Abstract
Uncomplicated urinary tract infections (UTI) are common in primary care. Whilst primary care physicians are called to be antimicrobial stewards, there is limited primary care antibiotic resistance surveillance and physician antibiotic prescription data available in southern Chinese primary care. The study aimed to investigate the antibiotic resistance rate and antibiotic prescription patterns in female patients with uncomplicated UTI. Factors associated with antibiotic resistance and prescription was explored. A prospective cohort study was conducted in 12 primary care group clinics in Hong Kong of patients presenting with symptoms of uncomplicated UTI from January 2012 to December 2013. Patients’ characteristics such as age, comorbidity, presenting symptoms and prior antibiotic use were recorded by physicians, as well as any empirical antibiotic prescription given at presentation. Urine samples were collected to test for antibiotic resistance of uropathogens. Univariate analysis was conducted to identify factors associated with antibiotic resistance and prescription. A total of 298 patients were included in the study. E. coli was detected in 107 (76%) out of the 141 positive urine samples. Antibiotic resistance rates of E. coli isolates for ampicillin, co-trimoxazole, ciprofloxacin, amoxicillin and nitrofurantoin were 59.8%, 31.8%, 23.4%, 1.9% and 0.9% respectively. E. coli isolates were sensitive to nitrofurantoin (98.1%) followed by amoxicillin (78.5%). The overall physician antibiotic prescription rate was 82.2%. Amoxicillin (39.6%) and nitrofurantoin (28.6%) were the most common prescribed antibiotics. Meanwhile, whilst physicians in public primary care prescribed more amoxicillin (OR: 2.84, 95% CI: 1.67 to 4.85, P<0.001) and nitrofurantoin (OR: 2.01, 95% CI: 1.14 to 3.55, P = 0.015), physicians in private clinics prescribed more cefuroxime and ciprofloxacin (P<0.05). Matching of antibiotic prescription and antibiotic sensitivity of E. coli isolates occurred in public than private primary care prescriptions (OR: 6.72, 95% CI: 2.07 to 21.80 P = 0.001) and for other uropathogens (OR: 6.19, 95% CI: 1.04 to 36.78 P = 0.034). Mismatching differences of antibiotic prescription and resistance were not evident. In conclusion, nitrofurantoin and amoxicillin should be used as first line antibiotic treatment for uncomplicated UTI. There were significant differences in antibiotic prescription patterns between public and private primary care. Public primary care practitioners were more likely to prescribe first line antibiotic treatment which match antibiotic sensitivity of E. coli isolates and other uropathogens. Further exploration of physician prescribing behaviour and educational interventions, particularly in private primary care may helpful. Meanwhile, development and dissemination of guidelines for primary care management of uncomplicated UTI as well as continued surveillance of antibiotic resistance and physician antibiotic prescription is recommended.
Introduction
Urinary tract infections (UTI) are one of the most common bacterial infections in primary care and classified as either complicated or uncomplicated [1]. Uncomplicated UTI occur in healthy individuals with either no structural or functional abnormalities of the urinary tract [1]. Bacterial pathogens are thought to be the cause of the presenting symptoms, and are often treated with antibiotics, which accounts for up to 95% of the antibiotic prescription for UTI in primary care settings [2]. Escherichia coli (E. coli) is the most common pathogen isolated in around 75% of uncomplicated UTI [3,4]. However, cases may be complicated by the E. coli producing extended-spectrum β-lactamase (ESBL), resulting in resistance to multiple β-lactam antibiotics [3]. Three international surveillance studies estimated that the prevalence of E. coli resistant to several types of antibiotics were around 8% (nitrofurantoin) to 48% (ampicillin) in North American, South American and European populations [4–6].
Meanwhile, primary care physicians are called to be antimicrobial stewards. Antibiotic resistance has been shown to be associated with patients’ age and sex, previous antimicrobial therapy and can be dynamic, responding to patterns of antibiotic treatment [7,8]. It is important to understand antibiotic prescribing behaviour of primary care physicians and antibiotic resistance patterns in primary care. Many studies has been conducted in the UK [8,9], however, there has been few studies to explore physician prescribing behaviour and antibiotic resistance in a Chinese primary care population. Antibiotic resistance of uropathogens and the prevalence of ESBL production of community-acquired UTI in Hong Kong is high (6.6% and 10% in 2004 and 2005 respectively) [10]. This study aimed to explore (1) the antibiotic resistance of urinary isolates of symptomatic patients presenting to primary care, (2) empirical antibiotic prescribing behaviours of primary care physicians and (3) factors associated with antibiotic resistance and physician antibiotic prescription.
Materials and methods
Design and setting
This was a prospective cohort study of public and private primary care clinics in Hong Kong. Public primary care clinics (government funded group primary care clinics) and private clinics provide more than 80% of primary care services for general population in Hong Kong [11]. Invitations were sent to all public and private group teaching practices in the New Territories and Kowloon region from January 2012 to December 2013. Group practices were chosen to increase yield of urinary samples. New Territories and Kowloon regions were selected to facilitate transportation of specimens.
Characteristics of patients
Eligible patients were women above the age of 16 presenting to primary care clinics with symptoms suggestive of UTI. The attending physician assessed eligibility criteria and invited patients with a provisional diagnosis of uncomplicated UTI based on UTI symptoms to participate in the study. Patients who were already on antibiotics or had antibiotics in the preceding 21 days, pregnant, having functional or structural abnormalities of the urinary tract, immunocompromised illness (e.g. AIDS, leukaemia, active viral hepatitis, multiple myeloma) or using immunosuppressants (e.g. oral corticosteroids, medication for modification of autoimmune conditions or transplanted organ and chemotherapy) were excluded from the study. Physicians were required to fill in a case report which included patient’s demographics, symptoms of UTI, hospitalisation history, antibiotic prescription for the present UTI, records of previous bacterial pathogens in the past one year and antibiotics prescribed in the past one year. Chronic medical conditions (e.g. diabetes mellitus, hypertension, ischaemic heart disease, chronic obstructive pulmonary disease and asthma, thyroid disease) were also recorded.
Ethical statement
This study was approved by the Clinical Research Ethics Committee of the Chinese University of Hong Kong and New Territories East Cluster of the Hospital Authority [CRE-2011.115]. Written formal consent was obtained from all participants.
Laboratory analysis
Participants were given verbal instructions on the steps of collecting a clean catch mid-stream urine (MSU) sample. The sample was collected in a sterile container refrigerated and delivered within the same day to the centralized accredited University Pathology laboratory for processing. Only one sample per patient was obtained.
In order to identify the major pathogen of UTI, the collected MSU specimens were processed for urine microscopy and cultures. Urine microscopy was performed by standard methods according to laboratory operating procedures (PHLS BSOP41, 1998; Urine for Microscopy, bacterial counts, identification and antibiotic susceptibilities, RCPA QAP Ltd). The urine sample was mixed well and a known quantity was examined under the inverted microscope at 20X power field in a flat-bottomed microtitre tray. The number of red and white cells were enumerated and reported as negative, moderate (10,000–100,000 cells/ml), large number (>100,000 cells/ml) of cells. Specimens were inoculated by the paper strip method (MAST) onto chromogenic medium (CPSE plate, Biomerieux) and incubated aerobically for 18–24 h at 35°C. The number of colonies were enumerated and reported with 0, <103, 103−4, 104−5, and >105 cfu/ml as no growth, insignificant, scanty, moderate and heavy growth respectively. Only urine cultures with heavy growth of a single bacterial type (>105 cfu/ml) were included in the analysis.
To identify antibiotic resistance patterns in the urinary isolates, the samples were further tested for antibiotic susceptibility to amoxicillin, ampicillin, ciprofloxacin, co-trimoxazole, gentamicin and nitrofurantoin and to detect ESBL positivity. Antibiotic susceptibilities were performed and interpreted as according to Clinical and Laboratory Standards Institute (CLSI) (Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing. CLSI document. Wayne). Specimens which were not processed immediately were stored at -4°C.
Data analysis
Descriptive statistics (mean±SD, percentages) were used to describe the frequency of bacterial pathogens, antibiotic resistance and prescription pattern.
Chi-squared test and univariate analysis were used for statistical analysis. E. coli antibiotic resistance was stratified with age group and chronic medical conditions and analysed by chi-square test. Odds ratios (OR) with 95% confidence intervals (95% CI) were calculated to identify factors associated with E. coli isolates resistance to antibiotics and for antibiotic prescription. A two tailed P< 0.05 was considered significant and all statistical analyses were performed by using SPSS v 21.0 Window (IBM Inc.,SPSS Inc, Chicago, IL USA).
Results
Patients and urine samples characteristics
Of the invited group clinics, 5 public and 12 private group clinics participated in the study and 306 female patient presentations were recruited into the study. Of these, eight clinical presentations were from previously recruited patients and only their first presentation were included in the study. Thus a total of 298 patients participated in the study of 145 (48.7%) and 153 (51.3%) were recruited from public and private clinics respectively (Table 1). The mean age of patients was 53.8±17.1 years old.
Table 1. Patients’ characteristics (n = 298).
| Patients’ characteristics | n (%)/mean±SDa | |||
|---|---|---|---|---|
| Total (n = 298) |
Public (n = 145) | Private (n = 153) | ||
| Age | All | 53.8±17.1 | 58.3±15.3 | 49.5±17.7 |
| 17–35 | 45 (15.1%) | 11 (7.6%) | 34 (22.2%) | |
| 36–50 | 75 (25.2%) | 29 (20%) | 46 (30.1%) | |
| 51–64 | 100 (33.6%) | 57 (39.3%) | 43 (28.1%) | |
| ≥65 | 78 (26.2%) | 48 (33.1%) | 30 (19.6%) | |
| Occupation | Professionals/ Officers/ Technicians |
16 (5.4%) | 7 (4.8%) | 9 (5.9%) |
| Server/Clericals | 62 (20.8%) | 28 (19.3%) | 34 (22.2%) | |
| Housewife/Unemployed | 107 (35.9%) | 69 (47.6%) | 38 (24.8%) | |
| Retired | 49 (16.4%) | 31 (21.4%) | 18 (11.8%) | |
| Students | 13 (4.4%) | 3 (2.1%) | 10 (6.5%) | |
| Missing | 51 (17.1%) | 7 (4.8%) | 44 (28.8%) | |
| Chronic medical conditions | Hypertension | 83 (27.9%) | 54 (37.2%) | 29 (19.0%) |
| Diabetes | 49(16.4%) | 33(22.8%) | 16(10.5%) | |
| Asthma | 4 (1.3%) | 3 (2.1%) | 1 (0.7%) | |
| Ischemic heart disease | 9 (3%) | 3 (2.1%) | 6 (3.9%) | |
| Thyroid disease | 6 (2%) | 2 (1.4%) | 4 (2.6%) | |
| COPD | 1 (0.3%) | 1 (0.7%) | 0 | |
| Gout | 1 (0.3%) | 1 (0.7%) | 0 | |
| Number of chronic medical conditions | 0 | 189 (63.4%) | 73 (50.3%) | 116 (75.8%) |
| 1 | 48 (16.1%) | 32 (22.1%) | 16 (10.5%) | |
| ≥2 | 61 (20.5%) | 40 (27.6%) | 21 (13.7%) | |
aData were presented as mean±SD for continuous variables and n (%) for categorical variables.
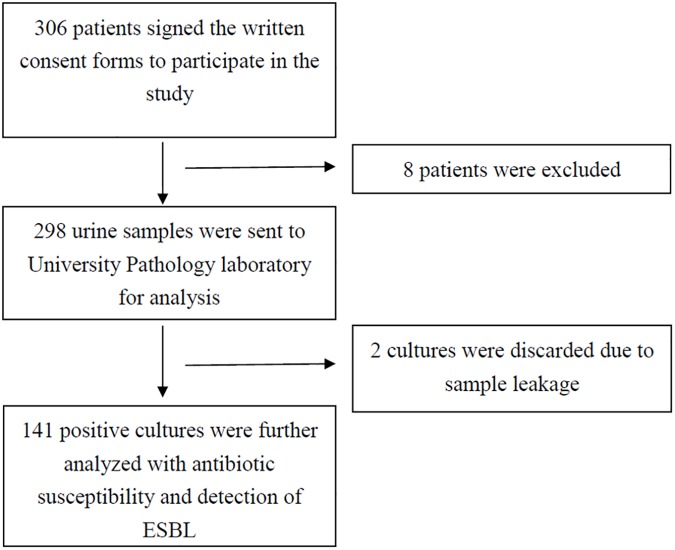
Of the samples received, two were discarded as there was leakage on transit and could not be processed. A positive urine culture was found in 141 (47.3%) of the collected samples (Fig 1) whilst 73 (24.5%) showed no growth, 68 (22.8%) as insignificant growth and 14 (4.7%) showed mixed bacteria and probable contamination.
Fig 1. Flow chart of the patient’s recruitment process and analysis of their urine samples.
Bacterial pathogens among positive urine isolates
Among the 141 positive urine isolates, the most common uncomplicated UTI pathogen was E. coli (75.9%). Other uropathogens included Klebsiella (9.2%), Enterococcus (3.5%), Proteus Mirabilis (3.5%) and Citrobacter (2.8%).
E. coli infection occurred mostly in women who aged 51 above (63.5%) and most likely to present with two or more symptoms (79.5%) of which dysuria, frequency and urgency were common symptoms experienced (Table 2).
Table 2. Patients’ characteristics of those with uncomplicated UTI with E. coli infection (n = 107).
| Patients’ characteristics | n (%) | |
|---|---|---|
| Age | mean±SDa 56.3±17.9 | |
| 17–35 | 13 (12.1%) | |
| 36–50 | 26 (24.3%) | |
| 51–64 | 38 (33.6%) | |
| 65 and above-80 | 34 (31.8%) | |
| Symptoms | Dysuria | 89 (83.2%) |
| Frequency | 71 (66.4%) | |
| Urgency | 48 (44.9%) | |
| Abdominal pain | 9 (8.4%) | |
| Haematuria | 18 (16.8%) | |
| Cloudy urine | 8 (7.5%) | |
| Nocturia | 4 (3.7%) | |
| Fever | 4 (3.7%) | |
| No. of symptoms | 1 | 22 (20.6%) |
| 2 | 69 (64.5%) | |
| ≥3 | 16 (15%) | |
aData were presented as mean±SD for continuous variables and n (%) for categorical variables.
Antibiotic resistance rates of E. coli isolates
Of the 107 E. coli isolates, the resistance rates were 59.8% for ampicillin, 31.8% for co-trimoxazole, 25.2% for gentamicin and 23.4% for ciprofloxacin. The majority of E. coli isolates were sensitive to nitrofurantoin (98.1%), amoxicillin (78.5%) and ciprofloxacin (76.6%) (Table 3).
Table 3. Susceptibility profile of E. coli, other uropathogens, ESBL producing isolates.
| Antibiotic agents | E. coli isolates n = 107/141 (75.9%) | Other uropathogens isolates n = 34/141 (24.1%) | ESBL producing isolates n = 14/141 (9.9%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Susceptibilitya n (%) | S | I | R | S | I | R | S | I | R |
| Amoxicillin | 84 (78.5%) | 21 (19.6%) | 2 (1.9%) | 32 (94.1%) | 1 (2.9%) | 1 (2.9%) | 6 (42.9%) | 8 (57.1%) | 0 (0%) |
| Ampicillin | 41 (38.3%) | 2 (1.9%) | 64 (59.8%) | 16 (47.1%) | 0 (0%) | 18 (52.9%) | 0 (0%) | 0 (0%) | 14 (100%) |
| Ciprofloxacin | 82 (76.6%) | 0 (0%) | 25 (23.4%) | 31 (91.2%) | 3 (8.8%) | 0 (0%) | 5 (35.7%) | 1 (7.1%) | 8 (57.1%) |
| Co-trimoxazole | 73 (68.2%) | 0 (0%) | 34 (31.8%) | 30 (88.2%) | 0 (0%) | 4 (11.8%) | 5 (35.7%) | 0 (0%) | 9 (64.3%) |
| Gentamicin | 80 (74.8%) | 0 (0%) | 27 (25.2%) | 30 (88.2) | 2 (5.9%) | 2 (5.9%) | 8 (57.1%) | 0 (0%) | 6 (42.9%) |
| Nitrofurantoin | 105 (98.1%) | 1 (0.9%) | 1 (0.9%) | 25 (73.5%) | 6 (17.6%) | 3 (8.8%) | 12 (85.7%) | 0 (0%) | 2 (14.3%) |
aS = sensitive; I = intermediate, R = resistant.
E. coli isolates with resistance to one drug can also be resistance to other drugs. Multidrug resistance (co-resistance to ampicillin, ciprofloxacin, and co-trimoxazole) occurred in 12.1% of E. coli isolates (n = 13).
ESBL producers
ESBL producers (n = 14) were found in 9.9% of the positive isolates (n = 141) and almost all ESBL producers (n = 13) were from E. coli isolates. 85.7% (n = 12) of ESBL producing isolates were found amongst older women who were aged 51 years and above. All ESBL producing isolates were resistant to ampicillin, whilst almost half were resistant to ciprofloxacin (n = 8, 53.3%) and co-trimoxazole (n = 8, 53.3%).
Antibiotic resistance rate of E. coli isolates and age and chronic medical conditions
Antibiotic resistance rates of ampicillin and multidrug resistance were statistically significant with increasing age. The OR of patients who aged >50 were 2.90 (95% CI: 1.28–6.55, P = 0.011) for having resistance with ampicillin. In addition, The OR of patients who aged >50 were 8.14 (95% CI: 1.02–65.26, P = 0.048) for having multidrug resistance. Meanwhile, although antibiotic resistance appear higher in those with more than two chronic medical conditions for most antibiotic types, this was not statistically significant (P = 0.134, P = 0.150P = 0.340 and P = 0.253 for ampicillin, ciprofloxacin, co-trimoxazole and multidrug resistance respectively) (Table 4).
Table 4. Odds ratio of patients’ characteristics and resistance to antibiotic agents (n = 107).
| Antibiotic resistance (E. coli isolates n = 107) | |||||
|---|---|---|---|---|---|
| Ampicillin | Ciprofloxacin | Co-trimoxazole | Multi-drug resistancea | ||
| Patients’ characteristics | OR (95% CI) P value | OR (95% CI) P value | OR (95% CI) P value | OR (95% CI) P value | |
| Age group | 17–50 | 1.00 | 1.00 | 1.00 | 1.00 |
| >50 | 2.90 (1.28–6.55) 0.011b | 2.13 (0.77–5.91) 0.145 | 2.40 (0.96–6.01) 0.062 | 8.14 (1.02–65.26) 0.048b | |
| No. of chronic medical conditions | 0 | 1.00 | 1.00 | 1.00 | 1.00 |
| 1 | 2.68 (1.00–7.17) 0.050b | 2.02 (0.76–5.39) 0.158 | 1.77 (0.70–4.42) 0.226 | 2.48 (0.72–8.49) 0.148 | |
| ≥2 | 2.75 (0.73–10.33) 0.134 | 3.42 (0.64–18.25) 0.150 | 1.94 (0.50–7.58) 0.340 | 3.65 (0.40–33.59) 0.253 | |
| Past UTI diagnosis | No | 1.00 | 1.00 | 1.00 | 1.00 |
| Yes | 1.19 (0.43–3.31) 0.743 | 3.04 (1.06–8.71) 0.033b | 1.32 (0.47–3.72) 0.601 | 1.46 (0.36–5.92) 0.592 | |
| Past antibiotic usec | None | 1.00 | 1.00 | 1.00 | 1.00 |
| In past 6 month | 1.64 (0.53–5.11) 0.391 | 2.21 (0.71–6.86) 0.163 | 3.29 (1.11–9.80) 0.027b | 2.96 (0.79–11.15) 0.096 | |
| In past 1 year | NA | 2.89 (1.09–7.68) 0.029b | 2.43 (0.97–6.14) 0.056 | 3.29 (0.99–10.95) 0.043b | |
aCo-resistance to ampicillin, ciprofloxacin and co-trimoxazole.
bStatistically significant at P<0.05.
cContains missing data.
Factors associated with antibiotic resistance of E. coli isolates
Patients who had taken antibiotics in the past year had OR of 2.89 (95% CI: 1.09 to 7.68; P = 0.029) for resistance to ciprofloxacin than those without antibiotics treatment. In addition, the OR of those exposed to antibiotics in the past year were 3.29 (95% CI: 0.99 to 10.95; P = 0.043) to have multidrug resistance than those without previous antibiotics treatment (Table 4).
The OR for those with previous UTI diagnosis in the past 6 months were 3.04 (95%CI: 1.06 to 8.71, P = 0.03) to have ciprofloxacin resistance (Table 4).
Antibiotic prescription
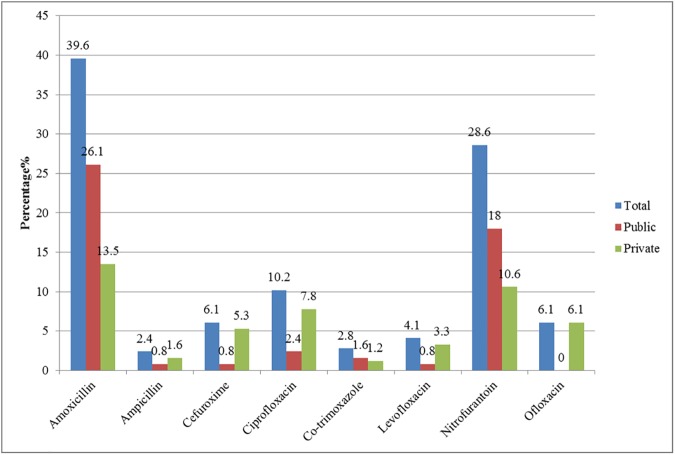
Of the 298 patients presenting with symptoms, 245 (82.2%) were prescribed antibiotics. Amoxicillin (39.6%) was most commonly prescribed, followed by nitrofurantoin (28.6%) and ciprofloxacin (10.2%). The mean duration of antibiotic prescription was 6.86±0.54 days (Fig 2).
Fig 2. Antibiotic prescription rate among patients (n = 245).
Antibiotic prescription rates among public and private clinics
The antibiotic prescription rates were 50.6% and 49.4% in public and private primary care clinics respectively. There was no significant difference in empirical antibiotic prescription between the two groups (P = 0.147). Meanwhile, the OR of public primary care physicians to private primary care physicians in prescribing amoxicillin were 2.84 (95% CI: 1.67 to 4.85) and 2.01 (95% CI: 1.14 to 3.55) for nitrofurantoin. The results were statistically significant (P<0.001 and 0.015) (S1 Table).
In contrast, private primary care physicians prescribed more cefuroxime (5.3 versus 0.8%, P = 0.003) and ciprofloxacin (7.8 versus 2.4%, P = 0.005) (Fig 2).
Factors for antibiotic prescription
There was no association between the rate of antibiotic prescription with age (P = 0.370). However, patients aged 65 years or above received more amoxicillin as empirical antibiotic treatment (P = 0.047), whilst patients aged between 17 and 50 years old received more cefuroxime (P<0.001) and ofloxacin (P = 0.005).
The OR of patients with co-existing medical conditions was 3.08 (95% CI: 1.60 to 5.94) to be prescribed amoxicillin than those without chronic medical conditions and this was significantly significant (P = 0.001).
The OR of prescribing ciprofloxacin was 2.17 (95% CI: 0.80 to 5.89, P = 0.122) and co-trimoxazole was 2.58 (95% CI: 0.48 to 13.84, P = 0.254) for patients with previous history of a UTI in the past 6 months than patients without a recent UTI. However the results were not statistically significant.
The odds of receiving an antibiotic prescription were greater with symptoms of dysuria or urgency. The OR of dysuria was 4.53 (95% CI: 2.33 to 8.83, P<0.001) and urgency (OR: 2.32, 95% CI: 1.18 to 4.58 P = 0.013). Meanwhile, The OR of patients presenting with more than two urinary symptoms were 0.33 (95% CI: 0.16 to 0.69, P = 0.003) time than those with one or two presenting symptoms to have empirical antibiotic treatment (S2 Table).
Empirical antibiotic prescription and uropathogen susceptibility
For the 141 positive isolates, 122 received empirical antibiotic treatment. The appropriate use of antibiotics was evident in public primary care. This was demonstrated by the matching of antibiotic susceptibility of the uropathogen and the prescribed antibiotics were statistically significant for both sensitivity and resistance of E. coli pathogens and for other uropathogens. The OR in matching antibiotic susceptibility and physician prescribing was 6.72 (95% CI: 2.07 to 21.80, P = 0.001) for E. coli isolates and 6.19 (95% CI: 1.04 to 36.78, P = 0.034) for other uropathogens between public primary care and private primary care physicians (Table 5).
Table 5. Antibiotic prescription and uropathogen sensitivity and resistance.
| E.coli isolates n = 107 | Other uropathogens n = 34 | |||
|---|---|---|---|---|
| Public n = 47 | Private n = 60 | Public n = 13 | Private n = 21 | |
|
Empirical antibiotics n (%) |
43 (91.5%) | 49 (81.7%) | 13 (100%) | 17 (81%) |
|
No antibiotic prescribed n (%) |
4 (8.5%) | 11 (18.3%) | 0 (0%) | 4 (19%) |
|
Antibiotic matchinga
(overall) n (%) |
39/43 (90.7%) | 29/49 (59.2%) | 11/13 (84.6%) | 8/17 (47.1%) |
| OR (95% CI), P value | 6.72 (2.07–21.80), p = 0.001c | 1.00 | 6.19 (1.04–36.78), p = 0.034c | 1.00 |
|
Antibiotic resistanceb (overall) n (%) |
1/43 (2.3%) | 1/49 (2.0%) | 0 (0%) | 2/17 (11.8%) |
| OR (95% CI), P value | 1.14 (0.07–18.84), p = 0.926 |
1.00 | NA, p = 0.201 | 1.00 |
aIsolates were sensitive to physicians prescribed antibiotics (amoxicillin, ampicillin, ciprofloxacin, co-trimoxazole, gentamicin and nitrofurantoin).
bIsolates were resistant to physicians prescribed antibiotics (amoxicillin, ampicillin, ciprofloxacin, co-trimoxazole, gentamicin and nitrofurantoin).
cStatistically significant at P<0.05.
Discussion
Antibiotic resistance
Our result showed that E. coli was the most common pathogen and present in 75.9% of the positive urine cultures, which was consistent with international studies [3,4,12–14].
Regarding antibiotic sensitivity and resistance rate, our results were consistent with a regional study of urinary isolates of outpatients from private hospital laboratories and community laboratories, the sensitivity rates of ampicillin (38.3% versus 37.4–37.6%), ciprofloxacin (76.6% versus 76.3–77.2%), co-trimoxamole (68.2% versus 64.7–65%) were similar. In addition, a lower antibiotic sensitivity to amoxicillin (Sensitivity rates of 81.3%-83.6% vs 78.5%) was observed, although resistance rates were low and comparable [10].
Reduced susceptibility to amoxicillin in our study may indicate patients presenting to primary care which may have less severe symptoms and likely to present earlier or may reflect changes in antibiotic susceptibilities due to physicians’ prescribing behaviour. The Centre of Health Protection in Hong Kong publishes sentinel surveillance information of outpatient urinary samples every month [15]. Meanwhile the sentinel data analyses around 500 routine samples per month, all of which are urine samples collected in 85 public primary care centres (GOPCs); this includes male and female who are likely to those who have complicated or recurrent urinary illness to warrant further investigation from urinary microscopy and culture. Over comparable periods in 2012–13, bacterial pathogens and antibiotic resistance to most antibiotics were similar to our results. However, the antibiotic resistance rates for co-trimoxazole as well as ESBL producers were lower in our study (31.8 versus 38% and 9.9% versus 18.0% respectively). Meanwhile, the website publishes resistance rates only, whilst sensitive and intermediate responses may also be helpful in primary care physicians choice of antibiotics given other factors e.g. co-morbidities and contraindication.
Previous studies have suggested an association between antibiotic resistance and increasing age [10] and whilst this trend was observed, only a significant finding of age and resistance to ampicillin and multiple drugs were evident in our study. Our study showed that public primary care clinics and past history of UTI diagnosis (within 6 months) were statistically significant associated with E. coli isolates resistance to ciprofloxacin. A study in Europe suggests that the prior use of antibiotics may be a contributory factor for antibiotic resistance; Sotto et al [16] found that a past diagnosis of UTI within the past 1 year was associated with E. coli antibiotic resistance to ciprofloxacin.
Antibiotic prescription
The results of our study suggest that nitrofurantoin is the most appropriate first line antibiotic of choice which had the lowest rate of resistance and the highest sensitivity. Second line treatment would be amoxicillin. Our result is similar with current guidelines on antibiotic prescription for UTI, such as Infectious Diseases Society of America guidelines [17,18] and inter-hospital guidelines in Hong Kong [19]. High antibiotic resistance rates to ampicillin, ciprofloxacin and co-trimoxazole (resistance rate > 20%) have been consistent to a local study [20] and demonstrate that these drugs should not be considered in the management of UTI unless there is proven resistance or allergy to nitrofurantoin or amoxicillin.
In our study, the most commonly prescribed antibiotic amongst primary care physicians is amoxicillin followed by nitrofurantoin. Selection of appropriate antibiotics should be based on differing resistance patterns in the locality [21], as well as patients’ medication, contraindications, allergy profile and cost [18]. All these considerations may affect the antibiotic prescription pattern, which may account for the differences in the antibiotic prescription rate. The use of nitrofurantoin may be limited as it is contraindicated in patients with renal impairment and creatinine clearance <60mL/min and may require careful consideration in the elderly [22] and this may explain the reluctance in prescribing. The threshold of creatinine clearance <60mL/min however, has been disputed due to non-existent evidence and suggests a lower threshold of creatinine clearance <40mL/min could be adapted [23] to improve nitrofurantoin use. Meanwhile, even with cautionary use, it may be likely that nitrofurantoin is underutilised and further exploration in clinician’s reluctance and other factors involved in the antibiotic decision making process.
Meanwhile, our result shows that private primary care physicians prescribed more ciprofloxacin (P = 0.005), while public primary care physicians prescribed more amoxicillin (P<0.001) and nitrofurantoin (P = 0.015). This may be attributed to guidelines, drug formulary and the cost of treatment. The public primary care clinics are governed by the Hospital Authority which has guidance for public primary care physicians on their clinical management system. Private group practices have their own clinical management systems and are not restricted by guidelines and have a wider drug formulary. Meanwhile, private group practices are able to absorb higher drug costs. In Hong Kong, a 3-day course of nitrofurantoin and ciprofloxacin costs 2.38 and 25.8 HKD respectively (1 USD = 7.8 HKD) [24]. The predominant use of nitrofurantoin and amoxicillin in public clinics may also be attributed to the use of available inter-hospital guidance [19] and clinical governance of its governing body the Hospital Authority and may reflect the benefits of governance on antibiotic prescribing behaviour. Surveillance of prescribing data of private doctors may enhance appropriate antibiotic prescribing, but this would logistically be difficult due to the heterogeneity of clinical systems and documentation by private practitioners and lack of a prescription tracking system as many private practitioners are dispensing practices.
International recommendations for treating uncomplicated UTI in primary care is a 3 day course of antibiotics [25], however 99.5% of the antibiotic prescriptions in this study were of 5–7 days duration. Our results show that patients who attended private clinics were younger (mean 49.50±17.66 years old) and had less comorbidity when compared to patients who attended public clinics (Table 1). Private primary care physicians also prescribed cefuroxime and ofloxacin which has been documented for use in young women [26,27]. Further exploration of physician’s prescribing behaviour and facilitators and barriers to appropriate prescribing is warranted.
Strength and limitations
This is the first study to investigate both antibiotic resistance and prescription rates of patients with uncomplicated UTI in Chinese primary care settings. Previous antibiotic resistance data was conducted in outpatients departments and public primary care clinics. Meanwhile, there have been no studies documenting physician prescribing habits in the region. The strength of this study has been to show the use of both antibiotic susceptibility data and antibiotic prescribing behaviour in identifying highlighting gaps associated with antibiotic appropriateness.
This study has several limitations. The participation of group practices in selected districts may limit the representativeness of patients across the Hong Kong S.A.R region. Due to the uncommon and sporadic presentation for UTI across a number of sites, the recruitment period was long and we were unable to document the number of patients who were approached but declined to participate in the study which may attribute to selection bias of the study sample. In addition, primary care physicians may also be more likely to prescribe an appropriate antibiotic as they were aware of being observed. Recall bias may also occur in patients recounting prior UTI episodes and prior antibiotic use. However these limitations are likely to be underestimating our study findings. The study was designed to reflect the pragmatic approach of UTI diagnosis and empirical antibiotic prescription in primary care. The use of urine dipstick in the diagnostic criteria, the follow up of presenting symptoms and analysis of urine culture yields of 103 and 104 cfu/ml may further enhance the study, particularly in detailing threshold for treatment and symptom resolution.
Our study has highlighted a gap between physician current prescribing habits and antibiotic resistance. There is a need for increased efforts for education and dissemination to improve appropriate antibiotic prescription. It may also be advantageous for the surveillance data to show intermediate susceptibility to particular antibiotics as well as resistance data to help guide primary care physicians on antibiotic choice. The prospective cohort and multicentre approach in this study yields a more comprehensive picture of the complexities in the patient presentation and doctors’ prescription of antibiotics not addressed in previous regional studies. Additional information on patient’s clinical response and recovery and physician’s decision on antibiotic prescription and choice can be considered in further studies.
Conclusions
This study identified antibiotic resistance amongst uropathogens and physician antibiotic prescription in primary care settings. There is scope to encourage nitrofurantoin as first line treatment in primary care settings particularly in private primary care and to explore its use and barriers for use. Enhancing antibiotic guidance for UTI and continued surveillance of antibiotic resistance and physician antibiotic prescription may improve appropriate antibiotic prescription for UTI in Chinese primary care.
Supporting information
aStatistically significant at P<0.05.
(PDF)
aStatistically significant at P<0.05.
(PDF)
Acknowledgments
We would like to thank the medical staff in all the participating primary care clinics and the laboratory technicians in helping out with the study.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The study was funded by Research Fund for the Control of Infectious Disease (Reference no: CU-10-01-03). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Foxman B. The epidemiology of urinary tract infection. Nat Rev Urol. 2010;7:653–660. doi: 10.1038/nrurol.2010.190 [DOI] [PubMed] [Google Scholar]
- 2.Ong DS, Kuyvenhoven MM, van Dijk L, Verheij TJ. Antibiotics for respiratory, ear and urinary tract disorders and consistency among GPs. J Antimicrob Chemother. 2008;62:587–592. doi: 10.1093/jac/dkn230 [DOI] [PubMed] [Google Scholar]
- 3.Flores-Mireles AL, Walker JN, Caparon M, Hultgren SJ. Urinary tract infections: epidemiology, mechanisms of infection and treatment options. Nat Rev Microbiol. 2015;13:269–284. doi: 10.1038/nrmicro3432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schito GC, Naber KG, Botto H, Palou J, Mazzei T, Gualco L. The ARESC study: an international survey on the antimicrobial resistance of pathogens involved in uncomplicated urinary tract infections. Int J Antimicrob Agents. 2009;34:407–413. doi: 10.1016/j.ijantimicag.2009.04.012 [DOI] [PubMed] [Google Scholar]
- 5.Karlowsky JA, Kelly LJ, Thornsberry C, Jones ME, Sahm DF. Trends in Antimicrobial Resistance among Urinary Tract Infection Isolates of Escherichia coli from Female Outpatients in the United States. Antimicrobial Agents and Chemotherapy. 2002;46:2540–2545. doi: 10.1128/AAC.46.8.2540-2545.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhanel GG, Hisanaga TL, Laing NM, DeCorby MR, Nichol KA, Palatnik LP, et al. Antibiotic resistance in outpatient urinary isolates: final results from the North American Urinary Tract Infection Collaborative Alliance (NAUTICA). Int J Antimicrob Agents. 2005;26:380–388. doi: 10.1016/j.ijantimicag.2005.08.003 [DOI] [PubMed] [Google Scholar]
- 7.Alós JI, Serrano MG, Gómez-Garcés JL, Perianes J. Antibiotic resistance of Escherichia coli from community-acquired urinary tract infections in relation to demographic and clinical data. Clinical Microbiology and Infection. 2005;11:199–203. doi: 10.1111/j.1469-0691.2004.01057.x [DOI] [PubMed] [Google Scholar]
- 8.Butler CC, Hillier S, Roberts Z, Dunstan F, Howard A, Palmer S. Antibiotic-resistant infections in primary care are symptomatic for longer and increase workload:outcomes for patients with E.coli UTIs. Br J Gen Pract. 2006;56:686–692. [PMC free article] [PubMed] [Google Scholar]
- 9.Little P, Merriman R, Turner S, Rumsby K, Warner G, Lowes JA, et al. Presentation, pattern, and natural course of severe symptoms, and role of antibiotics and antibiotic resistance among patients presenting with suspected uncomplicated urinary tract infection in primary care observational study. BMJ. 2010;340:b5633 doi: 10.1136/bmj.b5633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ho PL, Wong RC, Yip KS, Loke SL, Leung MS, Mak GC, et al. Antimicrobial resistance in Escherichia coli outpatient urinary isolates from women: emergning multidrug resistance phenotypes. Diagn Microbiol Infect Dis. 2007; 59:439–445. doi: 10.1016/j.diagmicrobio.2007.06.012 [DOI] [PubMed] [Google Scholar]
- 11.Wei X, Li H, Yang N, Wong SYS, Owolabi O, Xu J, et al. Comparing Quality of Public Primary Care between Hong Kong and Shanghai Using Validated Patient Assessment Tools. PLoS ONE. 2015;10:e0121269 doi: 10.1371/journal.pone.0121269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grude N, Tveten Y, Jenkins A, Kristiansen BE. Uncomplicated urinary tract infections. Bacterial findings and efficacy of empirical antibacterial treatment. Scand J Prim Health Care. 2005;23:115–119. doi: 10.1080/02813430510015287 [DOI] [PubMed] [Google Scholar]
- 13.Kahlmeter G. An international survey of the antimicrobial susceptibility of pathogens from uncomplicated urinary tract infections:the ECO.SENS Project. J Antimicrob Chemother. 2003;51:69–76. [DOI] [PubMed] [Google Scholar]
- 14.Ronald A. The etiology of urinary tract infection: traditional and emerging pathogens. Dis Mon. 2003;49:71–82. doi: 10.1067/mda.2003.8 [DOI] [PubMed] [Google Scholar]
- 15.Centre of Health Protection Hong Kong. Bacterial pathogen isolation and percentage of antimicrobial resistance- outpatient setting. Available: http://www.chp.gov.hk/en/data/1/10/641/697/5199.html. Accessed 18 February 2016.
- 16.Sotto A, De Boever CM, Fabbro-Peray P, Gouby A, Sirot D, Jourdan J. Risk factors for antibiotic-resistant Escherichia coli isolated from hospitalized patients with urinary tract infections: a prospective study. Journal of clinical microbiology. 2001;39:438–444. doi: 10.1128/JCM.39.2.438-444.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hooton TM. Uncomplicated Urinary Tract Infection. N Engl J Med. 2012;366:1028–1037. doi: 10.1056/NEJMcp1104429 [DOI] [PubMed] [Google Scholar]
- 18.Gupta K, Hooton TM, Naber KG, Wullt B, Colgan R, Miller LG, et al. International Clinical Practice Guidelines for the Treatment of Acute Uncomplicated Cystitis and Pyelonephritis in Women: A 2010 Update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis. 2011;52:e103–20. doi: 10.1093/cid/ciq257 [DOI] [PubMed] [Google Scholar]
- 19.Centre of Health Protection. Department of Health. Reducing bacterial resistance with IMPACT–Interhospital Multi-disciplinary Programme on Antimicrobial Chemotherapy. Available: http://www.chp.gov.hk/en/view_content/32062.html Accessed 18 February 2016.
- 20.Ho PL, Yip KS, Chow KH, Lo JY, Que TL, Yuen KY. Antimicrobial resistance among uropathogens that cause acute uncomplicated cystitis in women in Hong Kong: a prospective multicenter study in 2006 to 2008. Diagn Microbiol Infect Dis. 2010;66:87–93. doi: 10.1016/j.diagmicrobio.2009.03.027 [DOI] [PubMed] [Google Scholar]
- 21.SOGC Clinical Practice Guideline. Recurrent Urinary Tract Infection. Available: http://sogc.org/wp-content/uploads/2013/01/gui250CPG1011E_001.pdf. Accessed 31 August 2016.
- 22.Product information. Macrobid (nitrofurantoin monohydrate/macrocrystals). Cincinnati, OH: Procter & Gamble Pharmaceuticals, January 2009. [Google Scholar]
- 23.Oplinger M and Andrews O. Nitrofurantoin contraindication in patients with a creatinine clearance below 60mL/min: Looking for the evidence. Ann Pharmacother. 2013;47:106–111. doi: 10.1345/aph.1R352 [DOI] [PubMed] [Google Scholar]
- 24.Li Y, Yuen W. How efficient can we treat presumed acute uncomplicated lower urinary tract infection in women with 3-day antibiotic in the Accident & Emergency Department? Hong Kong Journal of Emergency Medicine. 2005;12:77–83. [Google Scholar]
- 25.Public Health England. Managing common infections: guidance for primary care. Published August 2010, Updated May 2016. Available: https://www.gov.uk/government/publications/managing-common-infections-guidance-for-primary-care. Accessed 31 August 2016.
- 26.Raz R, Genesin J, Gonen E, Shmilovitz M, Hefter H, Potasman I. Single low-dose ofloxacin for the treatment of uncomplicated urinary tract infection in young women. Journal of Antimicrobial Chemotherapy. 1988;22:945–949. [DOI] [PubMed] [Google Scholar]
- 27.Manka W, Solowiow R, Okrzeja D. Assessment of infant development during an 18-month follow-up after treatment of infections in pregnant women with cefuroxime axetil. Drug safety. 2000;22:83–88. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
aStatistically significant at P<0.05.
(PDF)
aStatistically significant at P<0.05.
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.