Abstract
Purpose of Review
Educate the reader of the multiple roles undertaken by the human epidermal lipidome and the experimental techniques of measuring them.
Recent Findings
Damage to skin elicits a wound healing process that is capped by the recreation of the lipid barrier. In addition to barrier function, lipids also undertake an active signaling role during wound healing. Achievement of these multiple functions necessitates a significant complexity and diversity in the lipidome resulting in a composition that is unique to the human skin. As such, any attempts to delineate the function of the lipidome during the wound healing process in humans need to be addressed via studies undertaken in humans.
Summary
The human cutaneous lipidome is unique and play a functionally significant role in maintaining barrier and regulating wound healing. Modern mass spectrometry and Raman spectroscopy based methods enable the investigation epidermal lipidome with respect to those functions.
Keywords: wound healing, bioactive lipids, analytical methods, sampling techniques, human
Introduction
Skin, the largest organ in the body, is protected by a continuous barrier of lipids that face the external environment. These lipids are a combination of those secreted by the sebaceous glands as well as those generated by the cells of the stratum corneum. The composition and distribution of lipids on the skin is unique to humans. Furthermore, skin lipids are often unique compared to lipids of internal tissues. In fact, the two key words used to characterize the human skin lipidome is “complexity and perversity”, where complexity is manifested by a large number of diverse lipid species and perversity is demonstrated by the uniqueness of skin lipids [1]. This is exampled by the fact that skin lipids contain significant amounts of both odd chain and branch chained lipids, a feature that is unique to the skin lipidome [1]. While there is significant spatial variability in the same person, the lipidome is also significantly altered during different stages of growth [2] and is also affected by environmental factors such as the different seasons, the skin microbiome and exposure to the elements [3–6]. Furthermore, significant variations exist between the skin lipidome of the different ethnic groups such as the Asian, African American and Caucasians [3]. This particular compositional spatial and temporal distribution of the lipidome found among humans are highly unique in the animal kingdom and cannot be replicated faithfully in any of the model organisms available for study. As such any studies that investigate the human dermal lipidome necessitates that those studies be undertaken using humans themselves as the model of choice. However, this requirement needs to be balanced with the potential for discomfort to subjects and minimizing invasive procedures wherever possible. In this regards, techniques such as micro sampling demonstrate significant advantages in the application towards studies investigating the human dermal lipidome. Furthermore, until recently, such detailed investigations of the human skin lipidome have not been feasible due to technological limitations. Technological advances in modern mass spectrometry based analytical methods has enabled micro sampling methods containing very small amounts of material to be used in the investigation of the variations in the human dermal lipidome during the wound healing process. Using these methods, as well as traditional techniques, a significant body of information has been derived with respect to the human skin lipidome.
Skin Surface Lipids
The primary sources of the human epidermal lipidome are the sebum and the cells of the stratum corneum. Triglycerides, free fatty acids, wax esters, squalene cholesterol esters and cholesterol constitute the primary human dermal lipids that are synthesized by the sebaceous glands [7]. The human sebum lipidome is especially unique in the fact some of the lipids such as squalene and wax esters are only found in the sebum secretions and nowhere else. Furthermore, the sebum derived skin lipidome is also unique in the presence of odd chain length and branched chain free fatty acids. An additional unique feature in the sebum derived human skin lipidome is the presence of Δ6 desaturase derived free fatty acids, namely sapinic acid [16:1 Δ6] which constitute almost 25% of the total fatty acids and demonstrate a significant level of antimicrobial activity [8, 9]. Additional elongation and desaturation also gives rise to unique sebaleic acid [18:2 Δ5,8] which has recently been described as being important in neutrophil recruitment following transcellular conversion to 5-oxo-[6E,8Z]-octadecadienoic acid [10]. The primary functions of the human sebum lipidome include photoprotection, antimicrobial activity [e.g. sapinic acid], and delivery of fat soluble antioxidants to the skin surface as well as lipid specific pro and anti-inflammatory activity [7]. Alterations to the sebum lipidome has been implicated in multiple dermal human dermal health complications including acne, asteatosis, comedone, furuncles, comedones, carbuncles, sebaceous hyperplasia, seborrhea, seborrheic dermatitis and steatomas [7, 11]. As such detailed investigation into changes of the sebum lipidome associated with such disease states has the potential towards novel treatment options that specifically target the lipid balance of the sebum derived skin lipidome.
Stratum Corneum Lipids
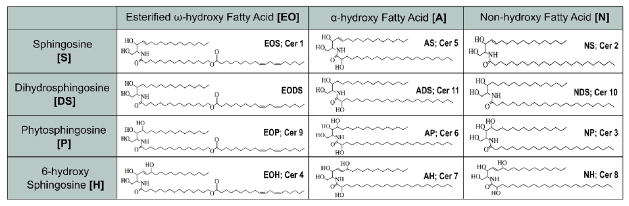
The second and equally important source of the skin lipidome is derived from the stratum corneum [SC] and is primarily involved in the maintenance of epidermal permeability barrier and prevention of trans epidermal water loss [TEWL] [12–14]. These SC derived lipids are primarily composed of ceramides [50% by mass] with the remainder made up of free fatty acids and cholesterol [14–16]. These lipids fill in the gaps between the spaces of the keratinocytes in a “brick and mortar” type of structure where the dead and terminally differentiated keratinocytes acting as the bricks and the SC derived lipids act as the mortar [16]. As in the case of the sebum lipidome, human SC lipidome is unique in that a significant fraction of this lipidome is only found on the skin. In this regards, SC barrier lipidome composed of 4 different sphingoid bases N-acylated to 3 different fatty acyls creating a combination of 12 classes of ceramides [numbered CER1-12]. The sphingoid bases are primarily composed of 18 carbons and include sphingosine, sphingenine, phytosphingosine and 4-hydroxy-sphingosine (Table 1). The fatty acyls include non-hydroxy, omega-hydroxy and esterified-Omega hydroxyl fatty acids (Table 1). A majority of the sphingoid bases demonstrated antibacterial activity [8].
Table 1.
Human skin ceramide structural variants identified to date. The different structural isomers of ceramides are depicted together with their commonly used names demonstrating the diversity of the human skein ceramides.
Signaling Lipids
In addition to the structural functions performed by the skin lipidome, a significant amount of signaling events are also mediated by the lipidome. We have demonstrated the importance of the sphingolipid ceramide-1-phosphate in the migration and proliferation of skin fibroblasts and demonstrated that this lipid species follow a temporal change during cutaneous wound healing in humans [17]. Additional studies by us and others have demonstrated the importance of eicosanoids in the mediating the signaling events during the wound healing process [17, 18]. Furthermore, we and others have demonstrated roles for sphingosine-1-phosphate in many of the aspects of wound healing biology [19]. Other lipids of relevance to the human dermal wound healing process include sphingophosphorylcholine [20], lysophosphatidic acid [21], protectins and resolvins [22].
Diseases Associated with Dysregulated Skin Lipid Metabolism
Dermatological research undertaken in the past few years have demonstrated a major role for the human dermal lipidome in the pathological conditions of the skin. In this regards, the role of ceramide metabolism with respect to atopic dermatitis is the most intensively investigated [15, 23–26]. For example, Yamato et al demonstrated that while the relative amounts of the major stratum corneum lipids remained unchanged, significant changes were observed within the ceramide class and the squalene, wax esters and triglycerides [24]. For example, the proportions of ceramides were demonstrated to be lower in the AD patients compared to their controls [24]. On the other hand, the sebaceous lipids were observed to be elevated in the AD patients compared to their controls [24]. The decrease in the ceramide content of the SC of the AD patients can be explained in part by the increased expression of sphingomyelin/glucosylceramide deacylase in the SC of AD patients [27]. This enzyme was demonstrated to hydrolyze sphingomyelin and glucosyl ceramides at the acyl site to liberate sphingophosphoroylcholine and glucosyl sphingosine which in turn lead to decreased production of SC ceramides [27]. In addition to the abnormalities in ceramides, significant changes were also observed in the cell membrane phospholipids in the epidermis of AD patients compared to their controls with a significant reduction observed in the phospholipid content of the epidermis of the AD patient [25]. These published studies demonstrate a close relationship between human skin lipid metabolism and AD. Psoriasis is another skin disorder that is due in part to lipid dysbiosis of the SC. In this regards, the generation of an abnormal skin ceramide composition leads to a disruption in the skin barrier function and elevated trans epithelial water loss [TEWL][12]. While the total ceramide content was demonstrated to remain unchanged, long chain ceramides containing ester linked fatty acids and those containing phytosphingoid backbones were demonstrated as being lower in psoriatic skin compared to normal skin [28]. These changes are attributed at least in part to decreased expression of the sphingolipid activation protein saposin [29, 30] which is a non-enzymatic component required for the hydrolysis of glucosyl ceramides. An inherited lipid related genetic disorder that leads to skin disease via dysregulated lipid metabolism is Gaucher disease. The disease is caused by a decrease in β-glucocerebrosidase and varies in clinical severity from asymptomatic to severe [31]. The decreased incidence of this enzyme manifests as an increase in the glucosyl ceramide and a decrease in the ceramide content [32] with a concomitant increase in the epidermal barrier function. Dry skin or Xerosis that often end up impairing barrier function is characterized by a deficiency primarily in the 6-hydroxy and 4-hydroxy backbone containing ceramides [33]. The fact that there is seasonal variation in skin ceramide content and the incidence in increases Xerosis in cooler seasons have been causally linked to each other [2]. Finally the most common of skin lipid mediated disorders would be acne. Alterations to the sebum lipidome has been heavily implicated in the outbreak of acne [11, 34–36]. Specifically, altered ratios between saturated and unsaturated fatty acids as well as altered amounts of specific fatty acids such as linoleic acid and the formation of squalene peroxides have all been linked to outbreaks of acne [37].
Pharmaceutical Modulation of the Skin Lipidome
Considering the primary roles played by the lipidome in the structure and function of the human skin, development of lipid formulations for both therapeutic and cosmetic purposes is a highly active pharmaceutical industry [38, 39]. As such, several formulations include the use of ceramides or their precursors. Primary among those are formulations containing hydroxypalmitoyl sphingenine [Cetaphil, RestoraDerm Skin restoring Moisturizer], ceramide 1, ceramide 3 [Eucerin, CeraVe] and pseudo ceramides. Additionally, lipid blends such as epicerum [13] consisting of a blend of ceramides, cholesterols and free fatty acids are also used as cosmetic and barrier repair agents.
Sampling Methods for the Investigation of the Skin Lipidome
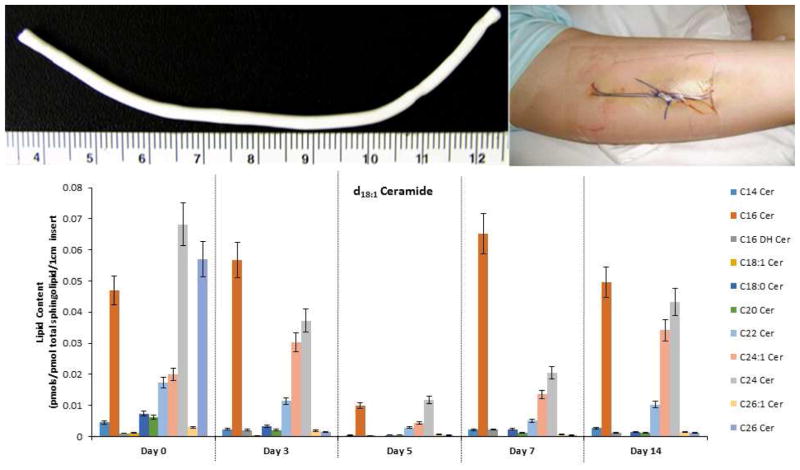
In order to explore different lipid components involved in human skin wound healing a variety of skin sampling techniques have been developed. The least invasive technique is called tape stripping [40]. One such process utilizes D-Squame® tape (CuDerm Corporation, Dallas, TX, USA) where the adhesive discs are applied to the skin using a set force and then ten or more successively samples are removed. The attached cells and lipid material on the disc are placed in an appropriate lipid extraction buffer followed by analysis of the lipids of interest [41]. Wound dressings are also a rich source of sampling material for research analyses of human wounds especially with respect to the signaling lipidome. A recent review by Widgerow et al., describes the usefulness of collecting wound fluids under film dressings as well as analyzing components obtained from the discarded primary dressing [42]. This technique is especially useful for the study and progression of the healing of human chronic and burn wounds. The wound fluids and contents of the dressing can be extracted and the specific target analyte can be isolated and analyzed. Another useful technique is the suction blister model of wound healing to obtain interstitial fluid as well as the epithelial “roof” layer for analyses [18]. One useful instrument to create suction blisters on the inner forearm is the Negative Pressure Instrument [Electronic Diversities, Finksburg MD]. Alternatively a simple chamber attached to a standard vacuum pump can be used as well [350 mmHg for 1 to 2 hours]. The procedure is relatively painless and does not leave a scar. Creation of partial-thickness skin wounds using a variety of dermatomes has also been used to study human skin reepithelization [43]. The “donor site” type of wound can be photographed over time to quantify the process of reepithelization with or without the topical application of test materials. The wound surface can also be sampled using a sterile swab or Whatman filter paper and then analytes can be isolated and analyzed [44]. Punch biopsy is the classical procedure to obtain epidermal and dermal tissues for analyses. The recent review by Yang and Kampp provides a complete “how to” procedure to obtain skin biopsies for research and analyses [45]. The various layers in the full thickness specimen can be isolated and analyzed by histology as well as specific biochemical techniques to quantify proteins and bioactive lipids. In addition, the open wound created by the tissue punch can be covered with an occlusive dressing such as Opsite® (Smith and Nephew, Fort Worth, TX) and the wound fluid can be collected over time for analyses [46]. Furthermore, the process of wound contraction can be measured by using standardized photography and image analysis. One of the more recent and highly valuable techniques to study human wound healing has been the development of implantable and retrievable high-porosity Polytetrafluoroethylene tubes (012-01-2 PTFE; International Polymer Engineering, Tempe AZ) [47–51]. Typically the PTFE tubes can be implanted using an 18 g, 3.5 inch spinal needle in an anesthetized area in the inner aspect of the subject’s upper arm [52]. The implants can be retrieved at sequential times such as 3, 5, 7 and 14 days after implantation and cut segments can be analyzed with respect to variations in the lipidome during the wound healing process (Figure 1). Using this technique, we have demonstrated that the sphingolipid ceramide-1-phosphate has a distinct variation during the human wound healing process and that it is likely acting as a master switch for the regulation eicosanoid signaling [17]. Additional and concurrent information can be gained by processing sections for histology, immuno-staining, matrix content, and with the use of an entropy-based automatic image analyzer system, specific cells and collagen deposition can also be quantified [53] as well as other signaling proteins that take part in the wound healing process [52].
Figure 1. Alteration in the NS (Cer 2) ceramide profile during wound healing in humans.
Lipids were extracted from a 1cm portion of PTFE implants inserted into the upper arm of healthy volunteers and removed on days 3, 5, 7 and 14. A 100 mm skin punch biopsy was used as the baseline (day 0). Lipids were extracted from those samples and subjected to targeted analysis via LC tandem mass spectrometry. The data shown is the average lipid content from 7 volunteers (n=7) normalized to total sphingolipids ± SD. The lipid content is depicted in pmol specific lipid/pmol total sphingolipids found in 1cm of PTFE insert.
Qualitative and Quantitative Analysis of the Human Skin Lipidome
Considering the fact that the lipidome is integrally involved in the function of the skin, its ability to heal and its various pathologies, the ability to quantitatively investigate its changes is highly describable. Furthermore, a majority of cosmetic products attempt to modulate the skin lipidome, and yet have ill-defined lipid compositions and is also worth investigating with respect to their claims in active lipid content [33, 39]. A significant body of information with respect to the quantitation of the skin lipidome has been obtained via analytical studies utilizing thin layer chromatography (TLC) [27, 28, 54, 54–59]. While TLC provides an affordable and low technology barrier method for analyzing the skin lipidome, it suffers from the inability to quantify individual lipid species. Furthermore the sensitivity of TLC towards determining the composition of the skin lipidome is also quite limited. Other analytical methods have been used over the years to obtain a more comprehensive understanding of the skin lipidome. These include p-nitrobenzoyl derivatization of skin ceramides followed by high-performance liquid chromatography with UV detection (HPLC-UV) [60] as well as gas chromatography coupled to mass spectrometry (GC-MS) [61, 61–63]. While better at quantitation of individual lipid species compared to TLC, these methods still suffer from limitations with respect to quantitatively capturing the full diversity of the skin lipidome. The most current technology for the analysis of the skin lipidome is atmospheric pressure ionization tandem mass spectrometry coupled to ultra-high performance liquid chromatography (UPLC API-MS/MS). Utilizing these methods, the human skin lipidome is currently being characterized extensively in many laboratories including ours [6, 17, 18, 56, 57, 64, 65, 65–70]. These studies have demonstrated unique changes in the skin lipidome during wound healing [17, 18, 67–70]. Application of such UPLC ESI-MS/MS methods have enabled our group to identify lipids that stimulate fibroblast growth in the presence of chronic wound fluid, a key requirement for cutaneous wound healing [19]. While, UPLC API-MS/MS methods are ideally suited for determination of the overall composition of the skin lipidome, these methods are not easily amenable for the investigation of the variations in the microscopic spatial distribution of the skin lipids. The most informative technique for determining the surface distribution of lipids of the skin surface is matrix associated laser desorption ionization mass spectrometry (MALDI-MS/MS) [71, 72]. While excellent in determining the spatial distribution of skin lipids, this method suffers from the primary drawback of being unable to distinguish between many of the isobaric lipid species and being limited in sensitivity for low abundant and low ionizing lipids. Furthermore, the method is only applicable for the investigation of excised samples. Techniques that enable the investigation of the spatial distribution of the human skin lipidome in vivo has great value in point of care diagnostics. In this regards, Raman Spectroscopy based methods have demonstrated great promise and have been demonstrated to be applicable for in vivo investigation of the human skin lipidome [73–75]. In summary, considering the diversity and variability of the human skin lipidome, a single analytical technique is insufficient to obtain a comprehensive understanding. A combination of methods utilizing UPLC ESI-MS/MS, MALDI MS/MS and Raman spectroscopy is needed for the most comprehensive understanding of the variations in the human skin lipidome with respect to cutaneous wound healing and other lipid related skin pathologies.
Conclusions
The bioactive lipids in the skin provide a critical function in protecting the skin and come into play when the skin is damaged to facilitate the repair process. Because of the uniqueness and complexity of the human skin lipidome, it has not been possible to investigate it using animal and cell culture model systems. Now with technological advances employing micro sampling plus the development of advanced analytical instrumentation we can now extensively explore the skin lipidome in humans. These new avenues of research are enabling for a more in-depth understanding of the skin bioactive lipids and foster the possibilities for new translational research to help develop broader and multi modal therapeutic strategies to treat skin disorder and repair.
Acknowledgments
Research reported in this publication was supported by research grants from National Institutes of Health under grant numbers HD087198 (DSW). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Additionally, this work was also supported by a Career Development Award 1 (to DSW) and via a Young Investigator Award from SCIEX for clinical lipidomic research (DSW). The project was also supported by CTSA award number UL1TR000058 from the National Center for Advancing Translational Sciences which provides tuition and stipend funds to UOW. Lastly, services and products in support of the research project were generated by the VCU Massey Cancer Center Lipidomics Shared Resource [Developing Core], supported, in part, with funding from NIH-NCI Cancer Center Support Grant P30 CA016059 as well as a shared resource grant [S10RR031535] from the National Institutes of Health. The contents of this manuscript do not represent the views of the Department of Veterans Affairs, National Center for Advancing Translational Sciences or the National Institutes of Health, the National Institute of Health nor the United States Government. We graciously acknowledge the generosity of Dr. Chalfant in allowing us to use the ceramide data generated by us and depicted in Figure 1.
Footnotes
Conflict of Interest
Dayanjan S Wijesinghe, Urszula Osinska Warncke, and Robert F. Diegelmann declare that they have no conflicts of interest.
Compliance with Ethics Guidelines
Human and Animal Rights and Informed Consent
This article does not contain any studies with animal subjects performed by any of the authors. All human studies were carried out under the approval of the Institutional Review Board (IRB) of VCU-School of Medicine (IRB number 11087) and written informed consent was obtained from all participants.
References
Recently published papers of particular interest have been highlighted as:
• Of importance
- 1.Nicolaides N. Skin lipids: their biochemical uniqueness. Science. 1974;186:19–26. doi: 10.1126/science.186.4158.19. [DOI] [PubMed] [Google Scholar]
- 2.Rogers J, Harding C, Mayo A, Banks J, Rawlings A. Stratum corneum lipids: the effect of ageing and the seasons. Arch Dermatol Res. 1996;288:765–770. doi: 10.1007/BF02505294. [DOI] [PubMed] [Google Scholar]
- 3.Muizzuddin N, Hellemans L, Van Overloop L, Corstjens H, Declercq L, Maes D. Structural and functional differences in barrier properties of African American, Caucasian and East Asian skin. J Dermatol Sci. 2010;59:123–128. doi: 10.1016/j.jdermsci.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 4•.Pappas A, Fantasia J, Chen T. Age and ethnic variations in sebaceous lipids. Dermatoendocrinol. 2013;5:319–324. doi: 10.4161/derm.25366. Demonstrates the variability of the skin surface lipidome among humans. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lampe MA, Burlingame AL, Whitney J, Williams ML, Brown BE, Roitman E, Elias PM. Human stratum corneum lipids: characterization and regional variations. J Lipid Res. 1983;24:120–130. [PubMed] [Google Scholar]
- 6.Tingstad JE, Wurster DE, Higuchi T. Investigation of human skin lipids II. J Am Pharm Assoc Sci Ed. 1958;47:192–193. doi: 10.1002/jps.3030470313. [DOI] [PubMed] [Google Scholar]
- 7.Picardo M, Ottaviani M, Camera E, Mastrofrancesco A. Sebaceous gland lipids. Dermatoendocrinol. 2009;1:68–71. doi: 10.4161/derm.1.2.8472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fischer CL, Drake DR, Dawson DV, Blanchette DR, Brogden KA, Wertz PW. Antibacterial Activity of Sphingoid Bases and Fatty Acids against Gram-Positive and Gram-Negative Bacteria. Antimicrob Agents Chemother. 2012;56:1157–1161. doi: 10.1128/AAC.05151-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drake DR, Brogden KA, Dawson DV, Wertz PW. Thematic Review Series: Skin Lipids. Antimicrobial lipids at the skin surface. J Lipid Res. 2008;49:4–11. doi: 10.1194/jlr.R700016-JLR200. [DOI] [PubMed] [Google Scholar]
- 10.Cossette C, Patel P, Anumolu JR, Sivendran S, Lee GJ, Gravel S, Graham FD, Lesimple A, Mamer OA, Rokach J, Powell WS. Human Neutrophils Convert the Sebum-derived Polyunsaturated Fatty Acid Sebaleic Acid to a Potent Granulocyte Chemoattractant. J Biol Chem. 2008;283:11234–11243. doi: 10.1074/jbc.M709531200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith KR, Thiboutot DM. Thematic review series: Skin Lipids. Sebaceous gland lipids: friend or foe? J Lipid Res. 2008;49:271–281. doi: 10.1194/jlr.R700015-JLR200. [DOI] [PubMed] [Google Scholar]
- 12.Motta S, Monti M, Sesana S, Mellesi L, Ghidoni R, Caputo R. Abnormality of water barrier function in psoriasis: Role of ceramide fractions. Arch Dermatol. 1994;130:452–456. [PubMed] [Google Scholar]
- 13.Hon KL, Leung AKC, Barankin B. Barrier Repair Therapy in Atopic Dermatitis: An Overview. Am J Clin Dermatol. 2013;14:389–399. doi: 10.1007/s40257-013-0033-9. [DOI] [PubMed] [Google Scholar]
- 14.Mizutani Y, Mitsutake S, Tsuji K, Kihara A, Igarashi Y. Ceramide biosynthesis in keratinocyte and its role in skin function. Biochimie. 2009;91:784–790. doi: 10.1016/j.biochi.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 15.Di Nardo A, Wertz P, Giannetti A, Seidenari S. Ceramide and cholesterol composition of the skin of patients with atopic dermatitis. Acta Derm Venereol. 1998;78:27–30. doi: 10.1080/00015559850135788. [DOI] [PubMed] [Google Scholar]
- 16.Elias PM. Epidermal lipids, barrier function, and desquamation. J Invest Dermatol. 1983;80:44s–9s. doi: 10.1038/jid.1983.12. [DOI] [PubMed] [Google Scholar]
- 17.Wijesinghe DS, Brentnall M, Mietla JA, Hoeferlin LA, Diegelmann RF, Boise LH, Chalfant CE. Ceramide kinase is required for a normal eicosanoid response and the subsequent orderly migration of fibroblasts. J Lipid Res. 2014;55:1298–1309. doi: 10.1194/jlr.M048207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18•.Nicolaou A. Eicosanoids in skin inflammation. Prostaglandins Leukot Essent Fat Acids PLEFA. 2013;88:131–138. doi: 10.1016/j.plefa.2012.03.009. Provides an excellent description of how eicosanoids are involved in the regulation of skin inflammation. [DOI] [PubMed] [Google Scholar]
- 19.Hoeferlin LA, Huynh QK, Mietla JA, Sell SA, Tucker J, Chalfant CE, Wijesinghe DS. The Lipid Portion of Activated Platelet-Rich Plasma Significantly Contributes to Its Wound Healing Properties. Adv Wound Care. 2015;4:100–109. doi: 10.1089/wound.2014.0589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun L, Xu L, Henry FA, Spiegel S, Nielsen TB. A New Wound Healing Agent—Sphingosylphosphorylcholine. J Invest Dermatol. 1996;106:232–237. doi: 10.1111/1523-1747.ep12340570. [DOI] [PubMed] [Google Scholar]
- 21.Xu K-P, Yin J, Yu F-SX. Lysophosphatidic Acid Promoting Corneal Epithelial Wound Healing by Transactivation of Epidermal Growth Factor Receptor. Invest Ophthalmol Vis Sci. 2007;48:636–643. doi: 10.1167/iovs.06-0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hellmann J, Tang Y, Spite M. Pro-resolving lipid mediators and diabetic wound healing. Curr Opin Endocrinol Diabetes Obes. 2012;19:104–108. doi: 10.1097/MED.0b013e3283514e00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ishikawa J, Narita H, Kondo N, Hotta M, Takagi Y, Masukawa Y, Kitahara T, Takema Y, Koyano S, Yamazaki S, Hatamochi A. Changes in the Ceramide Profile of Atopic Dermatitis Patients. J Invest Dermatol. 2010;130:2511–2514. doi: 10.1038/jid.2010.161. [DOI] [PubMed] [Google Scholar]
- 24.Yamamoto A, Serizawa S, Ito M, Sato Y. Stratum corneum lipid abnormalities in atopic dermatitis. Arch Dermatol Res. 1991;283:219–223. doi: 10.1007/BF01106105. [DOI] [PubMed] [Google Scholar]
- 25.Schäfer L, Kragballe K. Abnormalities in Epidermal Lipid Metabolism in Patients with Atopic Dermatitis. J Invest Dermatol. 1991;96:10–15. doi: 10.1111/1523-1747.ep12514648. [DOI] [PubMed] [Google Scholar]
- 26.Kleuser B, Japtok L. Sphingolipids and inflammatory diseases of the skin. Handb Exp Pharmacol. 2013 doi: 10.1007/978-3-7091-1511-4_18. [DOI] [PubMed] [Google Scholar]
- 27.Higuchi K, Hara J, Okamoto R, Kawashima M, Imokawa G. The skin of atopic dermatitis patients contains a novel enzyme, glucosylceramide sphingomyelin deacylase, which cleaves the N-acyl linkage of sphingomyelin and glucosylceramide. Biochem J. 2000;350:747–756. [PMC free article] [PubMed] [Google Scholar]
- 28.Motta S, Monti M, Sesana S, Caputo R, Carelli S, Ghidoni R. Ceramide composition of the psoriatic scale. Biochim Biophys Acta BBA - Mol Basis Dis. 1993;1182:147–151. doi: 10.1016/0925-4439(93)90135-n. [DOI] [PubMed] [Google Scholar]
- 29.Alessandrini F, Stachowitz S, Ring J, Behrendt H. The level of prosaposin is decreased in the skin of patients with psoriasis vulgaris. J Invest Dermatol. 2001;116:394–400. doi: 10.1046/j.1523-1747.2001.01283.x. [DOI] [PubMed] [Google Scholar]
- 30.Doering T, Holleran WM, Potratz A, Vielhaber G, Elias PM, Suzuki K, Sandhoff K. Sphingolipid Activator Proteins Are Required for Epidermal Permeability Barrier Formation. J Biol Chem. 1999;274:11038–11045. doi: 10.1074/jbc.274.16.11038. [DOI] [PubMed] [Google Scholar]
- 31.Grabowski GA. Gaucher disease and other storage disorders. ASH Educ Program Book. 2012;2012:13–18. doi: 10.1182/asheducation-2012.1.13. [DOI] [PubMed] [Google Scholar]
- 32.Holleran WM, Ginns EI, Menon GK, Grundmann JU, Fartasch M, McKinney CE, Elias PM, Sidransky E. Consequences of beta-glucocerebrosidase deficiency in epidermis. Ultrastructure and permeability barrier alterations in Gaucher disease. J Clin Invest. 1994;93:1756–1764. doi: 10.1172/JCI117160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Castiel-Higounenc I, Chopart M, Ferraris C. Stratum corneum lipids: specificity, role, deficiencies and modulation. Ol Corps Gras Lipides. 2004;11:401–406. [Google Scholar]
- 34.Mourelatos K, Eady EA, Cunliffe WJ, Clark SM, Cove JH. Temporal changes in sebum excretion and propionibacterial colonization in preadolescent children with and without acne. Br J Dermatol. 2007;156:22–31. doi: 10.1111/j.1365-2133.2006.07517.x. [DOI] [PubMed] [Google Scholar]
- 35.Harris HH, Downing DT, Stewart ME, Strauss JS. Sustainable rates of sebum secretion in acne patients and matched normal control subjects. J Am Acad Dermatol. 1983;8:200–203. doi: 10.1016/s0190-9622(83)70023-x. [DOI] [PubMed] [Google Scholar]
- 36.Piérard-Franchimont C, Piérard GE, Saint-Léger D, Lévêque JL, Kligman AM. Comparison of the kinetics of sebum secretion in young women with and without acne. Dermatologica. 1991;183:120–122. doi: 10.1159/000247650. [DOI] [PubMed] [Google Scholar]
- 37.Ottaviani M, Camera E, Picardo M, Ottaviani M, Camera E, Picardo M. Lipid Mediators in Acne, Lipid Mediators in Acne. Mediat Inflamm Mediat Inflamm 2010. 2010;2010:e858176. doi: 10.1155/2010/858176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meckfessel MH, Brandt S. The structure, function, and importance of ceramides in skin and their use as therapeutic agents in skin-care products. J Am Acad Dermatol. 2014;71:177–184. doi: 10.1016/j.jaad.2014.01.891. [DOI] [PubMed] [Google Scholar]
- 39.Rabasco Álvarez AM, González Rodríguez ML, et al. [Accessed July 6, 2016];Lipids in pharmaceutical and cosmetic preparations. 2000 [online] https://idus.us.es/xmlui/handle/11441/17554.
- 40.Clausen M-L, Slotved H-C, Krogfelt KA, Agner T. Tape Stripping Technique for Stratum Corneum Protein Analysis. Sci Rep. 2016;6:19918. doi: 10.1038/srep19918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hendrix SW, Miller KH, Youket TE, Adam R, O’Connor RJ, Morel JG, Tepper BE. Optimization of the skin multiple analyte profile bioanalytical method for determination of skin biomarkers from D-Squame tape samples. Skin Res Technol Off J Int Soc Bioeng Skin ISBS Int Soc Digit Imaging Skin ISDIS Int Soc Skin Imaging ISSI. 2007;13:330–342. doi: 10.1111/j.1600-0846.2007.00235.x. [DOI] [PubMed] [Google Scholar]
- 42.Widgerow AD, King K, Tocco-Tussardi I, Banyard DA, Chiang R, Awad A, Afzel H, Bhatnager S, Melkumyan S, Wirth G, Evans GRD. The burn wound exudate-an under-utilized resource. Burns J Int Soc Burn Inj. 2015;41:11–17. doi: 10.1016/j.burns.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Danielsen PL, Jorgensen LN, Jørgensen B, Karlsmark T, Agren MS. Erythema persists longer than one year in split-thickness skin graft donor sites. Acta Derm Venereol. 2013;93:281–285. doi: 10.2340/00015555-1455. [DOI] [PubMed] [Google Scholar]
- 44.Lawrence JC, Ameen H. Swabs and other sampling techniques. J Wound Care. 1998;7:232–233. doi: 10.12968/jowc.1998.7.5.232. [DOI] [PubMed] [Google Scholar]
- 45.Yang S, Kampp J. Common Dermatologic Procedures. Med Clin North Am. 2015;99:1305–1321. doi: 10.1016/j.mcna.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 46.Nwomeh BC, Liang HX, Diegelmann RF, Cohen IK, Yager DR. Dynamics of the matrix metalloproteinases MMP-1 and MMP-8 in acute open human dermal wounds. Wound Repair Regen. 1998;6:127–34. doi: 10.1046/j.1524-475x.1998.60206.x. [DOI] [PubMed] [Google Scholar]
- 47.Efron DT, Barbul A. In: Subcutaneous Sponge Models in Wound Healing: Methods and Protocols. DiPietro LA, editor. Humana Press Inc; Totowa, New Jersey: 2003. pp. 83–93. [DOI] [PubMed] [Google Scholar]
- 48.Alaish SM, Bettinger DA, Olutoye OO, Gould LJ, Yager DR, Davis A, Crossland MC, Diegelmann RF, Cohen I. Comparison of the polyvinyl alcohol [PVA] and ePTFE [Impra®] subcutaneous implants as models to evaluate wound healing potential in humans. Wound Repair Regen. 1995;3:292–298. doi: 10.1046/j.1524-475X.1995.30309.x. [DOI] [PubMed] [Google Scholar]
- 49.Jorgensen LN, Olsen L, Kallehave F, Karlsmark T, Diegelmann RF, Cohen IK, Gottrup F. The wound healing process in surgical patients evaluated by the ePTFE and the polyvinyl alcohol sponge: a comparison with special reference to the intra-patient variation. Wound Repair Regen. 1995;3:527–532. doi: 10.1046/j.1524-475X.1995.30419.x. [DOI] [PubMed] [Google Scholar]
- 50.Jorgensen LN, Madsen SM, Gottrup F. Implantable wound healing models and the determination of subcutaneous collagen deposition in expanded polytetrafluoroethylene implants. Methods Mol Med. 2003;78:263–273. doi: 10.1385/1-59259-332-1:263. [DOI] [PubMed] [Google Scholar]
- 51.Diegelmann RF, Lindblad WJ, Cohen IK. A subcutaneous implant for wound healing studies in humans. J Surg Res. 1986;40:229–37. doi: 10.1016/0022-4804(86)90156-3. [DOI] [PubMed] [Google Scholar]
- 52.Wijesinghe DS, Brentnall M, Mietla JA, Hoeferlin LA, Diegelmann RF, Boise LH, Chalfant CE. Ceramide kinase is required for a normal eicosanoid response and the subsequent orderly migration of fibroblasts. J Lipid Res. 2014;55:1298–1309. doi: 10.1194/jlr.M048207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Oswal V, Belle A, Diegelmann R, Najarian K. An entropy-based automated cell nuclei segmentation and quantification: application in analysis of wound healing process. Comput Math Methods Med. 2013;2013:592790. doi: 10.1155/2013/592790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Boniforti L, Passi S, Caprilli F, Porro MN. Skin surface lipids. Identification and determination by thin-layer chromatography and gas-liquid chromatography. Clin Chim Acta. 1973;47:223–231. doi: 10.1016/0009-8981(73)90319-7. [DOI] [PubMed] [Google Scholar]
- 55.Wertz PW, Schwartzendruber DC, Madison KC, Downing DT. Composition and Morphology of Epidermal Cyst Lipids. J Invest Dermatol. 1987;89:419–425. doi: 10.1111/1523-1747.ep12471781. [DOI] [PubMed] [Google Scholar]
- 56.Masukawa Y, Narita H, Sato H, Naoe A, Kondo N, Sugai Y, Oba T, Homma R, Ishikawa J, Takagi Y, Kitahara T. Comprehensive quantification of ceramide species in human stratum corneum. J Lipid Res. 2009;50:1708–1719. doi: 10.1194/jlr.D800055-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Weerheim A, Ponec M. Determination of stratum corneum lipid profile by tape stripping in combination with high-performance thin-layer chromatography. Arch Dermatol Res. 2001;293:191–199. doi: 10.1007/s004030100212. [DOI] [PubMed] [Google Scholar]
- 58.Long SA, Wertz PW, Strauss JS, Downing DT. Human stratum corneum polar lipids and desquamation. Arch Dermatol Res. 277:284–287. doi: 10.1007/BF00509081. [DOI] [PubMed] [Google Scholar]
- 59.Wertz PW, Miethke MC, Long SA, Strauss JS, Downing DT. The Composition of the Ceramides from Human Stratum Corneum and from Comedones. J Invest Dermatol. 1985;84:410–412. doi: 10.1111/1523-1747.ep12265510. [DOI] [PubMed] [Google Scholar]
- 60.Do UH, Pei PT, Minard RD. Separation of molecular species of ceramides as benzoyl andp. Lipids. 16:855–862. [Google Scholar]
- 61.Masukawa Y, Tsujimura H, Imokawa G. A systematic method for the sensitive and specific determination of hair lipids in combination with chromatography. J Chromatogr B. 2005;823:131–142. doi: 10.1016/j.jchromb.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 62.Pons A, Timmerman P, Leroy Y, Zanetta J-P. Gas-chromatography/mass-spectrometry analysis of human skin constituents as heptafluorobutyrate derivatives with special reference to long-chain bases. J Lipid Res. 2002;43:794–804. [PubMed] [Google Scholar]
- 63.Michael-Jubeli R, Bleton J, Baillet-Guffroy A. High-temperature gas chromatography-mass spectrometry for skin surface lipids profiling. J Lipid Res. 2011;52:143–151. doi: 10.1194/jlr.D008094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.van Smeden J, Hoppel L, van der Heijden R, Hankemeier T, Vreeken RJ, Bouwstra JA. LC/MS analysis of stratum corneum lipids: ceramide profiling and discovery. J Lipid Res. 2011;52:1211–1221. doi: 10.1194/jlr.M014456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.van Smeden J, Boiten WA, Hankemeier T, Rissmann R, Bouwstra JA, Vreeken RJ. Combined LC/MS-platform for analysis of all major stratum corneum lipids, and the profiling of skin substitutes. Biochim Biophys Acta BBA - Mol Cell Biol Lipids. 2014;1841:70–79. doi: 10.1016/j.bbalip.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 66.Masukawa Y, Narita H, Shimizu E, Kondo N, Sugai Y, Oba T, Homma R, Ishikawa J, Takagi Y, Kitahara T, Takema Y, Kita K. Characterization of overall ceramide species in human stratum corneum. J Lipid Res. 2008;49:1466–1476. doi: 10.1194/jlr.M800014-JLR200. [DOI] [PubMed] [Google Scholar]
- 67.Kendall AC, Nicolaou A. Bioactive lipid mediators in skin inflammation and immunity. Prog Lipid Res. 2013;52:141–164. doi: 10.1016/j.plipres.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 68•.Wijesinghe DS, Chalfant CE. Systems-Level Lipid Analysis Methodologies for Qualitative and Quantitative Investigation of Lipid Signaling Events During Wound Healing. Adv Wound Care. 2013;2:538–548. doi: 10.1089/wound.2012.0402. Provides detailed information with respect to tha application of mass spectrometry methods to study lipid changes during wound healing. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dhall S, Wijesinghe DS, Karim ZA, Castro A, Vemana HP, Khasawneh FT, Chalfant CE, Martins-Green M. Arachidonic acid-derived signaling lipids and functions in impaired healing. Wound Repair Regen Off Publ Wound Heal Soc Eur Tissue Repair Soc. 2015;23:644–656. doi: 10.1111/wrr.12337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dhall S, Do D, Garcia M, Wijesinghe DS, Brandon A, Kim J, Sanchez A, Lyubovitsky J, Gallagher S, Nothnagel EA, Chalfant CE, Patel RP, Schiller N, Martins-Green M. A novel model of chronic wounds: importance of redox imbalance and biofilm-forming bacteria for establishment of chronicity. PloS One. 2014;9:e109848. doi: 10.1371/journal.pone.0109848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.de Lima EO, de Macedo CS, Esteves CZ, de Oliveira DN, Pessolani MCV, da Nery JAC, Sarno EN, Catharino RR. Skin Imprinting in Silica Plates: A Potential Diagnostic Methodology for Leprosy Using High-Resolution Mass Spectrometry. Anal Chem. 2015;87:3585–3592. doi: 10.1021/acs.analchem.5b00097. [DOI] [PubMed] [Google Scholar]
- 72.Hart PJ, Francese S, Claude E, Woodroofe MN, Clench MR. MALDI-MS imaging of lipids in ex vivo human skin. Anal Bioanal Chem. 2011;401:115–125. doi: 10.1007/s00216-011-5090-4. [DOI] [PubMed] [Google Scholar]
- 73.Tfayli A, Jamal D, Vyumvuhore R, Manfait M, Baillet-Guffroy A. Hydration effects on the barrier function of stratum corneum lipids: Raman analysis of ceramides 2, III and 5. Analyst. 2013;138:6582–6588. doi: 10.1039/c3an00604b. [DOI] [PubMed] [Google Scholar]
- 74.Tfayli A, Guillard E, Manfait M, Baillet-Guffroy A. Raman spectroscopy: feasibility of in vivo survey of stratum corneum lipids, effect of natural aging. Eur J Dermatol EJD. 2012;22:36–41. doi: 10.1684/ejd.2011.1507. [DOI] [PubMed] [Google Scholar]
- 75.Chrit L, Hadjur C, Morel S, Sockalingum G, Lebourdon G, Leroy F, Manfait M. In vivo chemical investigation of human skin using a confocal Raman fiber optic microprobe. J Biomed Opt. 2005;10:44007. doi: 10.1117/1.2003747. [DOI] [PubMed] [Google Scholar]