Abstract
Objectives
Changes in estrogen receptor (ER) expression likely underlie differential metabolic effects of estrogen in pre-and postmenopausal women. The aim of the current study was to determine whether ER gene expression in abdominal and femoral subcutaneous adipose tissue (SAT) was associated with age, menopause, or regional adiposity.
Methods
We studied pre-and post-menopausal (n=23 and 22, respectively; age 35–65 y) normal weight (mean±SD; BMI 23.7±2.5 kg/m2) women with similar total fat mass. Abdominal and femoral SAT ERα (ESR1) and ERβ (ESR2) mRNA expression was determined by qPCR.
Results
Total fat mass did not differ between pre-and postmenopausal women (22.7±5.3 vs. 21.7±5.3 kg). Compared to premenopausal women, ESR1 and the ratio of ESR1 to ESR2 were lower (p≤0.05) in postmenopausal abdominal and femoral SAT. ESR1 and ESR1:ESR2 were inversely associated with age in abdominal SAT (r=−0.380 and r=−0.463, respectively; p<0.05) and femoral SAT (r=−0.353 and r=−0.472, respectively; p<0.05). ESR2 was not related to age or menopause. The inverse association between ESR1 and age persisted after adjusting for trunk fat mass, estradiol, or leptin.
Conclusion
Among healthy pre-and postmenopausal women, increased age was associated with a decreased balance of ERα to ERβ in abdominal and femoral subcutaneous adipose tissue.
Keywords: Aging, menopause, adipose tissue, estrogen receptor gene expression
Introduction
The majority of women spend more than a third of their life postmenopausal. Aging in women typically leads to adverse changes in regional adiposity (i.e., increasing abdominal and decreasing femoral fat mass) and increased cardiometabolic risk [1]. The extent to which the age-related changes in adiposity and cardiometabolic risk in women are mediated by the loss of endogenous estrogens remains elusive. Estrogens exert their biological effects through two estrogen receptor (ER) isoforms: ERα and ERβ [2–4]. Both ER subtypes appear to be present in most, if not all, tissues in the body, with different tissues displaying varying degrees of relative expression [5]. Gene expression of ERα (ESR1) is reportedly higher than ERβ (ESR2) in subcutaneous adipose tissue (SAT) [2]. Studies of ER receptors in tissues other than SAT suggest there may be declines in ER expression with increasing age [4], but age-related reductions in ER have not yet been reported in human SAT. In fact, one study reported higher, not lower, abdominal SAT ERβ expression in postmenopausal compared to premenopausal women [6]. Such menopause-related differences in ER expression could be the result of aging, estrogen deficiency, or changes in body fat distribution, but to our knowledge this has not yet been studied. Age-or menopause-related changes in ERs could have profound effects on estrogen physiology and the progression of metabolic syndrome [1]. ERα and ERβ have distinct, often opposing, actions suggesting the relative proportions of ERα and ERβ are important to the tissue-specific response to estrogen [2, 5]. Globally knocking out ESR1 in rodents promotes fat accumulation and metabolic impairments (e.g., insulin resistance, glucose intolerance) whereas knocking out ESR2 has none of these effects [3]. On the other hand, knocking out ESR1 specifically from adipocytes does not invoke the same metabolic impairments as seen in global knockouts, but results in adverse changes in adipose tissue function (e.g., inflammation, fibrosis); in the absence of ESR1, ESR2 plays a protective role [7]. Such preclinical observations highlight the importance of studying the relative balance of ERα and ERβ in specific tissues and across the lifespan in women. The aims of the current study were to: 1) compare abdominal and femoral SAT ESR1 and ESR2 expression in pre-and postmenopausal women, and 2) determine the independent contribution of age and regional adiposity.
Experimental
Participants
We retrospectively analyzed baseline tissue samples collected in healthy non-obese (BMI<30kg/m2) premenopausal (n=23, age 35–50y, regular menstrual cycles; 28±3d and early follicular follicle-stimulating hormone [FSH] 5–25 IU/L) and postmenopausal (n=22, age 48–60y, no menses ≥ 6mo or, if hysterectomized, FSH >30 IU/L) women previously enrolled in a study conducted by our laboratory [8]. None of the women used any type of oral contraceptive or postmenopausal hormone treatment. All participants provided written informed consent to participate in the study, which was approved by the Colorado Multiple Institutional Review Board.
Body composition
Total, trunk, and leg fat mass and fat-free mass were measured by dual-energy X-ray absorptiometry (DXA, Hologic Delphi-W, software v11.2, Hologic, Inc., Bedford, MA), using manufacturer’s recommendations to define the trunk and leg regions. Abdominal subcutaneous (SFA) and visceral (VFA) fat areas, femoral SFA and intramuscular fat area (IMFA) were determined by computed tomography as previously described [9].
Adipose Tissue Biopsies
Aspiration biopsies of abdominal and femoral SAT were collected in the fasted state using a mini-liposuction technique as previously described [10]. Collected adipose tissue was separated from the fluid and immediately flash frozen in liquid N2.
Quantification of Estrogen Receptor mRNA by Quantitative PCR (qPCR
Total RNA was isolated with the QIAGEN RNeasy (Lipid) Tissue Mini Kit (Qiagen, Inc., Valencia, CA). qPCR analysis was done as follows: 1) total RNA was analyzed and quantitated (Experion System, Bio-Rad, Hercules, CA); 2) 100 ng total RNA was reverse transcribed (iScript cDNA synthesis kit, Bio-Rad); 3) qPCR was performed in duplicate (primers: ESR1 5′AGATCTTCGACATGCTGCTGGCTA, 3′AGACTTCAGGGTGCTGGACAGAAA; ESR2 5′TTGGTTTGGGTGATTGCCAAGAGC, 3′ATGTTGAGCAGATGTTCCATGCCC; RPL13A 5′CCTGGAGGAGAAGAGGAAAGAGA, 3′TTGAGGACCTCTGTGTATTTGTCAA; iQ SYBR Supermix, Bio-Rad) following manufacturer’s protocol on an iQ5 Real-Time PCR Detection System (Bio-Rad) along with a no-template control per gene. RPL13A was used as an endogenous control; and RNA expression is expressed as 2ΔCT (ΔCT = RPL13A CT -gene of interest CT). Validation experiments confirmed that efficiencies of target and reference genes were approximately equal.
Bloodwork
Fasting serum samples were stored at −80°C and analyzed in batch. Leptin was assayed by radioimmunoassay (EMD Millipore, Corp., Billerica, MA), and estradiol (E2) by chemiluminescence (Beckman Coulter, Inc., Indianapolis, IN).
Data analysis
Pearson correlations were used to evaluate the associations among measures of ER, age, total and regional adiposity. Partial correlations tested whether the associations of ER with age remained after controlling for potential confounding variables (adiposity, E2, leptin). All statistical analyses were performed using SPSS software (IBM SPSS Statistics version 21.0). Data are presented as mean ± SD unless otherwise specified. Level of significance was set at p<0.05.
Results
Participant characteristics
Postmenopausal women were 13 years older than premenopausal women, and 7 years past menopause in average (Table 1). Participants were normal body weight (BMI 23.7±2.5 kg/m2). Total adiposity (i.e. %fat) was similar between pre-and postmenopausal women. Although total and trunk fat mass did not differ, postmenopausal women had less leg fat mass and fat free mass than premenopausal women. Abdominal SFA and VFA did not differ between groups, but femoral SFA was lower in postmenopausal compared to premenopausal women (Table 1).
Table 1.
Participant Characteristics
| Premenopausal n=23 | Postmenopausal n=22 | |
|---|---|---|
| Age (yr) | 42 ± 4 | 55 ± 4* |
| Years since menopause | n/a | 7 ± 6 |
| Weight (kg) | 67.6 ± 8.4 | 62.1 ± 8.2 |
| BMI (kg/m2) | 24.2 ± 2.5 | 23.3 ± 2.4 |
| %fat | 33.3 ± 4.8 | 34.5 ± 5.2 |
| Total Fat Mass (kg) | 22.7 ± 5.3 | 21.7 ± 5.3 |
| Trunk Fat Mass (kg) | 9.2 ± 2.6 | 9.7 ± 3.5 |
| Leg Fat Mass (kg) | 10.4 ± 2.5 | 8.8 ± 1.7* |
| Fat-free mass (kg) | 44.9 ± 4.6 | 40.5 ± 4.7* |
| VFA | 41 ± 25 | 58 ± 41 |
| Abdominal SFA (cm2) | 229 ± 62 | 221 ± 71 |
| Femoral SFA (cm2) | 212 ± 58 | 173 ± 40* |
| Femoral IMFA (cm2) | 17 ± 9 | 15 ± 6 |
| Estradiol (pg/mL) | 91 ± 76 | 13 ± 6 |
| Leptin (ng/mL) | 13 ± 6 | 12 ± 8 |
p<0.05; mean±SD; VFA = visceral fat area;
SFA = subcutaneous fat area, IMFA = intra-muscular fat area.
Menopause-related differences
Postmenopausal women had lower ESR1 expression than premenopausal women in abdominal SAT (0.96±0.58 vs. 1.58±1.17, p<0.05) and femoral SAT (0.97±0.49 vs. 1.44±0.98, p=0.05; Figure 1). ESR2 did not differ between pre-and postmenopausal women in either abdominal SAT (1.17±0.70 vs. 1.22±0.64) or femoral SAT (1.09±0.68 vs. 1.25±0.71). Postmenopausal women had lower ratio of ESR1:ESR2 than premenopausal women in both abdominal SAT (0.89±0.55 vs. 1.47±0.71, p<0.01) and femoral SAT (0.87±0.37 vs. 1.143±0.85, p<0.01; Figure 1).
Figure 1.
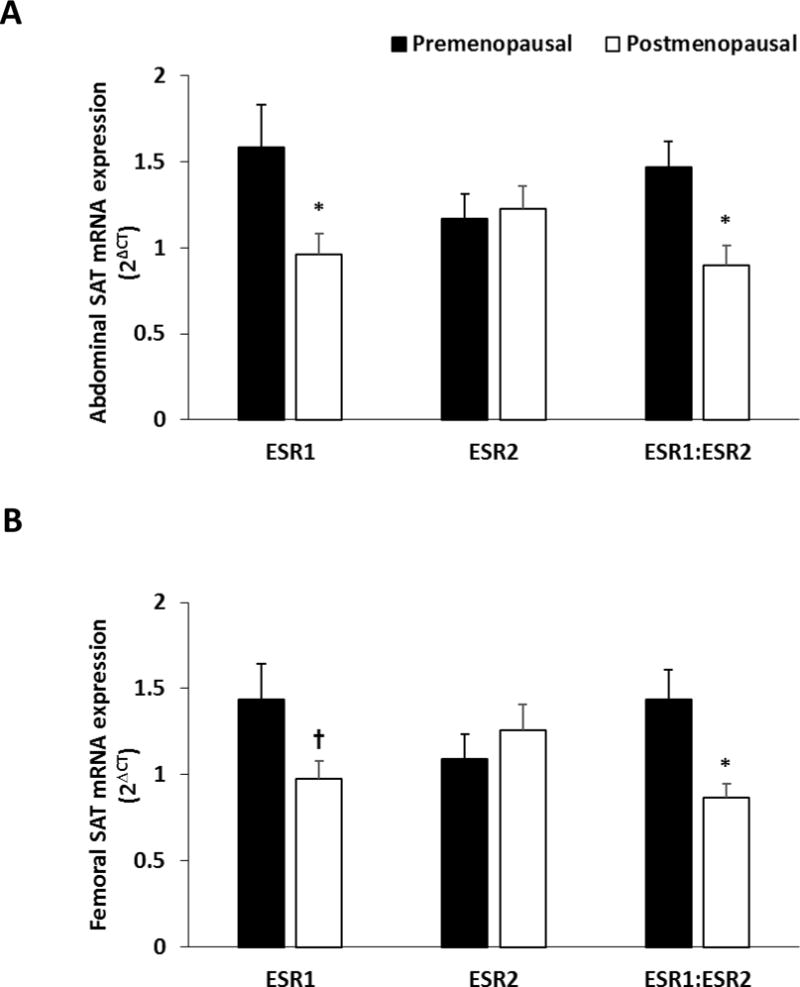
Relative mRNA expression of estrogen receptor (ER). Panel A: mRNA expression of ESR1 (ERα), ERβ (ESR2), and the balance of ESR1:ESR2 in abdominal subcutaneous adipose tissue (SAT); Panel B: mRNA expression of ESR1, ESR2, and the balance of ESR1:ESR2 in femoral SAT from premenopausal (n=23) and postmenopausal (n=22) women. * p<0.05, † p=0.05 for pre-vs post-menopausal women.
Regional adipose tissue differences
Measures of abdominal adiposity (i.e., trunk fat mass and abdominal VFA) were weakly inversely related to ESR1 and ESR1:ESR2 in abdominal and femoral SAT (r=−0.23 to −0.27, p≤0.15), but this did not reach statistical significance (Table 2). Measures of femoral adiposity (i.e., leg fat mass and femoral SFA) were not related to gene expression in either abdominal or femoral SAT. Serum E2 was positively associated with the ratio of ESR1:ESR2 in femoral SAT (r=0.496, p<0.01) but not abdominal SAT (r=0.258, p=0.15). Leptin, which was highly correlated with all measures of adiposity (data not shown), was inversely related to ESR1 in femoral SAT (−0.294, p<0.05; Table 2).
Table 2.
Correlations of abdominal and femoral subcutaneous adipose tissue estrogen receptor alpha (ESR1) and estrogen receptor beta (ESR2) mRNA expression with age, fat mass, serum estradiol and leptin among pre-and postmenopausal women.
| ESR1 | ESR2 | ESR1:ESR2 | |||||
|---|---|---|---|---|---|---|---|
| Abdominal | Femoral | Abdominal | Femoral | Abdominal | Femoral | ||
| Pearson Correlations | |||||||
| Age | −0.380* | −0.353* | 0.014 | 0.137 | −0.463** | −0.472** | |
| %fat | −0.291† | −0.219† | −0.028 | −0.053 | −0.359* | −0.211† | |
| Total fat mass | −0.178 | −0.160 | −0.139 | −0.061 | −0.127 | −0.156 | |
| Trunk fat mass | −0.270† | −0.256† | −0.162 | −0.048 | −0.247† | −0.264† | |
| Leg fat mass | −0.007 | −0.004 | −0.078 | −0.078 | 0.082 | −0.029 | |
| VFA | −0.233† | −0.248† | −0.129 | −0.055 | −0.224 | −0.229† | |
| Abdominal SFA | −0.155 | −0.179 | −0.038 | −0.032 | −0.296† | −0.250† | |
| Femoral SFA | 0.022 | 0.030 | 0.130 | −0.038 | −0.071 | 0.042 | |
| Serum E2 | 0.155 | 0.148 | −0.011 | −0.123 | 0.258† | 0.496** | |
| Serum Leptin | −0.248† | −0.294* | −0.215 | −0.082 | −0.130 | −0.218† | |
| Partial Correlations | |||||||
| Age (adjusted for %fat) | −0.336* | −0.320* | −0.021 | 0.152 | −0.417** | −0.446** | |
| Age (adjusted for E2) | −0.361* | −0.327* | 0.010 | 0.085 | −0.398* | −0.285+ | |
| Age (adjusted for leptin) | −0.417** | −0.405** | −0.005 | 0.129 | −0.480** | −0.511** | |
p≤0.05;
p≤0.01;
p≤0.15;
VFA = visceral fat area; SFA = subcutaneous fat area; E2 = estradiol.
Age-related differences
Among all women ESR1 and ESR1:ESR2, but not ESR2, were inversely associated with age in abdominal and femoral SAT (Table 2). In this cohort, age was inversely correlated with serum E2 (r=−0.496, p<0.001), fat free mass (r=−0.475, p<0.01), and positively correlated with abdominal VFA (r=0.301, p<0.05; data not shown). The inverse association of ESR1 and ESR1:ESR2 with age remained after adjusting for trunk fat mass, but was no longer significant in femoral SAT after accounting for variability in E2 (Table 2). While leptin was not correlated with age or E2 in this cohort (data not shown), the inverse associations of ESR1 and ESR1:ESR2 with age were strengthened after adjusting for leptin (Table 2).
Discussion
The primary new finding of the study was that there were menopause-and age-related differences in mRNA expression of ERα and ERα:ERβ, but not ERβ, in abdominal and femoral SAT. The inverse association of ERα and ERα:ERβ with age were independent of t otal and regional adiposity. Serum E2 was lower with increasing age in this cohort, but this did not appear to account for the lower ERα and ERα:ERβ with age.
Important role of ERα:ERβ
The relative expression of ERα and ERβ in adipose tissue has significant clinical relevance to women’s health via their regulation of adipose tissue distribution and function [1]. Presumably, maintenance of metabolically healthy SAT along with an adequate balance of ERs allows for efficient storage of potentially toxic lipids away from other important insulin-sensitive organs (i.e. skeletal muscle or liver). Alterations of ERα and ERβ in adipose tissue appears to affect adipose tissue inflammation and fibrosis irrespective of circulating E2 [7] with broad implications for the development of cardiometabolic disease [11, 12]. Thus, determining whether adipose tissue ER expression changes with aging or menopause (i.e., estrogen deficiency) in women is essential to: 1) understanding the progression of cardiometabolic risk after menopause; and 2) the development of safe and effective estrogen-based hormone therapies to attenuate risk.
It has been more than two decades since ERs were identified in human SAT [13], yet little is known about how expression of ERs changes with age or hormone exposure. Both ERα and ERβ are present in SAT in men and women, and while there do not appear to be sex differences in the expression of ERα, ERβ expression is higher in women than men [2]. ERα appears to regulate SAT homeostasis via growth and proliferation of adipocytes, whereas ERβ appears to regulate the sex-specific distribution of SAT [14]. While ERα expression is typically higher than ERβ, ERβ modulates ERα, and thus cellular estrogen sensitivity, highlighting the importance of ERα:ERβ [15].
Age-or menopause-related changes in ER expression
Studies assessing SAT ER expression across menopause are limited and data are conflicting. One study observed no menopause-or age-related difference in gene expression of either ER isoform in abdominal SAT from Korean women, nor any correlations between ER expression and anthropometric measures [16]. Others found an increase in abdominal SAT ERβ, but not ERα, with menopause, resulting in similar changes in ERα:ERβ observed in our study [6]. Discrepancies among studies may be complicated by racial differences in adipose tissue or hormone changes across menopause [17, 18]. We are the first to observe an age-related difference in gene expression for ERα and ERα:ERβ in human SAT from pre-and postmenopausal women not using any type of hormone therapy. Consistent with this, a study in rodents revealed a robust up-regulation of ERβ expression with age, regardless of estrogen status induced by ovariectomy [19]. However, the older rats were fatter than the young rats, making it difficult to separate the effects of chronological age from increased adiposity. It is generally believed that an increased fat mass might help to maintain local tissue production of estrogens due to aromatization of androgens [20]. Though evidence for this has yet to be demonstrated in human subcutaneous adipose tissue, elevated local estrogen production could in theory preserve or change ER in adipose tissue with aging. In the present study, pre-and postmenopausal women were similar in total adiposity, minimizing this potential confounding effect. Nevertheless, we evaluated whether regional adiposity was a predictor of estrogen receptor gene expression.
Relative expression of ER among adipose tissue depots
ER gene expression and protein content may differ across various SAT depots, but the data are mixed. One study reported no difference in gene expression for ERα, but lower ERβ, in abdominal compared to gluteal SAT from overweight premenopausal women [21]. Whereas another reported higher ERaα and lower ERβ protein in abdominal compared to gluteal SAT from overweight and obese premenopausal women [5]. In both reports, the balance of ERα:ERβ was higher in the abdominal compared to gluteal SAT depot. In contrast, the present study suggested no difference in ERα:ERβ between abdominal and gluteal SAT depot (p=0.86; data not shown). Our data are partially supported by an in vitro study [2] reporting that the gene expression for ERα and ERβ did not differ in mature adipocytes collected from abdominal subcutaneous and visceral adipose tissue. Ad ditional human studies are needed to confirm whether there are depot-specific differences in ER expression or the balance of ERα:ERβ.
Leptin and ER expression with age
Leptin is produced by adipocytes in response to AT expansion. Estrogen modulates leptin production and sensitivity to other organs in rodents and humans [22, 23]. Adjusting for leptin strengthened our observed inverse associations of ERα and ERα:ERβ with age, suggesting there may be bidirectional communication between leptin and ER in human SAT. This is partially supported by in vitro studies suggesting that stimulation of ERα increases leptin production, while stimulation of ERβ decreases it, in 3T3-L1 mature adipocytes [24]. Future studies are needed to further investigate the impact of circulating leptin on adipose tissue ER.
Study limitations
There are some limitations to the study that should be considered. First, the participants recruited in the study were non-obese and both groups had a similar BMI and adiposity. Increases in adiposity often occur with menopause; thus our outcomes may not be generalizable to women who become obese during the menopause transition. However, studying non-obese pre-and postmenopausal women matched for adiposity allowed us to investigate the effect of menopause and age independent of increased adiposity. Second, we did not collect muscle in this study. Although we recruited pre-and post-menopausal women having a similar adiposity, fat-free mass was lower in postmenopausal women. Future studies are needed to determine whether this loss of lean mass is accompanied by changes in muscle ER expression with age or menopause. Third, the present study only assessed gene expression. It will be important to measure protein content to further interrogate changes in ERs with age and menopause. Fourth, the present study used whole adipose tissues for mRNA analysis. There is a possibility that the cellular composition of the tissue (i.e. the proportion of adipocytes and stromal cells) varied by age or menopausal status, which may have influenced our results. Fifth, there may be differences in the association between ER and adipo sity within the pre-and postmenopausal groups that cannot be evaluated due to the limited sample size; larger studies are warranted. Lastly, postmenopausal women in the current study were relatively early postmenopausal (∼7 years since menopause on average). Future studies should include women who are more years past menopause to investigate whether duration of estrogen deficiency (i.e. time since menopause) further contributes to age-related declines in ER expression.
Conclusion
Among pre-and post-menopausal women, increasing age was associated with decreasing gene expression for ERα and ERα:ERβ in abdominal and femoral subcutaneous adipose tissue. This inverse correlation between ER and age was independent of adiposity and serum estradiol.
Highlights.
Menopause-related decrease in mRNA expression of ERα and ERα:ERβ in adipose tissue
Increasing age was associated with decreasing gene expression for ERα and ERα:ERβ
Inverse correlation between ER and age independent of adiposity and serum estradiol
Acknowledgments
The authors wish to thank the staffs of the University of Colorado Anschutz Medical Campus Clinical and Translational Research Center (CTRC), Department of Radiology, and Energy Balance Core of the Nutrition and Obesity Research Unit (NORC) for their assistanc e in conducting this study. The authors would also like to thank the members of their research group for carrying out the day-to-day activities of the project and the study volunteers for their time and efforts. All authors contributed extensively to the work presented in this paper. The following awards from the National Institutes of Health supported this research: R01 DK077992, R01 DK088105, P50 HD073063, T32 DK007446, P30 DK048520, UL1 TR000154
Funding source: NIH R01 DK077992, R01 DK088105, P50 HD073063, T32 DK007446, P30 DK048520, UL1 TR000154
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: None
Competing Interests:
None of the authors have any competing interests.
References
- 1.Hevener AL, Clegg DJ, Mauvais-Jarvis F. Impaired estrogen receptor action in the pathogenesis of the metabolic syndrome. Mol Cell Endocrinol. 2015;418(Pt 3):306–21. doi: 10.1016/j.mce.2015.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dieudonne MN, et al. Evidence for functional estrogen receptors alpha and beta in human adipose cells: regional specificities and regulation by estrogens. Am J Physiol Cell Physiol. 2004;286(3):C655–61. doi: 10.1152/ajpcell.00321.2003. [DOI] [PubMed] [Google Scholar]
- 3.Barros RP, Gustafsson JA. Estrogen receptors and the metabolic network. Cell Metabolism. 2011;14(3):289–99. doi: 10.1016/j.cmet.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 4.Wend K, Wend P, Krum SA. Tissue-Specific Effects of Loss of Estrogen during Menopause and Aging. Front Endocrinol (Lausanne) 2012;3:19. doi: 10.3389/fendo.2012.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gavin KM, Cooper EE, Hickner RC. Estrogen receptor protein content is different in abdominal than gluteal subcutaneous adipose tissue of overweight-to-obese premenopausal women. Metabolism: Clinical and Experimental. 2013;62(8):1180–8. doi: 10.1016/j.metabol.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 6.Mclnnes KJ, et al. Association of 11beta-hydroxysteroid dehydrogenase type I expression and activity with estrogen receptor beta in adipose tissue from postmenopausal women. Menopause. 2012;19(12):1347–52. doi: 10.1097/gme.0b013e318258aad7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davis KE, et al. The sexually dimorphic role of adipose and adipocyte estrogen receptors in modulating adipose tissue expansion, inflammation, and fibrosis. Mol Metab. 2013;2(3):227–42. doi: 10.1016/j.molmet.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bessesen DH, et al. Postprandial triglycerides and adipose tissue storage of dietary fatty acids: Impact of menopause and estradiol. Obesity (Silver Spring) 2015;23(1):145–53. doi: 10.1002/oby.20935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Pelt RE, et al. Lower-body adiposity and metabolic protection in postmenopausal women. J Clin Endocrinol Metab. 2005;90(8):4573–8. doi: 10.1210/jc.2004-1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coleman SR. Structural fat grafts: the ideal filler? Clin Plast Surg. 2001;28(1):111–9. [PubMed] [Google Scholar]
- 11.Mauvais-Jarvis F, Clegg DJ, Hevener AL. The role of estrogens in control of energy balance and glucose homeostasis. Endocrine reviews. 2013;34(3):309–38. doi: 10.1210/er.2012-1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rutkowski JM, Stern JH, Scherer PE. The cell biology of fat expansion. The Journal of cell biology. 2015;208(5):501–12. doi: 10.1083/jcb.201409063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pedersen SB, et al. Identification of oestrogen receptors and oestrogen receptor mRNA in human adipose tissue. Eur J Clin Invest. 1996;26(4):262–269. doi: 10.1046/j.1365-2362.1996.145278.x. [DOI] [PubMed] [Google Scholar]
- 14.Pallottini V, et al. Estrogen regulation of adipose tissue functions: involvement of estrogen receptor isoforms. Infectious disorders drug targets. 2008;8(1):52–60. doi: 10.2174/187152608784139631. [DOI] [PubMed] [Google Scholar]
- 15.Hall JM, McDonnell DP. The estrogen receptor beta-isoform (ERbeta) of the human estrogen receptor modulates ERalpha transcriptional activity and is a key regulator of the cellular response to estrogens and antiestrogens. Endocrinology. 1999;140(12):5566–78. doi: 10.1210/endo.140.12.7179. [DOI] [PubMed] [Google Scholar]
- 16.Shin JH, et al. The ratio of estrogen receptor alpha to estrogen receptor beta in adipose tissue is associated with leptin production and obesity. Steroids. 2007;72(6–7):592–9. doi: 10.1016/j.steroids.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 17.Gallagher D, et al. Adipose tissue distribution is different in type 2 diabetes. The American journal of clinical nutrition. 2009;89(3):807–14. doi: 10.3945/ajcn.2008.26955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hill JO, et al. Racial differences in amounts of visceral adipose tissue in young adults: the CARDIA (Coronary Artery Risk Development in Young Adults) study. The American journal of clinical nutrition. 1999;69(3):381–7. doi: 10.1093/ajcn/69.3.381. [DOI] [PubMed] [Google Scholar]
- 19.Tomicek NJ, Lancaster TS, Korzick DH. Increased estrogen receptor beta in adipose tissue is associated with increased intracellular and reduced circulating adiponectin protein levels in aged female rats. Gender medicine. 2011;8(5):325–33. doi: 10.1016/j.genm.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barakat R, et al. Extra-gonadal sites of estrogen biosynthesis and function. BMB Rep. 2016;49(9):488–96. doi: 10.5483/BMBRep.2016.49.9.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pedersen SB, et al. Demonstration of estrogen receptor subtypes alpha and beta in human adipose tissue: influences of adipose cell differentiation and fat depot localization. Molecular and Cellular Endocrinology. 2001;182(1):27–37. doi: 10.1016/s0303-7207(01)00557-3. [DOI] [PubMed] [Google Scholar]
- 22.Shimizu H, et al. Estrogen increases in vivo leptin production in rats and human subjects. The Journal of endocrinology. 1997;154(2):285–92. doi: 10.1677/joe.0.1540285. [DOI] [PubMed] [Google Scholar]
- 23.Clegg DJ, et al. Gonadal hormones determine sensitivity to central leptin and insulin. Diabetes. 2006;55(4):978–87. doi: 10.2337/diabetes.55.04.06.db05-1339. [DOI] [PubMed] [Google Scholar]
- 24.Yi KW, et al. Role of estrogen receptor-alpha and -beta in regulating leptin expression in 3T3-L1 adipocytes. Obesity. 2008;16(11):2393–9. doi: 10.1038/oby.2008.389. [DOI] [PubMed] [Google Scholar]


