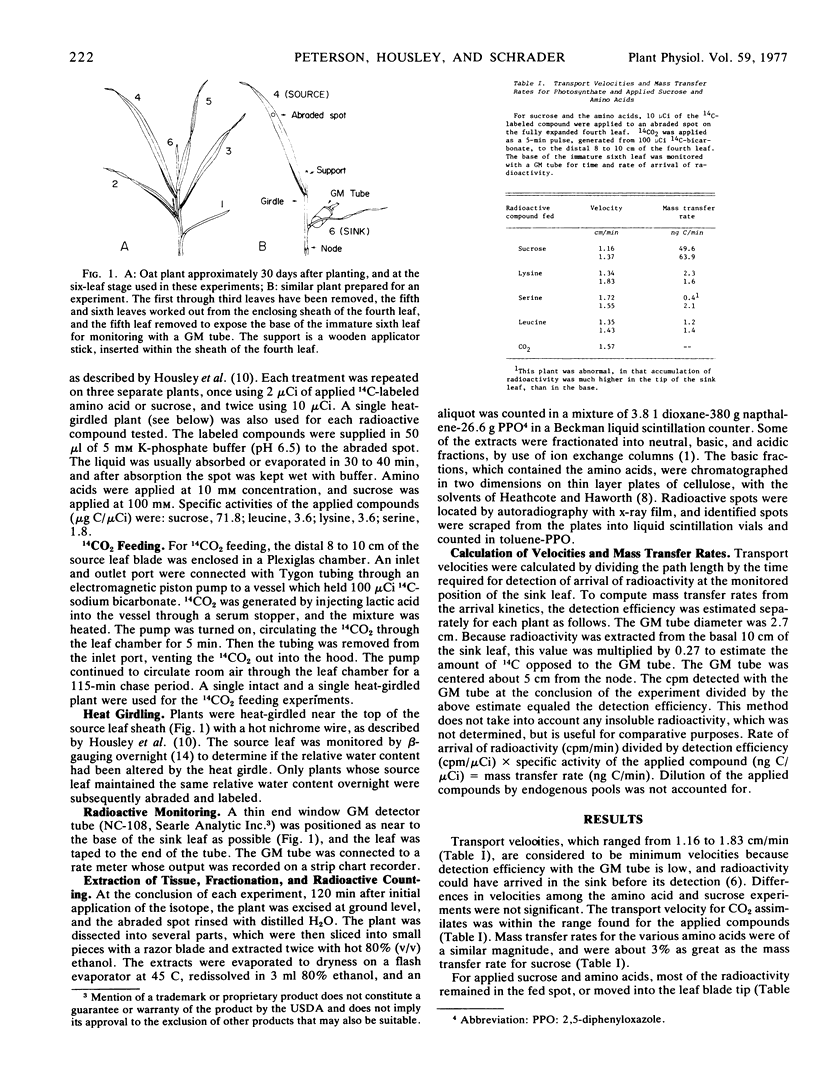
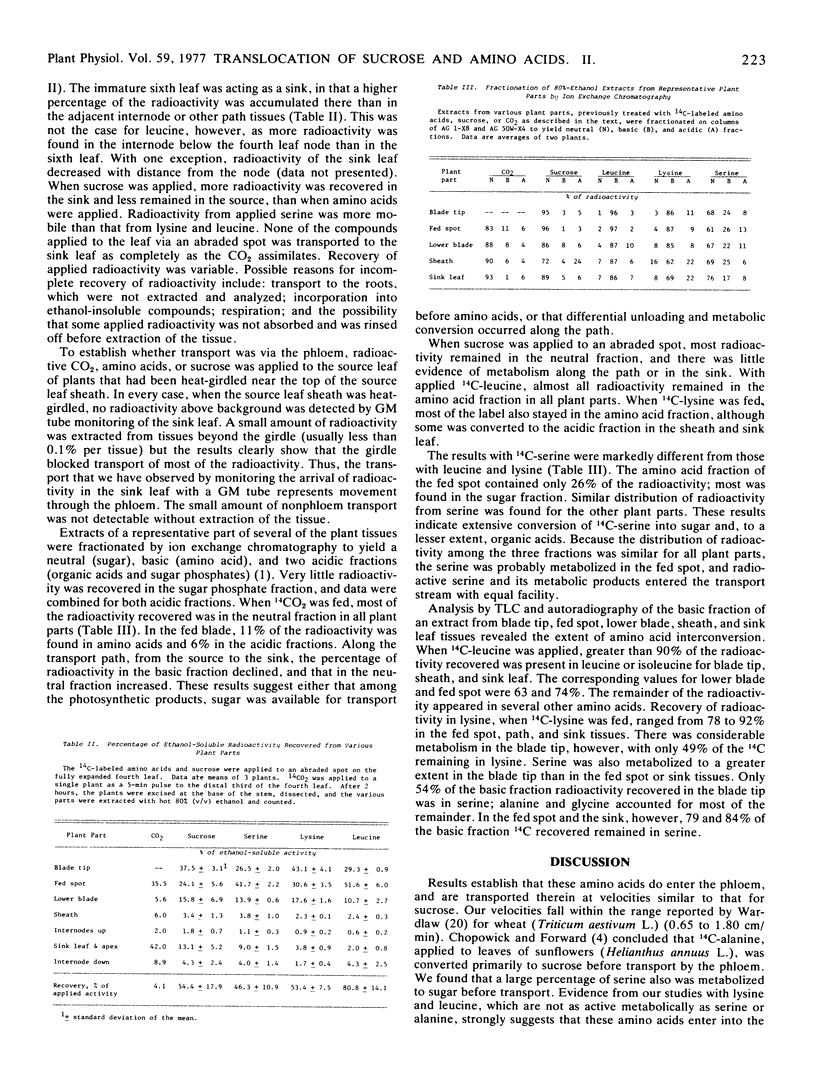
Abstract
To establish whether several amino acids were equally able to enter the phloem of oat (Avena sativa L.) plants and be transported, several 14C-labeled amino acids were applied individually to an abraded spot on a fully expanded source leaf. The base of an immature sink leaf was monitored with a GM tube for time and rate of arrival of radioactivity. Transport of 14C-sucrose and 14CO2 assimilates was measured for a comparison. The applied l-serine, l-lysine, and l-leucine, as well as sucrose, entered the phloem and were transported to the sink leaf at rates between 1.16 and 1.83 cm/min. Transport velocity for CO2 assimilates was 1.57 cm/min. A heat girdle near the top of the source leaf sheath blocked most transport, which indicated that transport was primarily through the phloem. Mass transfer rates for amino acids were only 3% as great as that for sucrose, suggesting different mechanisms of entry for sucrose than for amino acids into the phloem. The higher percentage of CO2 assimilates mobilized to the sink leaf was attributed to the greater surface area of minor veins accessible to loading, as compared to those compounds supplied via an abraded spot. Serine was extensively metabolized in the source leaf, and radioactive products in the sink leaf mirrored those in the source leaf. Most radioactivity of lysine and leucine remained within these compounds in the source, path, and sink tissues. We concluded that there was no barrier to entry of amino acids into the phloem and transport therein. Data do not suggest a specific mechanism for entry of amino acids into the phloem.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BIRT L. M., HIRD F. J. Kinetic aspects of the uptake of amino acids by carrot tissue. Biochem J. 1958 Oct;70(2):286–292. doi: 10.1042/bj0700286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopowick R. E., Forward D. F. Translocation of Radioactive Carbon after the Application of C-Alanine and CO(2) to Sunflower Leaves. Plant Physiol. 1974 Jan;53(1):21–27. doi: 10.1104/pp.53.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger D. R., Saunders M. A., Cataldo D. A. Translocation and accumulation of translocate in the sugar beet petiole. Plant Physiol. 1969 Dec;44(12):1657–1665. doi: 10.1104/pp.44.12.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger D. R., Sovonick S. A., Shock T. L., Fellows R. J. Role of free space in translocation in sugar beet. Plant Physiol. 1974 Dec;54(6):892–898. doi: 10.1104/pp.54.6.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heathcote J. G., Haworth C. An improved technique for the analysis of amino acids and related compounds on thin layers of cellulose. II. The quantitative determination of amino acids in protein hydrolysates. J Chromatogr. 1969 Aug 5;43(1):84–92. doi: 10.1016/s0021-9673(00)99169-6. [DOI] [PubMed] [Google Scholar]
- Joy K. W., Antcliff A. J. Translocation of amino acids in sugar beet. Nature. 1966 Jul 9;211(5045):210–211. doi: 10.1038/211210a0. [DOI] [PubMed] [Google Scholar]
- Nobel P. S., Cheung Y. S. Two amino-acid carriers in pea chloroplasts. Nat New Biol. 1972 Jun 14;237(76):207–208. doi: 10.1038/newbio237207a0. [DOI] [PubMed] [Google Scholar]
- Sovonick S. A., Geiger D. R., Fellows R. J. Evidence for active Phloem loading in the minor veins of sugar beet. Plant Physiol. 1974 Dec;54(6):886–891. doi: 10.1104/pp.54.6.886. [DOI] [PMC free article] [PubMed] [Google Scholar]



