exists a variety of possible mechanical and systemic complications. Knowledge about these complications might help orthopedic surgeons prevent and manage these phenomena.
References
- 1.Anagnostakos K, Kelm J. Enhancement of antibiotic elution from acrylic bone cement. J Biomed Mater Res B Appl Biomater. 2009;90:467–75. doi: 10.1002/jbm.b.31281. [DOI] [PubMed] [Google Scholar]
- 2.Anagnostakos K, Fürst O, Kelm J. Antibiotic-impregnated PMMA hip spacers: current status. Acta Orthop. 2006;77:628–37. doi: 10.1080/17453670610012719. [DOI] [PubMed] [Google Scholar]
- 3.Cui Q, Mihalko WM, Shields JS, Ries M, Saleh KJ. Antibiotic-impregnated cement spacers for the treatment of infection associated with total hip or knee arthroplasty. J Bone Joint Surg Am. 2007;89:871–82. doi: 10.2106/JBJS.E.01070. [DOI] [PubMed] [Google Scholar]
- 4.Guild GN 3rd, Wu B, Scuderi GR. Articulating vs. static antibiotic impregnated spacers in revision total knee arthroplasty for sepsis. A systematic review. J Arthroplasty. 2014;29:558–63. doi: 10.1016/j.arth.2013.08.013. [DOI] [PubMed] [Google Scholar]
- 5.Kuzyk PR, Dhotar HS, Sternheim A, Gross AE, Safir O, Backstein D. Two-stage revision arthroplasty for management of chronic periprosthetic hip and knee infection: techniques, controversies, and outcomes. J Am Acad Orthop Surg. 2014;22:153–64. doi: 10.5435/JAAOS-22-03-153. [DOI] [PubMed] [Google Scholar]
- 6.Pivec R, Naziri Q, Issa K, Banerjee S, Mont MA. Systematic review comparing static and articulating spacers used for revision of infected total knee arthroplasty. J Arthroplasty. 2014;29:553–7. doi: 10.1016/j.arth.2013.07.041. [DOI] [PubMed] [Google Scholar]
- 7.Voleti PB, Baldwin KD, Lee GC. Use of static or articulating spacers for infection following total knee arthroplasty. A systematic literature review. J Bone Joint Surg Am. 2013;95:1594–9. doi: 10.2106/JBJS.L.01461. [DOI] [PubMed] [Google Scholar]
- 8.Masri BA, Duncan CP, Beauchamp CP. Long-term elution of antibiotics from bone-cement. J Arthroplasty. 1998;13:331–8. doi: 10.1016/s0883-5403(98)90179-6. [DOI] [PubMed] [Google Scholar]
- 9.Masri BA, Duncan CP, Beauchamp CP, Paris NJ, Arntorp J. Tobramycin and Vancomycin elution from bone cement. An in vivo and in vitro study. Orthop Trans. 1994;18:130. [Google Scholar]
- 10.Isiklar ZU, Demirörs H, Akpinar S, Tandogan RN, Alparslan M. Two-stage treatment of chronic staphylococcal implant-related infections using Vancomycin- impregnated PMMA-Spacer and rifampicin containing antibiotic protocol. Bull Hosp Jt Dis. 1999;58:79–85. [PubMed] [Google Scholar]
- 11.Hsieh PH, Chang YH, Chen SH, Ueng SW, Shih CH. High concentration and bioactivity of vancomycin and aztreonam eluted from Simplex cement spacers in two-stage revision of infected hip implants: a study of 46 patients at an average follow-up of 107 days. J Orthop Res. 2006;24:1615–21. doi: 10.1002/jor.20214. [DOI] [PubMed] [Google Scholar]
- 12.Anagnostakos K, Wilmes P, Schmitt E, Kelm J. Elution of gentamicin and vancomycin from polymethylmethacrylate beads and hip spacers in vivo. Acta Orthop. 2009;80:193–7. doi: 10.3109/17453670902884700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fink B, Vogt S, Reinsch M, Büchner H. Sufficient release of antibiotic by a spacer 6 weeks after implantation in two-stage revision of infected hip prostheses. Clin Orthop Relat Res. 2011;469:3141–7. doi: 10.1007/s11999-011-1937-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mutimer J, Gillespie G, Lovering AM, Porteous AJ. Measurement of in vivo intra-articular gentamicin levels from antibiotic loaded articulating spacers in revision total knee replacement. Knee. 2009;16:39–41. doi: 10.1016/j.knee.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 15.Bertazzonnni Minelli E, Benini A, Magnan B, Bartolozzi P. Release of gentamicin and vancomycin from temporary human hip spacers in two-stage revision of infected arthroplasty. J Antimicrob Chemother. 2004;53:329–34. doi: 10.1093/jac/dkh032. [DOI] [PubMed] [Google Scholar]
- 16.Kelm J, Regitz T, Schmitt E, Jung W, Anagnostakos K. In vivo and in vitro studies of antibiotic release from and bacteria growth inhibition by antibiotic-impregnated polymethylmethacrylate hip spacers. Antimicrob Agents Chemother. 2006;50:332–5. doi: 10.1128/AAC.50.1.332-335.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McDonald DJ, Fitzgerald RH Jr, Ilstrup DM. Two-stage reconstruction of a total hip arthroplasty because of infection. J Bone Joint Surg Am. 1989;71:828–34. [PubMed] [Google Scholar]
- 18.Buttaro M, Valentini R, Piccaluga F. Persistent infection associated with residual cement after resection arthroplasty of the hip. Acta Orthop Scand. 2004;75:427–9. [PubMed] [Google Scholar]
- 19.Leunig M, Chosa E, Speck M, Ganz R. A cement spacer for two-stage revision of infected implants of the hip-joint. Int Orthop. 1998;22:209–14. doi: 10.1007/s002640050244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anagnostakos K, Köhler D, Schmitt E, Kelm J. The “glove”-technique: a modified method for femoral fixation of antibiotic-loaded hip spacers. Acta Orthop. 2009;80:386–8. doi: 10.3109/17453670903039551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Magnan B, Regis D, Biscaglia R, Bartolozzi P. Preformed acrylic bone cement spacer loaded with antibiotics. Use of two-stage procedure in 10 patients because of infected hips after total replacement. Acta Orthop Scand. 2001;72:591–4. doi: 10.1080/000164701317269003. [DOI] [PubMed] [Google Scholar]
- 22.Duncan CP, Beauchamp C. A temporary antibiotic-loaded joint replacement system for management of complex infections involving the hip. Orthop Clin North Am. 1993;24:751–9. [PubMed] [Google Scholar]
- 23.Ries MD, Jergesen H. An inexpensive molding method for antibiotic- impregnated cement spacers in infected total hip arthroplasty. J Arthroplasty. 1999;14:764–5. doi: 10.1016/s0883-5403(99)90234-6. [DOI] [PubMed] [Google Scholar]
- 24.Koo KH, Yang JW, Cho SH, Song HR, Park HB, Ha YC, Chang JD, Kim SY, Kim YH. Impregnation of vancomycin, gentamicin and cefotaxime in a cement spacer for two-stage cementless reconstruction in infected total hip arthroplasty. J Arthroplasty. 2001;16:882–92. doi: 10.1054/arth.2001.24444. [DOI] [PubMed] [Google Scholar]
- 25.Shin SS, Della Valle CJ, Ong BC, Meere PA. A simple method for construction of an articulating antibiotic-loaded cement spacer. J Arthroplasty. 2002;17:785–7. doi: 10.1054/arth.2002.33568. [DOI] [PubMed] [Google Scholar]
- 26.Takahira N, Itoman M, Higashi K, Utsiyama K, Miyabe M, Naruse K. Treatment outcome of two-stage revision total hip arthroplasty for infected hip arthroplasty using antibiotic-impregnated cement spacer. J Orthopedic Sci. 2003;8:26–31. doi: 10.1007/s007760300004. [DOI] [PubMed] [Google Scholar]
- 27.Jung J, Schmid NV, Kelm J, Schmitt E, Anagnostakos K. Complications after spacer implantation in the treatment of hip joint infections. Int J Med Sci. 2009;6:265–73. doi: 10.7150/ijms.6.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Struelens B, Claes S, Bellemans J. Spacer-related problems in two-stage revision knee arthroplasty. Acta Orthop Belg. 2013;79:422–6. [PubMed] [Google Scholar]
- 29.Faschingbauer M, Bieger R, Reichel H, Weiner C, Kappe T. Complications associated with 133 statuic, antibiotic-laden spacer after TKA. Knee Surg Sports Traumatol Arthrosc. 2015 doi: 10.1007/s00167-015-3646-0. [epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 30.Castelli CC, Gotti V, Ferrari R. Two-stage treatment of infected total knee arthroplasty: two to thirteen year experience using an articulating performed spacer. Int Orthop. 2014;38:405–12. doi: 10.1007/s00264-013-2241-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim YS, Bae KC, Cho CH, Lee KJ, Sohn ES, Kim BS. Two-stage revision using a modified articulating spacer in infected total knee arthroplasty. Knee Surg Relat Res. 2013;25:180–5. doi: 10.5792/ksrr.2013.25.4.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van Thiel GS, Berend KR, Klein GR, Gordon AC, Lombardi AV, Della Valle CJ. Intraoperative molds to create an articulating spacer for the infected knee arthroplasty. Clin Orthop Relat Res. 2011;469:994–1001. doi: 10.1007/s11999-010-1644-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johnson AJ, Sayeed SA, Naziri Q, Khanuja HS, Mont MA. Minimizing dynamic knee spacer complications in infected revision arthroplasty. Clin Orthop Relat Res. 2012;470:220–7. doi: 10.1007/s11999-011-2095-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Salvati EA, Callaghan JJ, Brause BD, Klein RF, Small RD. Reimplantation in infection: elution of gentamicin from cement and beads. Clin Orthop. 1986;207:83–93. [PubMed] [Google Scholar]
- 35.Springer BD, Lee GC, Osmon D, Haidukewych GJ, Hanssen AD, Jacofsky DJ. Systemic safety of high-dose antibiotic-loaded cement spacers after resection of an infected total knee arthroplasty. Clin Orthop Relat Res. 2004;427:47–51. doi: 10.1097/01.blo.0000144476.43661.10. [DOI] [PubMed] [Google Scholar]
- 36.Van Raaij TM, Visser LE, Vulto AG, Verhaar JA. Acute local failure after local gentamicin treatment of an infected total knee arthroplasty. J Arthroplasty. 2002;17:948–50. doi: 10.1054/arth.2002.34525. [DOI] [PubMed] [Google Scholar]
- 37.Patrick BN, Rivey MP, Allington DR. Acute renal failure associated with vancomycin- and tobramycin-laden cement in total hip arthroplasty. Ann Pharmacother. 2006;40:2037–42. doi: 10.1345/aph.1H173. [DOI] [PubMed] [Google Scholar]
- 38.Curtis JM, Sternhagen V, Batts D. Acute renal failure after placement of tobramycin-impregnated bone cement in an infected total knee arthroplasty. Pharmacotherapy. 2005;25:876–80. doi: 10.1592/phco.2005.25.6.876. [DOI] [PubMed] [Google Scholar]
- 39.Dovas S, Liakopoulos V, Papatheodorou L, Chronopoulou I, Papavasiliou V, Atmatzidis E, Giannopoulou M, Eleftheriadis T, Simopoulou T, Karachalios T, Stefanidis I. Acute renal failure after antibiotic-impregnated bone cement treatment of an infected total knee arthroplasty. Clin Nephrol. 2008;69:207–12. doi: 10.5414/cnp69207. [DOI] [PubMed] [Google Scholar]
- 40.Cabrita HB, Croci AT, Camargo OP, Lima AL. Prospective study of the treatment of infected hip arthroplasties with or without the use of an antibiotic-loaded cement spacer. Clinics. 2007;62:99–108. doi: 10.1590/s1807-59322007000200002. [DOI] [PubMed] [Google Scholar]
- 41.Wentworth SJ, Masri BA, Duncan CP, Southworth CB. Hip prosthesis of antibiotic-loaded acrylic cement for the treatment of infections following total hip arthroplasty. J Bone Joint Surg (Am) 2003;84:123–8. doi: 10.2106/00004623-200200002-00017. [DOI] [PubMed] [Google Scholar]
- 42.Scharfenberger A, Clark M, Lavoie G, O'Connor G, Masson E, Beaupre LA. Treatment of an infected total hip replacement with the PROSTALAC system. Part 1: infection resolution. Can J Surg. 2007;50:24–8. [PMC free article] [PubMed] [Google Scholar]
- 43.Fink B, Rechtenbach A, Büchner H, Vogt S, Hahn M. Articulating spacers used in two-stage revision of infected hip and knee prostheses abrade with time. Clin Orthop Relat Res. 2011;469:1095–1102. doi: 10.1007/s11999-010-1479-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hoberg M, Konrads C, Engelien J, Oschmann D, Holder M, Walcher M, Steinert A, Rudert M. Similar outcomes between two-stage revisions for infection and aseptic hip revisions. Int Orthop. 2016;40:459–64. doi: 10.1007/s00264-015-2850-3. [DOI] [PubMed] [Google Scholar]