Abstract
Brain abscess (BA) is a severe neurological emergency, which remains a challenge for physicians despite medical advancements. The purpose of this study is to describe the epidemiology of BA in Taiwan and to investigate potential factors affecting the survival of patients with BA. By using the Taiwan National Health Insurance Research Database, we identified hospitalized patients with a discharge diagnosis of pyogenic BA (324.X) between 2000 and 2013. The incidence and in-hospital mortality of BA were calculated based on both age and sex. A total of 6027 BA cases were identified. The overall incidence of BA was 1.88 (95% CI: 1.83–1.93) per 100,000 person-years and increased with age, from 0.58 per 100,000 person-years in individuals aged 0–14 years to 4.67 per 100,000 person-years in those over 60 years of age. The male-to-female incidence ratio was 2.37 (95% CI: 2.24–2.50), with a mountain-shaped distribution across ages peaking at 40–44 years. The in-hospital mortality also increased with age, from 4.22% (95% CI: 2.54–6.97) at 0–14 years to 17.34% (95% CI: 15.79–19.02) in individuals over 60 years of age, without a gender difference (11.9% for males, 12.5% for females). Age, stroke, septicemia, pneumonia, meningitis, and hepatitis were associated with increased risk of in-hospital mortality. There was a male predominance for BA, and both the incidence and in-hospital mortality rates increased with age. Infection-related disease such as septicemia, pneumonia and meningitis were important factors associated with in-hospital mortality. In addition to the original treatment of BA, we suggest paying close attention to potential infections to improve the outcome of BA patients.
Introduction
Brain abscess (BA) is a rare but life-threatening disease. The main strategies for BA treatment include surgical intervention and antibiotic therapy. Although previous studies examined various characteristics of BA, such as age, gender, location, symptoms, pathogens, and outcomes [1–6], few studies have assessed the incidence of BA. In addition, these studies included only a small number of cases [7, 8]. One recent study, which was conducted in Olmsted County, Minnesota, United States, reported the incidence of BA as 2.7 per 100,000 from 1935–1944 and 0.9 per 100,000 from 1965–1981[7].
Recently, newly diagnostic procedures, such as brain imaging techniques (i.e., magnetic resonance imaging [MRI] and computed tomography [CT]) and stereotactic biopsy, as well as administration of new antibiotics have considerably changed the management and outcome of patients with BA [2, 4, 8, 9]. The mortality of BA has declined from 40% in 1960 to 10%–20% during the past decade [1–4, 9, 10]. Unfortunately, many survivors continue to suffer from neurological deficits [1]. Moreover, most studies on BA outcomes focused on advances in new diagnostic procedures and neurosurgery, causative organisms, location, and clinical symptoms [7, 9–13]. No study investigated the impact of comorbidities on BA outcomes.
We are interested in whether improvements in the diagnosis and management of BA have changed the mortality rates of BA. This study aims to describe the trend in BA incidence over a 14-year period and to investigate the potential impact of predisposing factors and comorbidities on BA mortality.
Materials and methods
Data source and ethical approval
This investigation was a population-based cohort study using data obtained from the Taiwan National Health Insurance Research Database (NHIRD). The NHIRD is a claim dataset that has been used extensively for many studies [10–12]. Established in 1995, the National Health Insurance system in Taiwan is compulsory for all Taiwanese residents except for criminals and military personnel and covers over 99% of the total population of approximately 23 million people. The NHIRD contains registration files and original claims data for reimbursement, including records of demographic data; dates of clinical visits; diagnostic codes; details of prescriptions, examinations, and procedures; and medical expenditures. In each admission record, up to five discharge diagnoses are coded according to the International Classification of Disease, Ninth Revision, Clinical Modification (ICD-9-CM). This study was approved by the Institutional Review Board of the Ditmanson Medical Foundation Chiayi Christian Hospital, Taiwan (CYCH-IRB: 105003).
Study subjects and definitions
A BA was defined as a collection of purulent material within the cranial cavity that was confirmed by imaging (CT or MRI), biopsy, or surgery [9]. All patients who were hospitalized due to BA from January 1, 2000, to December 31, 2013, were included in the study. An ICD-9-CM code of 324.X, denoted as one of the first three diagnoses, was used to identify patients with BA. In patients with neurosurgical intervention, all materials from BA were cultured for aerobic and anaerobic bacteria, mycobacteria and fungi. In the patient without neurosurgical intervention, blood culture was performed for aerobic and anaerobic bacteria, mycobacteria and fungi. The age and sex of patients, calendar year of hospitalization, and in-hospital mortality related to BA were studied. The age at first diagnosis was categorized into five groups: 0–14, 15–29, 30–44, 45–59, and over 60 years of age. Comorbidities and predisposing factors were ascertained from the ICD-9-CM codes of the first five discharge diagnoses and the most common predisposing factors and comorbidities were included in further analyses.
Statistical analysis
A Chi-squared test was performed to compare the frequency of different variables between survival and deceased cases, whereas a Student’s t test was used to compare average values between the groups. A Wilcoxon rank-sum test was used to compare the median days of hospitalization. Multivariate logistic regression analysis was performed to estimate the odds ratio (OR) and 95% confidence intervals (CI) of in-hospital fatality of patients with BA. A p-value of less than .05 was considered to be statistically significant. Data management and analyses were performed using SAS/STAT® software, version 9.3 for Windows (SAS Institute Inc., Cary, NC, USA).
Results
Incidence and sex ratio
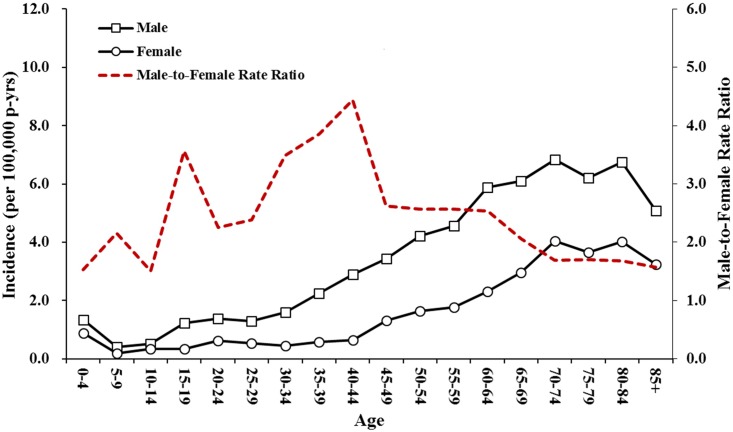
During the period between 2000 and 2013, there were 6027 patients hospitalized with BA (4265 males, 1762 females) in Taiwan. The overall incidence rate was 1.88 (95% CI: 1.83–1.93) per 100,000 person-years (Table 1). The incidence was lowest in the 0–14 year age group and increased with age (Table 1 and Fig 1). There was a male predominance (male-to-female rate ratio = 2.37; Table 1). The male-to-female incidence ratio changed with age and showed a mountain-shaped distribution with a peak at 40–44 years of age (Fig 1).
Table 1. Incidence rate (per 100,000 person-years) of hospitalized brain abscess in Taiwan, 2000–2013.
| Parameter | No. of case | % | Incidence Rate (95% CI) | Relative risk (95% CI) |
|---|---|---|---|---|
| All patients | 6027 | 100.00 | 1.88 (1.83–1.93) | - |
| Sex | ||||
| Female | 1762 | 29.24 | 1.11 (1.10–1.16) | Reference |
| Male | 4265 | 70.76 | 2.63 (2.55–2.71) | 2.37 (2.24–2.50) |
| Age, y | ||||
| 0–14 | 331 | 5.49 | 0.58 (0.52–0.65) | Reference |
| 15–29 | 677 | 11.23 | 0.91 (0.85–0.98) | 1.57 (1.38–1.79) |
| 30–44 | 1115 | 18.80 | 1.41 (1.33–1.49) | 2.42 (2.14–2.73) |
| 45–59 | 1800 | 29.87 | 2.77 (2.64–2.90) | 4.75 (4.23–5.34) |
| ≥60 | 2104 | 34.91 | 4.67 (4.47–4.87) | 8.01 (7.13–8.99) |
| Year | ||||
| 2000–2002 | 1158 | 19.21 | 1.72 (1.63–1.83) | Reference |
| 2003–2005 | 1346 | 22.33 | 1.98 (1.87–2.09) | 1.15 (1.06–1.24) |
| 2006–2008 | 1349 | 22.38 | 1.96 (1.86–2.07) | 1.14 (1.05–1.23) |
| 2009–2011 | 1349 | 22.38 | 1.94 (1.84–2.05) | 1.13 (1.04–1.22) |
| 2012–2013 | 825 | 16.69 | 1.77 (1.65–1.89) | 1.03(0.94–1.12) |
Fig 1. The incidence and male-to-female rate ratio of brain abscess by age in Taiwan from 2000 to 2013.
Age distribution of in-hospital mortality
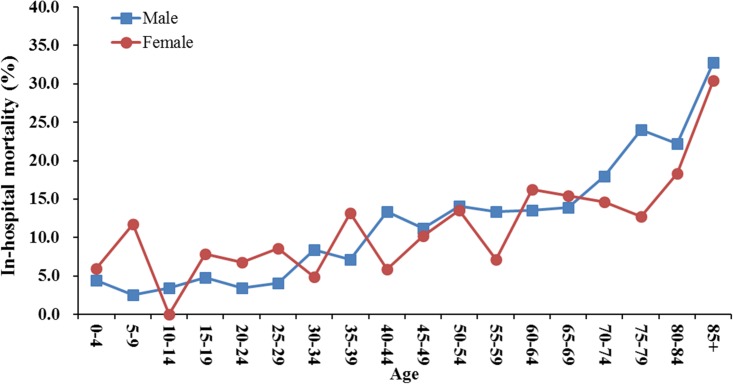
Among all cases, there were 741 deaths (531 males, 210 females), with an overall in-hospital mortality rate of 12.29% (95% CI: 11.49–13.15). The mortality rate also increased with age, from 4.22% (95% CI: 2.54–6.97) in patients 0–14 years of age to 17.34% (95% CI: 15.79–19.02) in patients older than 60 years of age (Fig 2). The mortality rate was similar between the two sexes (11.92% for males and 12.45% for females; Table 2). In addition, it revealed a declining trend from 13.0% to 10.2% over the study period, though without statistical significance (Table 2). The overall length-of-stay (LOS) in the hospital was 45.7 ± 45.5 days, with longer stays in the survival group than in the mortality group (47.3 ± 46.2 vs. 34.5 ± 38.5 days, respectively, P < .001; Table 2).
Fig 2. In-hospital mortality of brain abscess by age in Taiwan from 2000 to 2013.
Table 2. Demographic characteristics comparison between survival and death cases.
| Variables -No.(%) | Total | Survival | In-hospital death | P value |
|---|---|---|---|---|
| All patients | 6027 | 5286 (87.7%) | 741 (12.3%) | |
| Sex | ||||
| Male | 4265 | 3734 (87.5%) | 531 (12.5%) | 0.8360a |
| Female | 1762 | 1552 (88.1%) | 210 (11.9%) | |
| Age, years | ||||
| mean±SD | 50.6±20.6 | 49.5±20.3 | 58.5±18.1 | <0.0001b |
| 0–14 | 331 | 317 (95.7%) | 14 (4.3%) | <0.0001a |
| 15–29 | 677 | 643 (95.0%) | 34 (5.0%) | |
| 30–44 | 1115 | 1007 (90.3%) | 108 (9.7%) | |
| 45–59 | 1800 | 1580 (87.8%) | 220 (12.2%) | |
| ≥60 | 2104 | 1739 (80.5%) | 365 (19.5%) | |
| Year | ||||
| 2000–2002 | 1158 | 1007 (87.0%) | 151 (13.0%) | 0.2681a |
| 2003–2005 | 1346 | 1168 (86.8%) | 178 (13.2%) | |
| 2006–2008 | 1349 | 1183 (87.7%) | 166 (12.3%) | |
| 2009–2011 | 1349 | 1187 (88.0%) | 162 (12.0%) | |
| 2012–2013 | 825 | 741 (89.8%) | 84(10.2%) | |
| LOS, days | ||||
| mean±SD | 45.7±45.5 | 47.3±46.2 | 34.5±38.5 | <0.0001b |
| median (Q1-Q3) | 37 (18–59) | 39 (20–60) | 23 (10–46) | <0.0001c |
| Predisposing factors | ||||
| Septicemia | 593 | 349(58.9%) | 244(41.1%) | <0.0001a |
| Meningitis | 474 | 386(81.4%) | 88(18.6%) | <0.0001a |
| Pneumonia | 415 | 305(73.5%) | 112(26.5%) | <0.0001a |
| Systemic infection | 413 | 387(93.7%) | 26(6.3%) | 0.0001a |
| Comorbidity | ||||
| Pulmonary disease | 824 | 537 (65.2%) | 287 (34.8%) | <0.0001a |
| Diabetes mellitus | 866 | 735 (84.9%) | 131 (15.1%) | 0.0061a |
| Hypertension | 788 | 732(92.9%) | 56(7.1%) | <0.0001a |
| Hepatitis | 420 | 330(78.6%) | 90(21.4%) | <0.0001a |
| Other factors | ||||
| Head injury | 721 | 688 (95.4%) | 33 (4.6%) | <0.0001a |
| Stroke | 623 | 494 (79.3%) | 129 (20.7%) | <0.0001a |
| Brain tumor | 255 | 225(88.2%) | 30(11.8%) | 0.4347a |
SD: standard deviation; IQR: inter-quartile range.
aChi-Square.
bStudent’s t test.
cWilcoxon rank-sum test
Predisposing factors and comorbidities
Among all 6027 cases, the most common predisposing factors were septicemia in 593, meningitis in 474, pneumonia in 415 and systemic infection in 387 patients. The most common comorbidities were pulmonary disease in 824, diabetes mellitus in 866, hypertension in 788 and hepatitis in 420 patients (Table 2). Further analysis by multivariate logistic regression revealed that septicemia, pneumonia and meningitis all increase the risk of in-hospital mortality. The comorbidities, pulmonary disease and hepatitis, were associated with an increased risk of in-hospital mortality, whereas hypertension and head injury were associated with a decreased risk of in-hospital mortality (Table 3). Traumatic brain injury related BA was 12.5% (531/4265) in men and 10.8% (190/1762) in women, were not statistically different (p = .07). Further analysis of the data stratified by sex showed septicemia, pneumonia, and pulmonary disease increase the risk of in-hospital mortality in both men and women. In contrast meningitis and stroke increase the risk of in-hospital mortality in men but not in women, whereas both head injury and hypertension decrease the risk of in-hospital mortality in men but not in women.
Table 3. Multivariate logistic regression analysis of in-hospital fatality of brain abscess cases.
| Parameter | Case fatality (95% CI), % | Univariate | Multivariate | ||
|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | ||
| All patients | 12.29 (11.49–13.15) | - | - | - | - |
| Sex | |||||
| Female | 12.45 (11.49–13.48) | Reference | - | Reference | |
| Male | 11.92 (10.49–13.52) | 1.05 | 0.89–1.25 | 1.10 | 0.91–1.34 |
| Age, y | |||||
| 0–14 | 4.22 (2.54–6.97) | Reference | - | Reference | |
| 15–29 | 5.02 (3.62–6.94) | 1.20 | 0.63–2.26 | 1.98 | 1.01–3.89 |
| 30–44 | 9.69 (8.09–11.56) | 2.43 | 1.37–4.30 | 3.11 | 1.69–5.74 |
| 45–59 | 12.22 (10.79–13.82) | 3.15 | 1.81–5.48 | 3.84 | 2.12–659 |
| ≥60 | 17.34 (15.79–19.02) | 4.75 | 2.75–8.21 | 5.68 | 3.15–10.25 |
| Year | |||||
| 2000–2002 | 13.04 (11.22–15.10) | Reference | - | Reference | |
| 2003–2005 | 13.22 (11.52–15.14) | 1.02 | 0.81–1.28 | 0.95 | 0.73–1.22 |
| 2006–2008 | 12.31 (10.66–14.17) | 0.94 | 0.74–1.19 | 0.81 | 0.63–1.06 |
| 2009–2011 | 12.00 (10.38–13.85) | 0.91 | 0.72–1.15 | 0.78 | 0.60–1.02 |
| 2012–2013 | 10.18(8.30–12.43) | 0.76 | 0.57–1.00 | 0.65 | 0.47–0.88 |
| Predisposing factors | |||||
| Septicemia | 41.14 (37.26–45.15) | 6.95 | 5.76–8.38 | 5.61 | 4.56–6.90 |
| Meningitis | 18.57 (15.32–22.31) | 1.71 | 1.34–2.19 | 1.56 | 1.18–2.06 |
| Pneumonia | 26.51 (22.49–30.95) | 2.85 | 2.26–3.60 | 1.73 | 1.33–2.26 |
| Systemic infection | 6.30 (4.33–9.06) | 0.46 | 0.31–0.69 | 0.70 | 0.46–1.07 |
| Comorbidity | |||||
| Pulmonary disease | 34.83 (31.65–38.15) | 5.59 | 4.70–6.64 | 3.78 | 3.12–4.58 |
| Hypertension | 7.11 (5.51–9.12) | 0.51 | 0.38–0.68 | 0.60 | 0.44–0.81 |
| Diabetes mellitus | 15.13 (12.90–17.67) | 1.33 | 1.08–1.63 | 1.22 | 0.97–1.53 |
| Hepatitis | 21.43 (17.77–25.61) | 2.08 | 1.62–2.66 | 2.11 | 1.60–2.79 |
| Other factors | |||||
| Stroke | 20.71 (17.71–24.06) | 2.05 | 1.66–2.53 | 1.66 | 1.31–2.10 |
| Head injury | 4.58 (3.28–6.36) | 0.31 | 0.22–0.45 | 0.50 | 0.34–0.72 |
| Brain tumor | 11.76 (8.37–16.30) | 0.95 | 0.64–1.40 | 1.27 | 0.84–1.94 |
Multivariable logistic regression analysis
Discussion
This nationwide, population-based study on BA revealed a steady annual incidence (1.72–1.98 per 100,000 person-years) and a slight decline in mortality rates from 13.0% to 10.2% throughout the study period. The incidence of BA had a male predominance (male-to-female rate ratio of 2.37) and increased with age. The in-hospital fatality also increased with age but without a gender difference. Lastly, the multivariate regression analysis found that concomitant infection-related predisposing factors, such as septicemia, meningitis, and pneumonia, might increase the risk of in-hospital mortality in patients with BA.
The incidence rate in this study was higher than that reported previously [7, 8]. The incidence of BA was 3–5 per million population-years in a study conducted in Northern Ireland [8], which identified BA cases based on pathology and excluded patients with abscesses smaller than 15 mm in diameter. A more recent investigation conducted in Olmsted County in Minnesota showed a decline in the incidence of BA from 2.7 per 100,000 persons during 1935–1944 to 0.9 per 100,000 persons during 1965–1981 [7]. However, these studies were performed over 20 years ago in Western countries [7, 8]. The difference in the incidence between previous studies and the present study could be due to different inclusion criteria, ethics, and advances in diagnostic technology introduced during recent decades [13–16].
The male predominance in BA noted in this study was consistent with previous studies [1–4, 7, 8]. Moreover, the dynamic change in the male-to-female rate ratio of BA cases, which exhibited a mountain-shaped distribution and a peak at 40–45 years of age, was similar to our previous study on the incidence of status epilepticus, which also showed a mountain-shaped distribution of the male-to-female rate ratio [12]. It is noteworthy that this age period corresponds to the female premenopausal period since the protective effects of estrogen in neurons were proposed previously [17]. However, the role of reproductive hormones in the development of BA needs further investigation.
There are several predisposing factors of BA such as being immunocompromised, having existing medical conditions, and distant infection [1, 9, 18]. Sharma et al. reported that an adjacent focus of infection (i.e., sinusitis, otogenic, odontogenic, and post-meningitis) was found in 42.5% of patients with BA, followed by neurosurgical procedures and distant infections [4]. Nathoo et al. reported that otorhinogenic infections (38.5%) and trauma (32.8%) were the two most common causes of BA in South Africa [16]. Otorhinogenic infection, which appears to be the most important etiology of BA, is often observed in the first decade of life [2, 4, 16, 19]. However, its contribution to the incidence of BA was only 3.1% (185/6,027) in the present study, which might explain the low number of BA cases found in the young age group in Taiwan (Fig 1). The age-specific incidence of BA in this study was similar to a report by Tsou et al. [3], but was different from previous studies conducted in Olmsted County and South Africa [7, 16]. In the Olmsted County study, the highest incidence of BA was in the 5–9 year age group [7]. Similarly, in the study from South Africa, approximately 70% of patients were in the first three decades of life, and 43% were younger than 18 years of age [16]. Taken together, we propose that the low number of BA cases in the young age group may be associated with fewer severe otorhinogenic infections in Taiwan. Because most patients can access health care conveniently that would reduce the spread of pathogens from otorhinogenic infections to the brain.
The in-hospital mortality rate of BA in our analysis (12.3%) was similar to the recently reported rates of 9%–15% [2, 14, 16]. However, it was much lower than the 38–53% of earlier reports [6–8, 20]. Most previous studies were based on a single or small number of institutes, unlike the present study that use a national representative sample. One rational finding was the fatality rate increased with age. However, despite the higher incidence of BA in males, the case-fatality rate was similar in both genders. This finding is in agreement with previous studies that showed no gender difference in BA mortality [7, 16, 21]. In addition, we found a significantly longer LOS in the hospital for those who survived compared with those who died. This result might be because survivors usually suffer from neurological sequelae that need subsequent treatment in the hospital, whereas fatal cases likely followed a faster clinical course occurring within a certain time period.
Preexisting comorbidities and predisposing factors can greatly affect the survival of patients with BA [7,19, 21,]. Our results, consistent with previous studies, found that infection-related factors, such as meningitis [18], pneumonia and septicemia [21] were associated with increased mortality. Consistent with the finding of previous studies that pulmonary disease and hepatitis can increase the risk of in-hospital mortality in trauma patients [22, 23]; our study found pulmonary disease and hepatitis increase the risk of in-hospital mortality in BA patients. In contrast, non-infection-related comorbidities did not affect BA mortality. For example, head injury patients had an even lower fatality rate [16], as head injuries occur more frequently in younger patients, who are usually healthier prior to the BA episode, and are mostly due to traffic accidents, [24]. Hypertension may change the upper and lower blood pressure limit of brain autoregulation. A previous study showed that during hypoxia, the cerebral blood flow is higher in hypertensive patients than in normotensive patients [25]. Lower fatality rate in BA patients with hypertension suspected related to hypertensive patients have more cerebral blood flow than normotensive patients. However, this needs further investigation.
There are several limitations of this study. First, case determination was based on the claims dataset, which might raise the question of diagnostic accuracy. Taiwan has a comprehensive healthcare coverage with the national health insurance system. All patients with suspected BA are usually referred to hospitals with available neurologists, where the diagnosis can be confirmed by brain CT or MRI and pus or blood culture. Therefore, the coding of BA is highly reliable. Second, various factors affect mortality of BA, such as consciousness at the time of admission, nature of infection, and location and multiplicity of abscesses [2, 8, 16, 26]. However, the NHIRD as a claims dataset was not designed for academic research, and clinical information on symptoms, number, location, and size of lesions, and mode of neurosurgical intervention was not available in the study dataset. In addition, data on the pathogenic etiology of BA, such as the species of bacteria, fungi, parasite, culture rate, and functional outcome, was not available and remains beyond the scope of the current study.
Conclusions
This nationwide population cohort study revealed the epidemiological information about BA in Taiwan. Both the incidence and mortality rates both increased with age. Although incidence had a male predominance, mortality showed no sex difference. Infection-related diseases such as septicemia, pneumonia, and meningitis, significantly increased the risk of in-hospital mortality. In contrast, non-infection-related comorbidities, such as diabetes mellitus and a brain tumor, did not affect mortality. Therefore, we suggest paying attention to potential infections to improve the outcome of BA patients.
Supporting information
(DOC)
Abbreviations
- CI
confidence interval
- ICD-9-CM
International Classification of Diseases, 9th Revision, Clinical Modification
- NHIRD
National Health Insurance Research Database
- OR
odds ratio
Data Availability
The data obtained from the Taiwan National Health Insurance Research Database (NHIRD). Only citizens of the Republic of China (Taiwan) who fulfill the requirements of conducting research projects are eligible to apply for the NHIRD. The use of NHIRD is limited to research purposes only. Applicants must follow the Computer-Processed Personal Data Protection Law (http://www.winklerpartners.com/?p=987) and related regulations of National Health Insurance Administration and NHRI, and an agreement must be signed by the applicant and his/her supervisor upon application submission. All applications are reviewed for approval of data release.
Funding Statement
The author received no specific funding for this work.
References
- 1.Carpenter J, Stapleton S, Holliman R. Retrospective analysis of 49 cases of brain abscess and review of the literature. Eur J Clin Microbiol Infect Dis 2007; 26 (1): 1–11. 10.1007/s10096-006-0236-6 [DOI] [PubMed] [Google Scholar]
- 2.Menon S, Bharadwaj R, Chowdhary A, Kaundinya D, Palande D. Current epidemiology of intracranial abscesses: a prospective 5 year study. J Med Microbiol. 2008; 57 (10): 1259–1268. [DOI] [PubMed] [Google Scholar]
- 3.Tsou TP, Lee PI, Lu CY, Chang LY, Huang LM, Chen JM, et al. Microbiology and epidemiology of brain abscess and subdural empyema in a medical center: a 10-year experience. J Microbiol Immunol Infect 2009; 42 (5): 405–12. [PubMed] [Google Scholar]
- 4.Sharma R, Mohandas K, Cooke RP. Intracranial Abscesses: Changes in Epidemiology and Management Over Five Decades in Merseyside. Infection 2009; 37 (1): 39–43. 10.1007/s15010-008-7359-x [DOI] [PubMed] [Google Scholar]
- 5.Shachor-Meyouhas Y, Bar-Joseph G, Guilburd JN, Lorber A, Hadash A, Kassis I. Brain abscess in children—epidemiology, predisposing factors and management in the modern medicine era. Acta Paediatr. 2010; 99 (8): 1163–1167. 10.1111/j.1651-2227.2010.01780.x [DOI] [PubMed] [Google Scholar]
- 6.Zhang C, Hu L, Wu X, Hu G, Ding X, Lu Y. A retrospective study on the aetiology, management, and outcome of brain abscess in an 11-year, single-centre study from China. BMC Infec Dis. 2014; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nicolosi A, Hauser WA, Musicco M, Kurland LT. Incidence and prognosis of brain abscess in a defined population: Olmsted County, Minnesota, 1935–1981. Neuroepidemiology 1991; 10(3): 122–31. [DOI] [PubMed] [Google Scholar]
- 8.McClelland CJ, Craig BF, Crockard HA. Brain abscesses in Northern Ireland: a 30 year community review. J Neurol Neurosurg Psychiatry 1978; 41 (11): 1043–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brouwer MC, Tunkel AR, McKhann II GM, van de Beek D. Brain Abscess. N Engl J Med 2014; 371 (5): 447–56. 10.1056/NEJMra1301635 [DOI] [PubMed] [Google Scholar]
- 10.Lai C-H, Tseng H-F. Nationwide population-based epidemiological study of myasthenia gravis in Taiwan. Neuroepidemiology 2010; 35(1): 66–71. 10.1159/000311012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuo CF, See LC, Yu KH, Chou IJ, Chang HC, Chiou MJ, et al. Incidence, cancer risk and mortality of dermatomyositis and polymyositis in Taiwan: a nationwide population study. Br J Dermatol 2011; 165 (6): 1273–1279. 10.1111/j.1365-2133.2011.10595.x [DOI] [PubMed] [Google Scholar]
- 12.Ong C-T, Sheu S-M, Tsai C-F, Wong Y-S, Chen SC-C. Age-Dependent Sex Difference of the Incidence and Mortality of Status Epilepticus: A Twelve Year Nationwide Population-Based Cohort Study in Taiwan. PloS one 2015; 10 (3): e0122350 10.1371/journal.pone.0122350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mathisen GE, Johnson JP. Brain abscess. Clin Infec Dis 1997: 763–779. [DOI] [PubMed] [Google Scholar]
- 14.Brouwer MC, Coutinho JM, van de Beek D. Clinical characteristics and outcome of brain abscess: systematic review and meta-analysis. Neurology; 82 (9): 806–13. 10.1212/WNL.0000000000000172 [DOI] [PubMed] [Google Scholar]
- 15.Cartes-Zumelzu FW, Stavrou I, Castillo M, Eisenhuber E, Knosp E, Thurnher MM. Diffusion-weighted imaging in the assessment of brain abscesses therapy. ANJR Am J neuroradiol 2004; 25 (8): 1310–1317. [PMC free article] [PubMed] [Google Scholar]
- 16.Nathoo N, Nadvi SS, Narotam PK, van Dellen JR. Brain Abscess: Management and Outcome Analysis of a Computed Tomography Era Experience with 973 Patients. World Neurosurg 2011; 75 (5–6): 716–726. 10.1016/j.wneu.2010.11.043 [DOI] [PubMed] [Google Scholar]
- 17.Datto J, Yang J, Dietrich WD, Pearse DD. Does being female provide a neuroprotective advantage following spinal cord injury? Neural Regen Res 2015; 10(10):1533–6. 10.4103/1673-5374.165213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Landriel F, Ajler P, Hem S, Bendersky D, Goldschmidt E, Garategui L, et al. Supratentorial and infratentorial brain abscesses: surgical treatment, complications and outcomes—a 10-year single-center study. Acta neurochi 2012; 154 (5): 903–911. [DOI] [PubMed] [Google Scholar]
- 19.Helweg-Larsen J, Astradsson A, Richhall H, Erdal J, Laursen A, Brennum J. Pyogenic brain abscess, a 15 year survey. BMC Infec Dis; 12: 332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hakan T, Ceran N, Erdem İ, Berkman MZ, Göktaş P. Bacterial brain abscesses: An evaluation of 96 cases. J Infect 2006; 52 (5): 359–366. 10.1016/j.jinf.2005.07.019 [DOI] [PubMed] [Google Scholar]
- 21.Lu CH, Chang WN, Lin YC, Tsai NW, Liliang PC, Su TM, et al. (2002) Bacterial brain abscess: microbiological features, epidemiological trends and therapeutic outcomes. Qjm 2002; 95: 501–509. [DOI] [PubMed] [Google Scholar]
- 22.Wutzler S, Maegele M, Marzi I, Spanholtz T, WafaiSade A, Lefering R, et al. Association of preexisting medical conditions with In-Hospital mortality in multiple-trauma Patients. J Am Coll Surg 2009; 209: 75–81. 10.1016/j.jamcollsurg.2009.03.015 [DOI] [PubMed] [Google Scholar]
- 23.Shoko T, Shiraishi A, Kaji M, Otomo Y. Effect of pre-existing conditions on in-hospital mortality: analysis of 20,257 trauma patients in Japan. J Am Coll Surg 2010; 211:338–346. 10.1016/j.jamcollsurg.2010.04.010 [DOI] [PubMed] [Google Scholar]
- 24.Kuo CY, Chiou HY, Lin JW, Tsai SY, Chiang YH, Lin CM, et al. Seatbelt use and traumatic brain injury in Taiwan: A 16 years study. Iran J Public health 2015; 44: 470–478. [PMC free article] [PubMed] [Google Scholar]
- 25.Strandgaard S, Olesen J, Skinhoj E, Lassen NA. Autoregulation of brain circulation in severe arterial hypertension. Br Med J. 1973; 1 (5852): 507–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beller AJ, Sahar A, Praiss I. Brain abscess: Review of 89 cases over a period of30 years. J Neurol Neurosurg Psychiatry 1973; 36 (5): 757–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
Data Availability Statement
The data obtained from the Taiwan National Health Insurance Research Database (NHIRD). Only citizens of the Republic of China (Taiwan) who fulfill the requirements of conducting research projects are eligible to apply for the NHIRD. The use of NHIRD is limited to research purposes only. Applicants must follow the Computer-Processed Personal Data Protection Law (http://www.winklerpartners.com/?p=987) and related regulations of National Health Insurance Administration and NHRI, and an agreement must be signed by the applicant and his/her supervisor upon application submission. All applications are reviewed for approval of data release.