Abstract
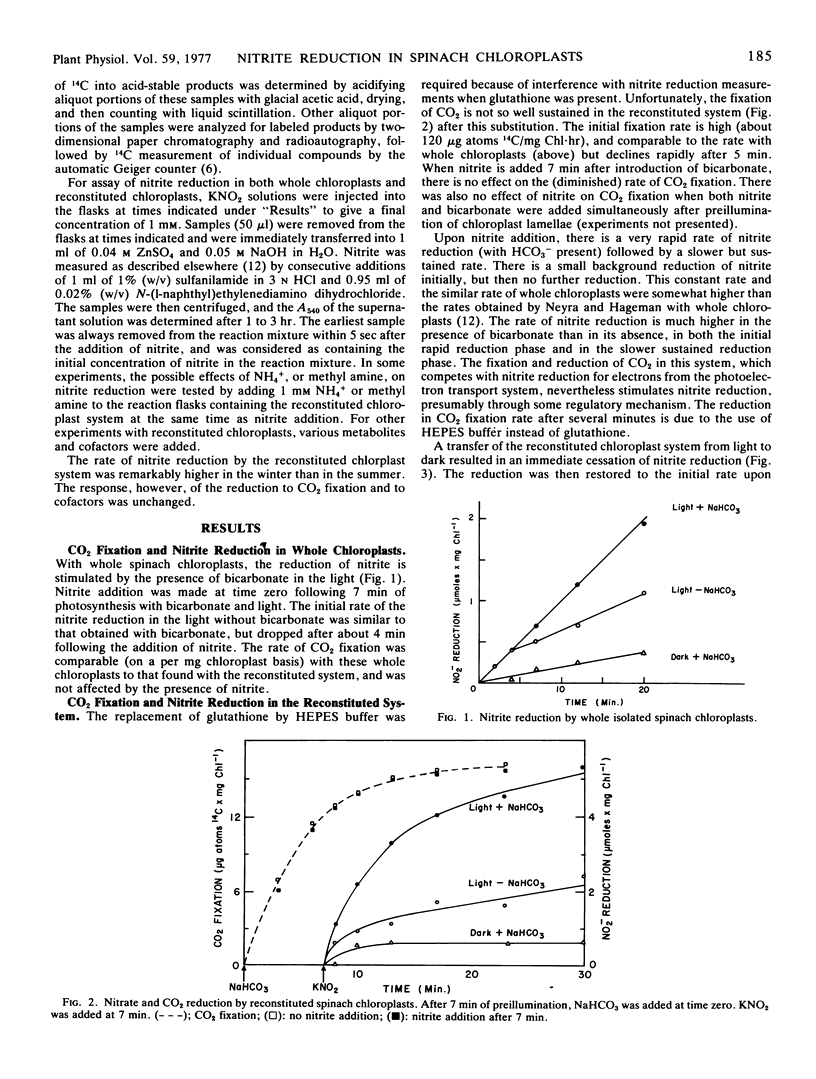
Nitrite reduction in either whole, isolated spinach chloroplasts (Spinacia oleracea L.) or in reconstituted spinach chloroplasts is stimulated by a short period of photosynthetic CO2 fixation in the light prior to nitrite addition. With reconstituted chloroplasts, a similar stimulation can be obtained in nitrite reduction without CO2 fixation by the addition of dihydroxyacetone phosphate or fructose 6-phosphate. Specific intermediate metabolites of the photosynthetic carbon reduction cycle may have a regulatory role in nitrite reduction in chloroplasts in the light.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dalling M. J., Tolbert N. E., Hageman R. H. Intracellular location of nitrate reductase and nitrite reductase. I. Spinach and tobacco leaves. Biochim Biophys Acta. 1972 Dec 14;283(3):505–512. doi: 10.1016/0005-2728(72)90266-6. [DOI] [PubMed] [Google Scholar]
- Jensen R. G., Bassham J. A. Photosynthesis by isolated chloroplasts. Proc Natl Acad Sci U S A. 1966 Oct;56(4):1095–1101. doi: 10.1073/pnas.56.4.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanazawa T., Kirk M. R., Bassham J. A. Regulatory effects of ammonia on carbon metabolism in photosynthesizing Chlorella pyrenoidosa. Biochim Biophys Acta. 1970 Jun 30;205(3):401–408. doi: 10.1016/0005-2728(70)90106-4. [DOI] [PubMed] [Google Scholar]
- Losada M., Ramírez J. M., Paneque A., Del Campo F. F. Light and dark reduction of nitrate in a reconstituted chloroplast system. Biochim Biophys Acta. 1965 Sep 27;109(1):86–96. doi: 10.1016/0926-6585(65)90093-2. [DOI] [PubMed] [Google Scholar]
- MEDINA A., NICHOLAS D. J. Interference by reduced pyridine nucleotides in the diazotization of nitrite. Biochim Biophys Acta. 1957 Feb;23(2):440–442. doi: 10.1016/0006-3002(57)90355-4. [DOI] [PubMed] [Google Scholar]
- Magalhaes A. C., Neyra C. A., Hageman R. H. Nitrite assimilation and amino nitrogen synthesis in isolated spinach chloroplasts. Plant Physiol. 1974 Mar;53(3):411–415. doi: 10.1104/pp.53.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell C. A., Stocking C. R. Kinetics and Energetics of Light-driven Chloroplast Glutamine Synthesis. Plant Physiol. 1975 Jan;55(1):59–63. doi: 10.1104/pp.55.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neyra C. A., Hageman R. H. Dependence of nitrite reduction on electron transport chloroplasts. Plant Physiol. 1974 Oct;54(4):480–483. doi: 10.1104/pp.54.4.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritenour G. L., Joy K. W., Bunning J., Hageman R. H. Intracellular localization of nitrate reductase, nitrite reductase, and glutamic Acid dehydrogenase in green leaf tissue. Plant Physiol. 1967 Feb;42(2):233–237. doi: 10.1104/pp.42.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson G. W., Cocking E. C. Enzymic Assimilation of Nitrate in Tomato Plants. I. Reduction of Nitrate to Nitrite. Plant Physiol. 1964 May;39(3):416–422. doi: 10.1104/pp.39.3.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholl R. L., Harper J. E., Hageman R. H. Improvements of the nitrite color development in assays of nitrate reductase by phenazine methosulfate and zinc acetate. Plant Physiol. 1974 Jun;53(6):825–828. doi: 10.1104/pp.53.6.825. [DOI] [PMC free article] [PubMed] [Google Scholar]


