Abstract
Background
Psoriasis and inflammatory bowel disease (IBD) are chronic inflammatory diseases sharing similar pathogenic pathways. Intestinal microbial changes such as a decrease of bakers’ yeast Saccharomyces cerevisiae have been reported in IBD, suggesting the presence of a gut-skin axis.
Objective
To investigate whether the S. cerevisiae abundance was altered in psoriasis patients versus healthy controls, and whether dimethylfumarate (DMF) interacted with this yeast.
Methods
Using qPCR, faecal samples were compared between psoriasis patients without DMF (n = 30), psoriasis patients with DMF (n = 28), and healthy controls (n = 32).
Results
Faecal S. cerevisiae abundance was decreased in psoriasis compared to healthy controls (p<0.001). Interestingly, DMF use raised S. cerevisiae levels (p<0.001). Gastrointestinal adverse-effects of DMF were correlated with a higher S. cerevisiae abundance (p = 0.010). In vitro, a direct effect of DMF on S. cerevisiae growth was observed. In addition, anti-Saccharomyces cerevisiae antibodies were not elevated in psoriasis.
Conclusion
The abundance of baker’s yeast S. cerevisiae is decreased in psoriasis patients, but appears to be restored upon DMF use. S. cerevisiae is generally classified as a yeast with beneficial immunomodulatory properties, but may also be involved in the occurrence of DMF’s gastrointestinal adverse-effects. Potentially, DMF might be a new therapy for IBD.
Introduction
Psoriasis and inflammatory bowel disease (IBD) are chronic inflammatory diseases sharing similar pathogenic pathways[1]. Both diseases are characterized by an increased inflammatory response at the epithelial barrier. Secondly, similar genetic susceptibility plays an important role in the development of both disease entities[2, 3]. A third contributing factor to IBD development is an altered intestinal microbial composition, with a decreased abundance of several commensal microbes (e.g. Faecalibacterium prausnitzii) and an increase of pathogens (e.g. adherent invasive Escherichia coli)[4]. Changes in the fungal microbial community are also common, with a decreased Saccharomyces cerevisiae abundance as one of the most prominent observations[5]. S. cerevisiae, also called “baker’s yeast” or “brewer’s yeast” because of its usage in fermentative production of bread, beer or wine, is the most intensively studied eukaryotic organism in literature and one of the most abundant yeasts in our gut. S. cerevisiae is known to possess anti-inflammatory properties by being able to stimulate IL-10 and inhibit TNF-α[5, 6]. Whether the fungal microbiome also plays a role in psoriasis is as yet unknown.
Dimethylfumarate (DMF) is an effective therapy for psoriasis, and an emerging therapy for multiple sclerosis (MS)[7, 8]. There is growing interest for the implementation of DMF for other chronic diseases such as IBD[9], although the exact mechanisms of action, besides immunomodulation, have not yet been fully elucidated[10]. DMF has been also applied as a biocide in shoe soles, clothes and furniture for prevention of mold growth. However, due to allergic eczematous reactions, its use in these applications has been discontinued[11, 12].
As IBD and psoriasis share similar pathogenic pathways, including similar bacterial disturbances in the gut as we have recently shown[13], we speculated that, as in IBD, S. cerevisiae abundance might be decreased in psoriasis. Interestingly, in Crohn’s disease, anti-Saccharomyces cerevisiae antibodies (ASCAs) are significantly elevated compared to healthy controls[14, 15]. Recently, elevated ASCA levels have also been demonstrated in patients with spondyloarthritis[16], but in psoriasis this has not been studied.
In this study we investigated whether psoriasis patients harbor an altered faecal S. cerevisiae abundance compared to healthy controls. Furthermore, in vivo and in vitro, we investigated whether DMF had impact on the S. cerevisiae abundance. In addition, ASCA levels were measured.
Materials and methods
Patients
All patients were included at the outpatient clinic of the Department of Dermatology, Erasmus MC in Rotterdam, the Netherlands. The study was approved by the medical ethical committee of the Erasmus MC (MEC-2014-371). Written informed consent was obtained for all participants. Inclusion criteria were a confirmed psoriasis diagnosis by a dermatologist, and age between 18–75 years. A minimum use of 6 weeks of DMF was required before inclusion in the study. Exclusion criteria were oral antibiotic use 8 weeks prior to inclusion, IBD-comorbidity, history of bowel resection, pregnancy and active infection. Clinical data was collected regarding medical history, comorbidities, medication, and disease characteristics. Duration of DMF use and presence of adverse-effects were recorded. Psoriasis Area and Severity Index (PASI) was used to assess disease activity (<10 mild, 10–20 moderate, >20 severe). From every participant, the faecal sample was sent by mail and stored at -80° Celsius within 48 hours.
A total of 49 psoriasis patients were included, of which the characteristics are depicted in Table 1. In total 30 samples were collected from psoriasis patients without DMF. A total of 28 samples were collected from psoriasis patients with DMF. Of nine of these patients, two samples were collected, one sample before and one sample after (6–9 weeks) start of DMF.
Table 1. Patients’ characteristics.
| Psoriasis - DMF |
Psoriasis + DMF |
Healthy controls |
P-value1 | |
|---|---|---|---|---|
| N (patients) | 30 | 28 | 32 | |
| Age (mean yrs, SD) | 46.1 (13.9) | 42.7 (14.1) | 42.6 (14.1) | P = 0.54 |
| Gender (% female) | 60.0 | 50.0 | 62.5 | P = 0.52 |
| Smoking (%) | 20.0 | 28.6 | 6.3 | P = 0.07 |
| BMI (mean, SD) | 27.8 (5.3) | 27.2 (4.5) | 25.3 (4.8) | P = 0.11 |
| Caucasian (%) | 80.0 | 82.1 | 81.3 | P = 0.98 |
|
Age at diagnosis (mean yrs, SD) |
30.8 (12.2) | 25.4 (11.8) | NA | P = 0.09 |
|
Disease duration (mean yrs, SD) |
15.7 (11.6) | 17.0 (11.1) | NA | P = 0.67 |
| Psoriasis type n (%) | ||||
| Vulgaris | 25 (83.3) | 25 (89.3) | NA | |
| Guttate | 3 (10.0) | 2 (7.1) | NA | |
| Palmoplantaris | 2 (6.7) | 1 (3.6) | NA | |
| Psoriasis therapy2 | ||||
| Immunosuppressant | 2 | 1 | NA | |
| Local therapy | 15 | 14 | NA | |
| UVB therapy | 2 | 0 | NA | |
| Duration DMF (wks, mean, SD) | NA | 66.8 (94.0) | NA | |
| PASI3 | ||||
| < 10 | 16 (64.0) | 20 (80.0) | NA | |
| >10 en ≤ 20 | 8 (32.0) | 5 (20.0) | NA | |
| >20 | 1 (5.0) | 0 (0) | NA |
DMF, dimethylfumarate; BMI, body mass index; NA, non-applicable
1 P-value calculated by one-way ANOVA for age, BMI; unpaired-t-test for age at diagnosis, disease duration; chi-square test for gender, smoking, ethnicity
2 Patient could have been on concomitant drugs
3 PASI, Psoriasis Area Severity Index for plaque type at time of sample collection (<10 mild, 10–20 moderate, >20 severe). Note that 9 of the 28 patients on DMF are paired with the no-DMF group.
DNA isolation and quantitative polymerase chain reaction (qPCR)
DNA was extracted from 20 mg faeces per sample as described previously[13]. Briefly, 1 ml of cell lysis buffer (Ambion, Life Technologies) was added, followed by 15 min incubation period. Full cell lysis was achieved by bead-beating [three times 30s]. The samples were centrifuged, whereupon 3:1 protein precipitation buffer (Promega) was added; 100% isopropanol [1:1] was used to precipitate DNA from the supernatant; 100μl 70% ethanol was used to wash the DNA pellet. Finally, DNA was resuspended in 50μl TE-buffer, DNA concentration was measured on a Nanodrop spectrophotometer [Isogen Life Science BV, De Meern, The Netherlands] and diluted to 10 ng/μl.
QPCR was performed two times as described[13]. QPCRs were performed in duplicate for S. cerevisiae using the following primer:
F-AGGAGTGCGGTTCTTTG; R-TACTTACCGAGGCAAGCTACA[5].
Bacterial abundance analysis was performed by SybrGreen based qPCR reaction containing 20ng DNA [2μl of 10ng/μl], 9μL SYBR®Select MasterMix for CFX (ThermoFisher Scientific), 7μl dH20, 1μl 10μM forward primer, and 1μl 10μM reverse primer.
Thermocycle conditions comprised: denaturation step 10 min at 95°C; 40 cycles of 95°C denaturation for 15s; 56°C primer annealing for 30s; and 72°C extension for 30s followed by a standard melting
curve analysis. The mean was calculated from the two qPCRs and the S. cerevisiae abundance was expressed in log10copies/gram faeces.
XTT assay
In vitro analysis was performed to establish whether the change in S. cerevisiae abundance was directly or indirectly induced by DMF. The yeast S. cerevisiae (MycoBank#:163963) was obtained from CBS-KNAW Fungal Biodiversity Centre, institute of the Royal Netherlands Academy of Arts and Sciences. The yeast was cultured at 30°C for 48 hours and 100μl of a 0.1McFarland yeast suspension was pipetted into a 96-well plate. Serial dilutions of DMF, fumaric acid and an antimycotic (amphotericine-B-deoxycholate) were added to the cells in triplicate. H20 and ethanol were used as vehicle control. XTT/PMS was added to the suspension as per manufacturers’ directions (Molecular Probes/Thermo Scientific) and the plate was read after 24h at OD415 nm. Six independent experiments were performed.
ASCA measurement
Serum was collected of 30 psoriasis patients, including 18 without DMF and 12 with DMF, and 17 healthy controls, for the determination of anti-Saccharomyces cerevisiae antibodies (ASCAs), IgA and IgG. Results were categorized as following: ASCAs (units) <20 negative; 20–25 borderline positive; >25 positive. ASCAs were determined using QUANTALite ASCA IgA/IgG ELISA (Inova Diagnostics, San Diego, CA). ELISA was performed according to the manufacturer’s instructions, without modifications.
Statistical analysis
Patients’ characteristics were compared between psoriasis patients without DMF use, psoriasis patients with DMF use, and healthy controls by using the one-way analysis of variance (ANOVA), unpaired t-test and chi-square test, depending of the presence of a normal distribution. IBM SPSS 21.0 statistical software, Armonk, NY, USA was used for the statistical analyses. In vitro data were analyzed using Graphpad Prism (version 5.1), performing paired-t-tests.
Results
Patients’ characteristics
There were no significant clinical differences between the groups (Table 1). Nine participants (10%) followed a specific diet. In the psoriasis patients without DMF, one followed a sugar-free and one a cow-milk- and wheat-free diet. In the psoriasis patients with DMF, one followed a gluten-free, one a low-carbohydrate, one a both vegetarian and low-carbohydrate diet and one a dairy-free diet. Of the healthy controls, three were vegetarian. In the electronic charts, none of the included patients were reported to have used oral antifungal or antibiotic medication 8 weeks before the study or during the study. In the psoriasis patients without DMF, the majority had plaques on multiple body sites (diffuse) (n = 28, 88%). Two had solely plaques on their feet, and two solely on their head. In the psoriasis patients with DMF, plaques were located diffusely in 26 patients (93%). One had solely psoriasis plaques on the neck and scalp, and one on the feet.
Significant reduction of S. cerevisiae abundance in psoriasis, corrected by DMF treatment
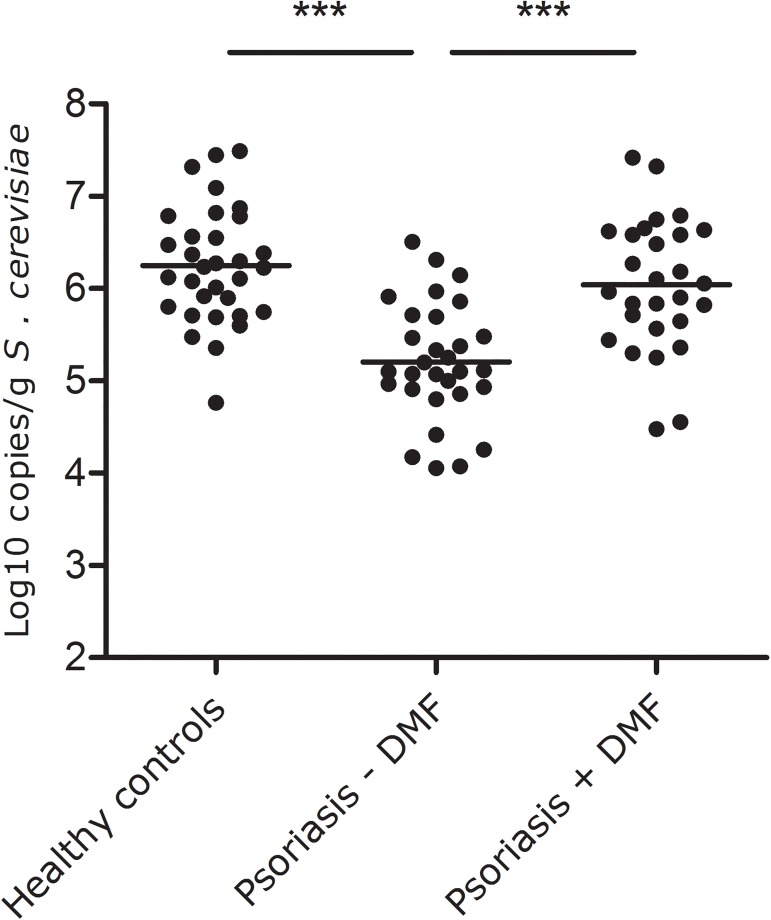
A significant difference in S. cerevisiae abundance was demonstrated by one-way ANOVA of the S. cerevisiae abundance between the three groups (1. psoriasis patients without DMF, 2. psoriasis patients with DMF, 3. healthy controls; p<0.001). Psoriasis patients without DMF had a significantly lower S. cerevisiae abundance than healthy controls (mean log10 copies/g± SD: 5.20±0.64 vs 6.25±0.63, p<0.001). Psoriasis patients using DMF had a significant higher S. cerevisiae abundance than patients without DMF (6.04±0.72, p<0.001), reaching levels that were similar to those of healthy controls (p = 0.233, Fig 1). Excluding the psoriasis patients using systemic immunosuppressant’s (n = 2 without DMF, n = 1 with DMF) did not affect the results. A subgroup analysis was performed for the patients for whom paired samples were available, before and after DMF (n = 9). Within this group there was also a trend observed towards an increased S. cerevisiae abundance upon DMF treatment (before DMF, 4.99±0.64; after DMF 5.62±0.60; p = 0.086). All patients showed clinical response to DMF, and PASI scores did not correlate to the S. cerevisiae abundance.
Fig 1. Psoriasis patients without DMF had a significantly lower faecal Saccharomyces cerevisiae abundance than healthy controls and psoriasis patients on DMF (both p<0.001).
Psoriasis patients using DMF had similar S. cerevisiae abundance compared to healthy controls (p = 0.233). The middle line represents the average abundance.
The presence of side-effects of DMF correlate with a higher S. cerevisiae abundance
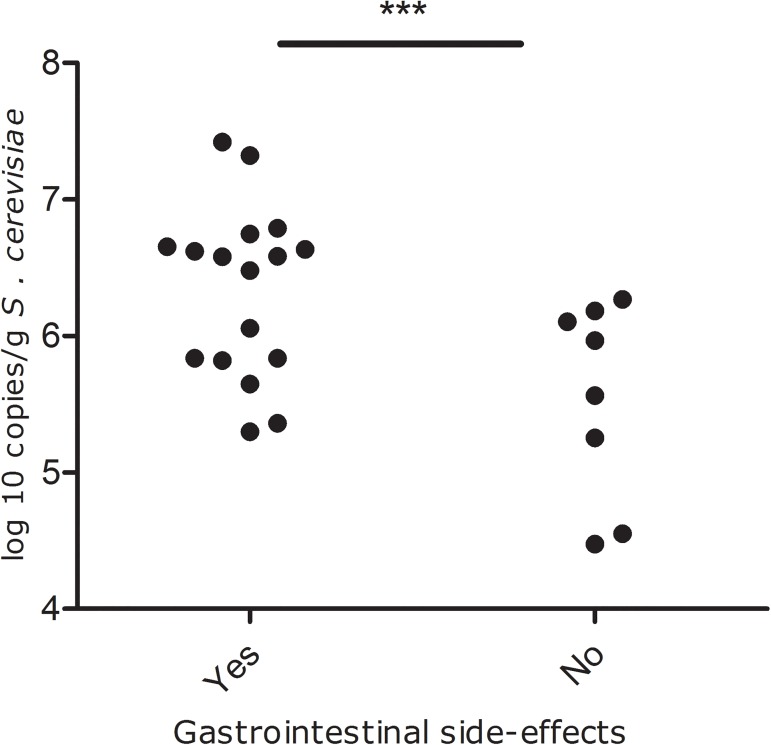
The majority (20 of 25 patients (80%), n = 3 missing) of the psoriasis patients who used DMF reported adverse effects of DMF use. The most reported adverse effects were flushing (42.9%), diarrhea (32.1%), abdominal pain (25.0%) and nausea (14.3%). Gastrointestinal side-effects (abdominal pain, diarrhea and/or nausea), were reported in 17 patients on DMF, while 8 patients had no side-effects. Patients with gastrointestinal side-effects had a significant higher S. cerevisiae abundance than patients without these side-effects (6.33±0.63 vs 5.55±0.72; p = 0.010, Fig 2). When we included all adverse-effects, including flushing, similar results were observed (6.26±0.63 vs 5.37±0.81; p = 0.013).
Fig 2. Psoriasis patients on DMF who had gastrointestinal side-effects had a significantly higher S. cerevisiae abundance than psoriasis patients without the side-effects (p = 0.010).
DMF directly stimulates S. cerevisiae growth in vitro
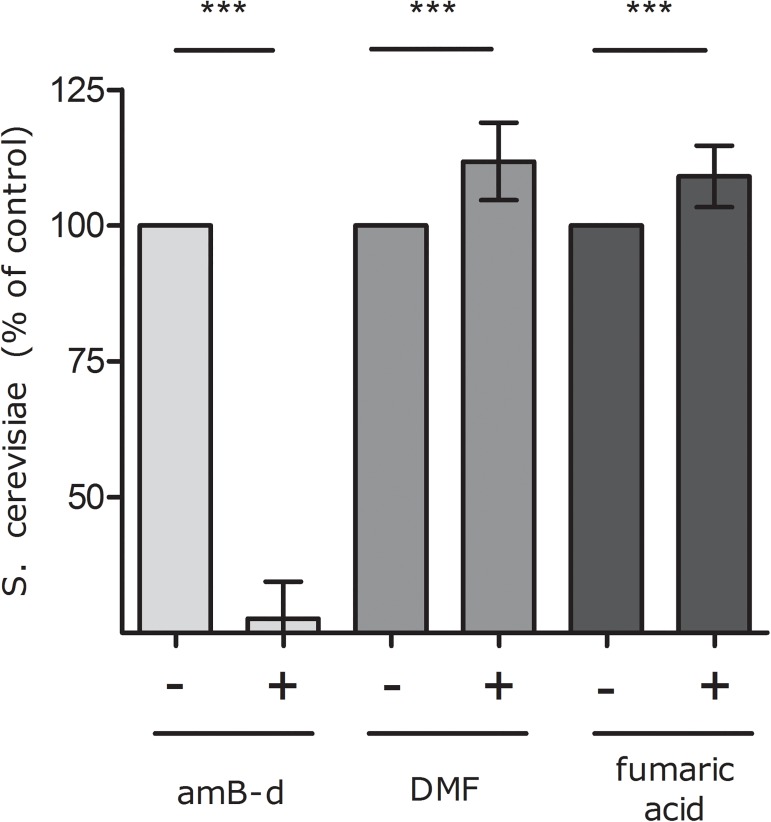
To investigate whether the increase in S. cerevisiae in faeces from patients treated with DMF was a direct effect of DMF on the yeast itself, we treated S. cerevisiae in vitro with DMF. Results show that DMF significantly induces S. cerevisiae growth after 24 hours in comparison to untreated cells (p = 0.0128; Fig 3). DMF is a dimethyl ester of fumaric acid, and addition of fumaric acid directly to S.cerevisiae also resulted in a significant increase in yeast growth (p = 0.010). Administration of the antimycotic amphotericine-B-deoxycholate resulted, as expected, in a significant decrease of cultured S. cerevisiae (p<0.001). In addition, to exclude the possibility that the depletion of S. cerevisiae in psoriasis was due to lower faecal fumaric acid levels in these patients, fumaric acid measurement in faeces was performed in 16 paired psoriasis samples, without DMF use and with DMF, and in 8 healthy controls. No significant differences in fumaric acid levels between these groups were found (see supplementary data, in particular S2 Fig).
Fig 3. DMF (1.6 mg/ml) and fumaric acid (0.46 mg/ml) significantly stimulate S. cerevisiae growth (p = 0.01), amphotericine B-deoxycholate (amB-d) inhibits S. cerevisiaeas expected.
Maximal concentration fumaric acid used (0.46 mg/ml) was limited by the dissolvent, ethanol. Data are expressed as mean, SD.
Anti-Saccharomyces cerevisiae antibodies (ASCAs)
The ASCA IgA level was elevated in one (3.3%) of the 30 psoriasis patients. This patient used DMF. Two patients (6.7%) (both not on DMF) had a borderline positive ASCA IgA, compared to one (5.9%) healthy subject (total n = 17).
The ASCA IgG level was elevated in 3 out of 30 patients (10%), of which two with and one without DMF. One of them had also a positive IgA level. Of the healthy subjects two (11.8%) had a positive IgG ASCA, which were different subjects than the healthy control with a positive IgA. No correlation between a positive ASCA and faecal S. cerevisiae abundance was found.
Discussion
This study demonstrates a significant depletion of S. cerevisiae in psoriasis, which appears to be restored in psoriasis patients on DMF. Additionally, our study confirmed that, in vitro, DMF can directly stimulate S. cerevisiae growth.
To date, information on the impact of medication on the (bacterial or fungal) microbiome is still scarce, and this study shows we should not underestimate the consequences of medication use, whether they are beneficial or harmful to our microbiome. It is unclear whether microbes and medication act synergistically or whether microbial changes could be a result of the disease remission and the anti-inflammatory milieu caused by the medication. Our in vitro data showing that DMF directly stimulates the growth of S. cerevisiae suggests that the increased colonisation is not only the result of an anti-inflammatory environment. Recent evidence shows that S. cerevisiae itself exhibits several immunomodulatory properties. The cell wall of S. cerevisiae consists mainly of β-glucans, which has immunomodulatory effects[17], and TNF-α reduction and IL10 stimulation by S. cerevisiae have recently been shown[5, 6]. TNF-α (pro-inflammatory) and IL10 (anti-inflammatory) are important cytokines in the pathogenesis of chronic inflammatory immune-mediated diseases such as psoriasis and IBD. Thus, a higher S. cerevisiae abundance might contribute to the anti-inflammatory effect of DMF. In addition to the immunomodulatory effects of DMF itself, a DMF-induced increase of S. cerevisiae might contribute to achievement (and maintenance) of stable remission in patients, providing a beneficial milieu for colonization. The finding that DMF seems to restore a (fungal) disturbance in psoriasis, could also be extrapolated to the S. cerevisiae depletion observed in IBD[5, 9], or potentially, MS. Psoriasis and IBD share common pathophysiological processes, including common genetic risk factors [18, 19]. Interestingly, it was recently demonstrated that in IBD, a risk-associated single nucleotide polymorphism (SNP) in the CARD9 gene negatively correlates with S. cerevisiae abundance, suggesting that genetic variants affecting immune cell function can modulate microbial abundance [5]. However, to the best of our knowledge, the CARD9 IBD-risk allele has so far not been associated with psoriasis [19, 20]. Furthermore, while SNPs in other immunity genes may potentially contribute to an altered microbial balance, it is important to note that such disease-associated alleles, while more prevalent in disease, are also present in healthy individuals, and are therefore unlikely to (solely) explain the decreased S. cerevisiae abundance in the psoriasis cohort as compared to healthy controls.
S. cerevisiae is one of the most abundant members of the fungal microbiota and might exert favorable immunologic effects in immunological diseases. However, it is important to note that from a greater perspective DMF potentially also interacts with other members of the fungal and/or bacterial microbiome. Thus, while our in vitro data show a direct effect of DMF on S. cerevisiae growth, it is also conceivable that other microbes might be able to influence S. cerevisiae and vice versa. Therefore more research into the other members of the microbiome is warranted.
It is of interest to note that the benefit of S. cerevisiae when used as skin-conditioning agent has been demonstrated earlier[21]. Our study outlines the potential beneficial role of S. cerevisiae in skin (and gut) homeostasis. At present, S. cerevisiae is also used as dietary supplement because of its nutrients (rich in amino acids) and functions. It is unknown whether S. cerevisiae supplemented in food colonizes the intestine similarly as the stimulation of (probably resident) S. cerevisiae by DMF. Some of the patients included in our study followed a particular diet which might potentially impact the response of DMF, its side-effects and/or the S. cerevisiae abundance, although we did not observe such trend. Interestingly, a variant of S. cerevisiae, Saccharomyces boulardii, is already used as probiotic for diarrhea. Besides S. cerevisiae, other members of the fungal microbiome could be of interest, and moreover, bacteria could be influenced by DMF as well.
However, while the potential use of S. cerevisiae (and DMF) as probiotic is interesting, it should, in the light of our study, be carefully implemented after further investigations into its effect and safety[22]. Our study showed that a higher S. cerevisiae abundance might also have less favorable effects: the presence of gastrointestinal side-effects, such as diarrhea, nausea and abdominal pain, which are often reported side-effects of DMF therapy, were correlated with a significant higher S. cerevisiae abundance. Nevertheless, although in the past decades an increasing incidence of (opportunistic) infections involving S. cerevisiae have been described[23], S. cerevisiae, which is used in baking and brewing, is still considered to be safe and non-pathogenic.
A strength of this patient cohort was that 93% did not use additional systemic medication, excluding other medication bias. Future studies should include a complete paired-sample set, and also investigate other members of the fungal microbiome in psoriasis.
In conclusion, S. cerevisiae is depleted in psoriasis patients. In in vitro and in vivo experiments, DMF is able to stimulate the growth of S. cerevisiae, which might play a role in the etiopathogenesis of psoriasis and possible also other diseases such as MS and IBD. It is also conceivable that S. cerevisiae acts as a bystander, however not innocently, as it might be the cause the gastrointestinal adverse-effects of DMF. Further investigations should look into the immunologic and therapeutic functions of S. cerevisiae and the mechanistic effect of DMF and other medications on the inhabitants of our gut. S. cerevisiae as probiotic might be a potential candidate for novel treatments in patients with chronic inflammatory diseases such as psoriasis.
Supporting information
(DOCX)
(TIF)
(TIF)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.Skroza N, Proietti I, Pampena R, La Viola G, Bernardini N, Nicolucci F, et al. Correlations between psoriasis and inflammatory bowel diseases. Biomed Res Int. 2013;2013:983902 Epub 2013/08/24. PubMed Central PMCID: PMC3736484. doi: 10.1155/2013/983902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ellinghaus D, Ellinghaus E, Nair RP, Stuart PE, Esko T, Metspalu A, et al. Combined Analysis of Genome-wide Association Studies for Crohn Disease and Psoriasis Identifies Seven Shared Susceptibility Loci. American Journal of Human Genetics. 2012;90(4):636–47. doi: 10.1016/j.ajhg.2012.02.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jostins L, Ripke S, Weersma RK, Duerr RH, McGovern DP, Hui KY, et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature. 2012;491(7422):119–24. doi: 10.1038/nature11582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kostic AD, Xavier RJ, Gevers D. The microbiome in inflammatory bowel disease: current status and the future ahead. Gastroenterology. 2014;146(6):1489–99. Epub 2014/02/25. PubMed Central PMCID: PMC4034132. doi: 10.1053/j.gastro.2014.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sokol H, Leducq V, Aschard H, Pham HP, Jegou S, Landman C, et al. Fungal microbiota dysbiosis in IBD. Gut. 2016. Epub 2016/02/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jawhara S, Habib K, Maggiotto F, Pignede G, Vandekerckove P, Maes E, et al. Modulation of intestinal inflammation by yeasts and cell wall extracts: strain dependence and unexpected anti-inflammatory role of glucan fractions. PLoS One. 2012;7(7):e40648 Epub 2012/08/01. PubMed Central PMCID: PMC3407157. doi: 10.1371/journal.pone.0040648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Atwan A, Ingram JR, Abbott R, Kelson MJ, Pickles T, Bauer A, et al. Oral fumaric acid esters for psoriasis: Abridged Cochrane systematic review including GRADE assessments. Br J Dermatol. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu Z, Zhang F, Sun F, Gu K, Dong S, He D. Dimethyl fumarate for multiple sclerosis. Cochrane Database Syst Rev. 2015;4:CD011076. Epub 2015/04/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Casili G, Cordaro M, Impellizzeri D, Bruschetta G, Paterniti I, Cuzzocrea S, et al. Dimethyl Fumarate Reduces Inflammatory Responses in Experimental Colitis. J Crohns Colitis. 2016;10(4):472–83. Epub 2015/12/23. doi: 10.1093/ecco-jcc/jjv231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mrowietz U, Asadullah K. Dimethylfumarate for psoriasis: more than a dietary curiosity. Trends Mol Med. 2005;11(1):43–8. Epub 2005/01/15. doi: 10.1016/j.molmed.2004.11.003 [DOI] [PubMed] [Google Scholar]
- 11.Gimenez-Arnau A, Silvestre JF, Mercader P, De la Cuadra J, Ballester I, Gallardo F, et al. Shoe contact dermatitis from dimethyl fumarate: clinical manifestations, patch test results, chemical analysis, and source of exposure. Contact Dermatitis. 2009;61(5):249–60. Epub 2009/11/03. doi: 10.1111/j.1600-0536.2009.01628.x [DOI] [PubMed] [Google Scholar]
- 12.D'Erme AM, Bassi A, Lotti T, Gola M. Dimethyl fumarate contact dermatitis of the foot: an increasingly widespread disease. Int J Dermatol. 2012;51(1):42–5. Epub 2011/09/15. doi: 10.1111/j.1365-4632.2011.04916.x [DOI] [PubMed] [Google Scholar]
- 13.Eppinga H, Sperna Weiland CJ, Thio HB, van der Woude CJ, Nijsten TE, Peppelenbosch MP, et al. Similar depletion of protective Faecalibacterium prausnitzii in psoriasis and Inflammatory Bowel Disease, but not in Hidradenitis Suppurativa. J Crohns Colitis. 2016. Epub 2016/03/14. [DOI] [PubMed] [Google Scholar]
- 14.Forcione DG, Rosen MJ, Kisiel JB, Sands BE. Anti-Saccharomyces cerevisiae antibody (ASCA) positivity is associated with increased risk for early surgery in Crohn's disease. Gut. 2004;53(8):1117–22. Epub 2004/07/13. PubMed Central PMCID: PMC1774147. doi: 10.1136/gut.2003.030734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Z, Li C, Zhao X, Lv C, He Q, Lei S, et al. Anti-Saccharomyces cerevisiae antibodies associate with phenotypes and higher risk for surgery in Crohn's disease: a meta-analysis. Dig Dis Sci. 2012;57(11):2944–54. Epub 2012/06/07. doi: 10.1007/s10620-012-2244-y [DOI] [PubMed] [Google Scholar]
- 16.Maillet J, Ottaviani S, Tubach F, Roy C, Nicaise-Rolland P, Palazzo E, et al. Anti-Saccharomyces cerevisiae antibodies (ASCA) in spondyloarthritis: Prevalence and associated phenotype. Joint Bone Spine. 2016. Epub 2016/03/20. [DOI] [PubMed] [Google Scholar]
- 17.Takada Y, Nishino Y, Ito C, Watanabe H, Kanzaki K, Tachibana T, et al. Isolation and characterization of baker's yeast capable of strongly activating a macrophage. FEMS Yeast Res. 2014;14(2):261–9. Epub 2013/10/15. doi: 10.1111/1567-1364.12098 [DOI] [PubMed] [Google Scholar]
- 18.Lees CW BJ, Parkes M, Satsangi J. New IBD genetics: common pathways with other diseases. Gut 2011. December;60(12):1739–53. doi: 10.1136/gut.2009.199679 [DOI] [PubMed] [Google Scholar]
- 19.Ellinghaus D JL, Spain SL, Cortes A, Bethune J, Han B, et al. Analysis of five chronic inflammatory diseases identifies 27 new associations and highlights disease-specific patterns at shared loci. Nat Genet 2016. May;48(5):510–8. doi: 10.1038/ng.3528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aterido A JA, Ferrándiz C, Puig L, Fonseca E, Fernández-López E, et al. Genome-Wide Pathway Analysis Identifies Genetic Pathways Associated with Psoriasis. J Invest Dermatol 2016. March;136(3):593–602. doi: 10.1016/j.jid.2015.11.026 [DOI] [PubMed] [Google Scholar]
- 21.Gaspar LR, Camargo FB Jr., Gianeti MD, Maia Campos PM. Evaluation of dermatological effects of cosmetic formulations containing Saccharomyces cerevisiae extract and vitamins. Food Chem Toxicol. 2008;46(11):3493–500. Epub 2008/09/23. doi: 10.1016/j.fct.2008.08.028 [DOI] [PubMed] [Google Scholar]
- 22.Palma ML, Zamith-Miranda D, Martins FS, Bozza FA, Nimrichter L, Montero-Lomeli M, et al. Probiotic Saccharomyces cerevisiae strains as biotherapeutic tools: is there room for improvement? Appl Microbiol Biotechnol. 2015;99(16):6563–70. Epub 2015/07/05. doi: 10.1007/s00253-015-6776-x [DOI] [PubMed] [Google Scholar]
- 23.Perez-Torrado R, Querol A. Opportunistic Strains of Saccharomyces cerevisiae: A Potential Risk Sold in Food Products. Front Microbiol. 2015;6:1522 Epub 2016/01/19. PubMed Central PMCID: PMC4705302. doi: 10.3389/fmicb.2015.01522 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(TIF)
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.