Abstract
Background:
Women have higher morning serum zolpidem concentrations than men after taking an evening dose, potentially leading to increased risk of harm. On 19 April 2013, the United States Food and Drug Administration required labeling changes for zolpidem, recommending an initial dose of no greater than 5 mg (immediate release) or 6.25 mg (controlled release) per night in women.
Objectives:
The primary objective of this study was to compare prescribing practices before and after the 2013 zolpidem labeling change. A secondary objective was to evaluate serious adverse events potentially related to zolpidem.
Methods:
Electronic medical records of adults receiving care through the University of Colorado Health system were accessed for study inclusion if patients were provided a first-time prescription for zolpidem either prior to or after the Food and Drug Administration labeling change. Patients were randomly chosen from eight strata based on age, gender, and date of zolpidem initiation (before/after the labeling change). Demographic and zolpidem prescribing data were collected. Low-dose zolpidem was considered 5 mg (immediate release) or 6.25 mg (controlled release) daily or less. Documentation of potentially related serious adverse events within the patients’ records was also evaluated.
Results:
A total of 400 patients were included in the study. The overall percentage of patients prescribed low-dose zolpidem increased from 44% to 58% after the labeling change (p = 0.0020). In a pre-specified subgroup analysis, the percentage of patients prescribed low-dose zolpidem increased in all groups, including young men (38%–50%, p = 0.23), elderly men (34%–40%, p = 0.53), and elderly women (60%–74%, p = 0.14), but the change was only significant in young women (42%–70%, p = 0.0045).
Conclusion:
After Food and Drug Administration–mandated labeling changes for zolpidem in 2013, the percentage of overall patients in our health system, and specifically young women, with initial prescriptions for low-dose zolpidem significantly increased as compared to before the labeling change.
Keywords: Zolpidem, insomnia, sleep initiation and maintenance disorders, United States Food and Drug Administration, drug safety
Background
Zolpidem is the most widely prescribed sedative-hypnotic in the United States. It was initially approved by the Food and Drug Administration (FDA) in 1992 in an immediate release (IR) formulation under the trade name Ambien® (Sanofi-Aventis, Bridgewater, NJ) for the short-term treatment of insomnia. Due to concern regarding morning psychomotor impairment and interpatient variability of adverse effects, the first product label encouraged providers to individualize the dose of zolpidem and specifically recommended a lower initial dose of 5 mg for elderly, debilitated patients, and patients with hepatic impairment.1 As additional zolpidem dosage forms were studied and approved for use, further variations in morning psychomotor impairment were discovered.
A modified-release formulation of zolpidem (Ambien CR®, Sanofi-Aventis) was approved in 2005 for the treatment of insomnia characterized by difficulties with sleep onset and/or sleep maintenance. Subsequently, a sublingual, lower-dose zolpidem tablet (Intermezzo®, Purdue Pharma, Stamford, CT) was approved in 2011 for patients who had difficulty falling back to sleep after waking in the middle of the night. Although there were previous data that observed higher serum zolpidem concentrations in women than men, this was the first zolpidem dosage form labeled with different recommended doses for men and women (3.5 vs 1.75 mg, respectively) at the time of initial approval.2,3 This recommendation to individualize the dose of sublingual zolpidem based on gender was apparently due to data that showed that women, when given the same dose as men, had higher serum concentrations and lower clearance of zolpidem compared to men.4 The presumed goal of the gender-specific dosing recommendations was to minimize next-day psychomotor impairment, while preserving the efficacy for middle-of-the-night insomnia, in both men and women.
Safety concerns regarding zolpidem also prompted the FDA to re-analyze pharmacokinetic (PK) data for other zolpidem dosage forms to determine if the serum concentrations from alternative formulations were also gender dependent. The FDA’s review was described by Farkas5 and Farkas et al.6 and found that the serum drug concentrations differed between men and women for both zolpidem IR and controlled release (CR). When analyzing the PK data from abbreviated new drug application studies for zolpidem IR, the FDA found that approximately 15% of women and 3% of men had serum drug concentrations ⩾50 ng/mL 8–9 h after taking zolpidem IR 10 mg.5 These PK data also showed about one-third of women and one-quarter of men had serum drug concentrations ⩾50 ng/mL 8–9 h after taking zolpidem CR 12.5 mg.5 Farkas5 describes in the FDA safety communication that the serum zolpidem concentration of 50 ng/mL was considered significant by the FDA due to driving simulation and laboratory studies submitted to the FDA indicating the degree of impairment in patients with serum zolpidem concentrations ⩾50 ng/mL was similar to that observed in patients with blood alcohol concentrations (BACs) considered illegal for driving.7 Although there are no published driving data to support the recommendation, this conclusion apparently guided the FDA to the required label change.
Given their concerns about differing serum zolpidem drug concentrations in women versus men, the FDA required a labeling change effective 19 April 2013 that reduced the initial recommended dose for zolpidem in women from 10 to 5 mg (IR) daily and from 12.5 to 6.25 mg (CR) daily.7 The dosing recommendations for men and the elderly remained unchanged. The goal of this study was to assess whether the 2013 dosing recommendations based on gender impacted zolpidem prescribing practices in our health system.
Methods
This was a retrospective, pre/post electronic medical record review of patients with first-time prescriptions for zolpidem. Inclusion criteria included (1) adults aged 18–89 years receiving continuous care at a University of Colorado (CU) Health ambulatory care clinic since 1 April 2011 and (2) new initiation of zolpidem IR or CR between the dates of 1 April 2011 to 1 April 2013 or 1 June 2013 to 1 June 2015. Although the zolpidem labeling was changed on 19 April 2013, 1 June 2013 was chosen as the start date for the “after” group to allow time for dissemination of information to providers. Patients were excluded if they were previously prescribed zolpidem at any time through the CU health system. This study was approved by the Colorado Multiple Institutional Review Board.
An electronic list of patients meeting inclusion criteria was generated and then stratified into eight groups based on age, gender, and date of zolpidem initiation. The eight groups included young men, elderly men, young women, and elderly women before the labeling change; and young men, young women, elderly men, and elderly women after the labeling change. “Elderly” was defined as age ⩾65 years old. The “before” group was defined as patients who were initially prescribed zolpidem within 2 years before the labeling change, and the “after” group defined as within the 2 years after the labeling change. After stratification into eight groups, 50 subjects were randomly selected within each of the eight strata using a random number generator within Microsoft® Excel® (v14.5.0). Exclusions were made during the manual electronic medical review for patients with historical zolpidem prescriptions, as it was not possible to determine when these patients were initially prescribed zolpidem.
The primary objective of this study was to compare the proportion of patients prescribed low-dose zolpidem before the 2013 labeling change to the proportion of patients prescribed low-dose zolpidem after the 2013 labeling change. Low dose was considered to be zolpidem IR ⩽5 mg or zolpidem CR ⩽6.25 mg daily. If a patient was prescribed a range of zolpidem (e.g. zolpidem IR, 5–7.5 mg), the highest dose prescribed (e.g. zolpidem IR, 7.5 mg) was the documented dose. Secondary objectives included (1) a subgroup analysis to determine and compare the proportion of subjects prescribed low-dose zolpidem before the labeling change to the proportion after the labeling change in each of four demographic groups: young men, young women, elderly men, and elderly women and (2) to compare documented serious adverse events, potentially due to zolpidem, before and after the labeling change.
The following data were collected for each patient: gender, age, ethnicity, race, zolpidem index date, initial zolpidem dose, history of falls or fractures, history of cognitive impairment or dementia, number and description of falls, fractures, or motor vehicle accidents (MVAs) during the study period, number of emergency department (ED) visits for falls, fractures, or MVAs during the study period, and if the patient was prescribed low-dose or high-dose zolpidem when the adverse event occurred.
The baseline characteristics were compared using a two-sample t-test assuming unequal variances (age) and chi-square tests (race and ethnicity). A multivariate logistic regression model was used to examine the association of age (elderly vs non-elderly), gender, and zolpidem index date (before vs after labeling change) with receiving a low-dose zolpidem prescription. This model was used to determine the overall effect of the labeling change on low-dose zolpidem prescriptions and if the effect of the labeling change was different between genders and/or between age groups. A descriptive stratified analysis was used to examine the association between the labeling change and initial zolpidem dose within each sample strata (elderly men, young men, elderly women, and young women). For all analyses, an α of 0.05 was considered statistically significant. All statistical analyses were executed in R statistical software (version 3.3.0). The glm function for estimating generalized linear models in the stats package was used for logistic regression analyses.
Results
A total of 4513 patients met study criteria with 200 patients randomly selected for the “before” group and 200 patients randomly selected for the “after” group. Groups were similar with regard to baseline characteristics (Table 1). While a greater number of patients had a history of falls/fractures/MVAs and cognitive impairment/dementia in the “after” group, as compared to the “before” group, the difference was not statistically significant.
Table 1.
Characteristics of the study population before and after the 2013 zolpidem labeling change.
| Before (n = 200) | After (n = 200) | |
|---|---|---|
| Mean age, years | 56.8 | 57.5 |
| Ethnicity, n (%) | ||
| Non-Hispanic | 168 (84) | 174 (87) |
| Hispanic | 15 (7.5) | 15 (7.5) |
| Unknown | 17 (8.5) | 11 (5.5) |
| Race, n (%) | ||
| White | 154 (77.0) | 159 (79.5) |
| Black | 12 (6.0) | 13 (6.5) |
| Other | 20 (10.0) | 20 (10.0) |
| Unknown | 14 (7.0) | 8 (4.0) |
| History of falls, fractures, or MVAs, n (%) | 17 (8.5) | 21 (10.5) |
| History of cognitive impairment or dementia, n (%) | 4 (2) | 7 (3.5) |
MVA: motor vehicle accident.
In the initial multivariate model, which included the three-way interaction between age, gender, and index prescription date and all pairwise interactions, none of the interaction effects that included zolpidem index date were significant (p = 0.46). This indicates that the magnitude of the effect of the labeling change was not statistically different between the four subgroups. The final multivariate model did not include these interaction effects but did include age, gender, zolpidem index date, and the interaction between age and gender. This model indicated that the percentage of patients prescribed low-dose zolpidem increased significantly after the labeling change (p = 0.0020; Figure 1). In the stratified analysis, the percentage of patients prescribed low-dose zolpidem increased in all subgroups, including young men (p = 0.23), young women (p = 0.0045), elderly men (p = 0.53), and elderly women (p = 0.14), but the increase was only statistically significant in young women (Figure 1).
Figure 1.
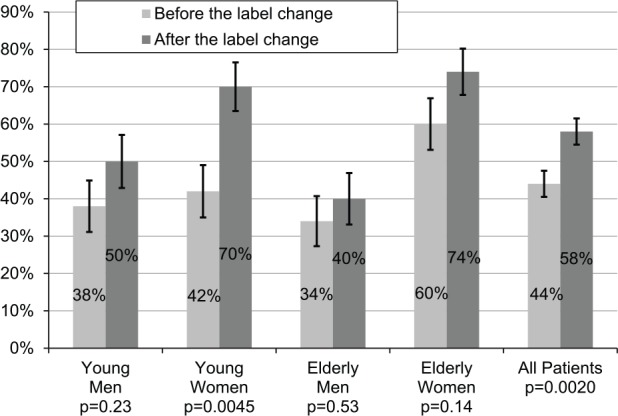
Percentage of patients prescribed low-dose zolpidem.
Although rare, there were more documented serious adverse events after the labeling change, including falls/fractures/MVAs and ED visits for falls, fractures, and MVAs (7 in the “before” group vs 12 in the “after” group). Several patients experienced more than one adverse event. The seven adverse events in the “before” group occurred in six patients (3%). The 12 adverse events in the “after” group occurred in nine patients (4.5%). Based upon dose, 3.4% (7 out of 204) of patients prescribed low-dose zolpidem and 4.1% (8 out of 196) of patients prescribed high-dose zolpidem experienced serious adverse events. The majority of patients who suffered an adverse event were women (60%; 9 out of 15 patients) and/or elderly (73%; 11 out of 15 patients).
Discussion
Zolpidem prescribing practices in our health system appeared to be affected by the FDA drug safety communication and updated zolpidem label in 2013.7 The percentage of patients prescribed low-dose zolpidem increased overall and in all subgroups. However, the percentage increase was statistically significant only in the subgroup of young women. Although our providers may have previously been aware of differences in serum zolpidem concentrations between men and women, our data indicate that providers changed their prescribing habits and followed the updated dosing recommendations closely for young women. The data also suggest that the labeling change may have served as a reminder to prescribe the lowest zolpidem dose possible in all patients. While the product labeling change did not specifically state that only young women should initially be prescribed zolpidem IR 5 mg or zolpidem CR 6.25 mg, if providers were following recommended dosing, elderly men and women should have already been prescribed low-dose zolpidem based on previous dosing recommendations from 2008.8 While the community may assume that providers always follow FDA dosing recommendations closely, there are data to the contrary. This was illustrated in a study describing simvastatin prescribing patterns before and after FDA-mandated dosing restrictions.9 The study found that 41% and 93% of patients continued to be prescribed against-label regimens of simvastatin-calcium channel blocker and simvastatin-non-calcium channel blocker combinations, respectively, 9 months after the simvastatin product labeling was updated with dosing restrictions. We are pleased that 70% of young women in our study had appropriate initial zolpidem doses prescribed after the labeling change. By following governmental dosing recommendations closely, providers may have prevented patients from sustaining adverse reactions.
Although there is no demonstrated difference in zolpidem safety or efficacy based on gender,10,11 evidence suggests there is a dose–response relationship with zolpidem adverse events, supporting the rationale to prescribe the lowest effective dose of zolpidem for a short duration.12 A matched cohort study, including over 80,000 patients, compared patients prescribed zolpidem with patients who were not prescribed zolpidem to estimate the risk of head injury or fracture requiring hospitalization.12 The adjusted hazard ratio (HR) for major injury in the zolpidem group was 1.67 (95% confidence interval (CI), 1.19–2.34). The authors found the HR increased as the mean dose of zolpidem per year increased, supporting a dose–response relationship. For groups prescribed zolpidem 71–800, 801–1600, and >1600 mg/year, HRs (95% CI) for major injury were 2.04 (1.32–3.13), 4.37 (2.12–9.01), and 4.74 (2.38–9.42), respectively.
In addition to an increased risk of head injury and fracture, zolpidem has also been associated with increased automobile crashes due to hangover sedation.13 A new user cohort study of over 400,000 adults in Washington State enrolled in an integrated health care system estimated the association between sedative-hypnotic use, including temazepam, trazodone, and zolpidem, and motor vehicle crash risk. The study found new users of all three sedatives were at an increased risk of crash relative to nonusers. Zolpidem had a higher risk of crash compared to temazepam and trazodone (HR (95% CI), 2.20 (1.64–2.95), 1.27 (0.85–1.91), and 1.91 (1.62–2.25), respectively). The risk estimates are equivalent to the risk while driving with BACs between 0.06% and 0.11%, indicating some of the drivers would be considered too impaired to legally operate an automobile.13 Furthermore, several studies evaluating driving simulation and psychomotor tests support that the driving impairment with zolpidem is often not recognizable to the affected patient.6,14,15 Therefore, it is not feasible to tell patients to wait to drive until they feel capable. In fact, this could cause more accidents by giving patients the false sense that they will know when they are capable of driving.
While there is an increased risk of automobile crashes potentially due to hangover sedation from zolpidem, there are also data that associate sleepiness with decreased driving performance and increased risk of automobile crashes.16–18 Furthermore, the efficacy of zolpidem is shown to be dose dependent in most studies.19 Therefore, it is important for providers to remember that the product labeling recommends both (1) to prescribe an initial dose of zolpidem IR 5 mg or zolpidem CR 6.25 mg in women and (2) to use the lowest effective dose for the patient.7 In some instances, it may be appropriate to increase the zolpidem dose beyond the starting dose to ensure the medication is effective and the patient’s insomnia is appropriately treated. To our knowledge, there are no published studies comparing the safety of zolpidem and the incidence of adverse effects before and after the labeling change.
Limitations of this study are largely due to its retrospective nature. We were not able to determine whether clinical decisions regarding the appropriate initial zolpidem dose for a patient were due to knowledge of the FDA safety announcement and zolpidem labeling change in 2013 or due to other patient-specific factors. Second, this study had a relatively small sample size and only large differences in the proportion of patients prescribed low-dose zolpidem were expected to reach statistical significance. Another potential limitation is that the data were gathered retrospectively from electronic health records. Medical problem lists and ED notes were utilized to determine if patients had a history of falls, fractures, MVAs, a history of cognitive impairment/dementia, or if a serious adverse event occurred. These factors may not have been added to patients’ problem lists and therefore may be underreported in this study. We were unable to gather any ED data from other hospitals in our area, so the incidence of ED visits is likely underestimated. Moreover, the adverse event data are hypothesis generating only and cannot be used to draw conclusions regarding the safety of zolpidem. Our study was not powered to detect a difference in adverse events in the “before” group versus the “after” group and there was no control group. Furthermore, we cannot prove any of the previously discussed adverse events were caused by zolpidem given the retrospective nature of the study. Finally, we were not able to collect evidence (e.g. prescription claims data, interview patients, zolpidem serum levels) that the patient was actually taking their zolpidem at the time of their adverse event. Patients may have taken more zolpidem than prescribed, which could have precipitated their adverse events.
Future research is needed to further examine the effect of the labeling changes and the impact on clinical outcomes. Our population was within an academic medical center where updated evidence is easily disseminated to providers, so our results may not be generalizable to all practice settings. Larger cohorts and the use of prescription claims data and patient interviews are needed to determine if the labeling changes have truly affected the safety or efficacy of zolpidem products in young women.
Conclusion
Within the University of Colorado Health system, the percentage of new zolpidem prescriptions initiated at a low dose significantly increased after the 2013 FDA required labeling change. However, consistent with the specifics of the labeling change, when subgroups were evaluated only the subgroup of young women had a statistically significant increase in low-dose zolpidem prescriptions after the labeling change.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval: Ethical approval for this study was obtained from the Colorado Multiple Institutional Review Board (COMIRB #15-1651).
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Informed consent: Informed consent was not sought for this study because it was retrospective.
References
- 1. Sanofi-Aventis. Ambien (package insert). Bridgewater, NJ: Sanofi-Aventis, 1992. [Google Scholar]
- 2. Olubodun JO, Ochs HR, Von Moltke LL, et al. Pharmacokinetic properties of zolpidem in elderly and young adults: possible modulation by testosterone in men. Br J Clin Pharmacol 2003; 56: 297–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Purdue Pharma. Intermezzo (package insert). Stamford, CT: Purdue Pharma, 2011. [Google Scholar]
- 4. Greenblatt DJ, Harmatz JS, Singh NN, et al. Gender differences in pharmacokinetics and pharmacodynamics of zolpidem following sublingual administration. J Clin Pharmacol 2014; 54: 282–290. [DOI] [PubMed] [Google Scholar]
- 5. Farkas RH. Clinical review: dosing for zolpidem products (Reference ID: 3137324). Silver Spring, MD: Food and Drug Administration (FDA), 2013, pp. 1–15. [Google Scholar]
- 6. Farkas RH, Unger EF, Temple R. Zolpidem and driving impairment—identifying persons at risk. N Engl J Med 2013; 369: 689–691. [DOI] [PubMed] [Google Scholar]
- 7. Food and Drug Administration. FDA drug safety communication: FDA approves new label changes and dosing for zolpidem products and a recommendation to avoid driving the day after using Ambien CR, http://www.fda.gov/Drugs/DrugSafety/ucm352085.htm (2013, accessed 10 August 2015).
- 8. Sanofi-Aventis. Ambien (package insert). Bridgewater, NJ: Sanofi-Aventis, 2008. [Google Scholar]
- 9. Tuchscherer RM, Nair K, Ghushchyan V, et al. Simvastatin prescribing patterns before and after FDA dosing restrictions: a retrospective analysis of a large healthcare claims database. Am J Cardiovasc Drugs 2015; 15: 27–34. [DOI] [PubMed] [Google Scholar]
- 10. Roehrs TA, Roth T. Gender differences in the efficacy and safety of chronic nightly zolpidem. J Clin Sleep Med 2016; 12: 319–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Roth T, Steinberg F, Singh NN, et al. Gender influences on efficacy and safety of sublingual zolpidem tartrate for middle-of-the-night awakening in insomnia. Hum Psychopharmacol 2014; 29: 25–30. [DOI] [PubMed] [Google Scholar]
- 12. Lai MM, Lin CC, Lin CC, et al. Long-term use of zolpidem increases the risk of major injury: a population-based cohort study. Mayo Clin Proc 2014; 89: 589–594. [DOI] [PubMed] [Google Scholar]
- 13. Hansen RN, Boudreau DM, Grossman DC, et al. Sedative hypnotic medication use and the risk of motor vehicle crash. Am J Public Health 2015; 105: 64–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mallila MJ, Matiila-Evenden ME. Effects of alcohol and hypnosedative drugs on digit-symbol substitution: a comparison of two different computerized tests. J Psychopharmacol 1997; 11: 313–317. [DOI] [PubMed] [Google Scholar]
- 15. Verster JC, Roth T. Driver can poorly predict their own driving impairment: a comparison between measurements of subjective and objective driving quality. Psychopharmacology 2011; 219: 775–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Connor J, Norton R, Ameratunga S, et al. Driver sleepiness and risk of serious injury to car occupants: population based case control study. Br Med J 2002; 324: 1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Philip P, Sagaspe P, Moore N, et al. Fatigue, sleep restriction, and driving performance. Accident Anal Prev 2003; 37: 473–478. [DOI] [PubMed] [Google Scholar]
- 18. Sagberg F. Road accidents caused by drivers falling asleep. Accident Anal Prev 1999; 31: 639–649. [DOI] [PubMed] [Google Scholar]
- 19. Greenblatt DJ, Roth T. Zolpidem for insomnia. Pharmacotherapy 2012; 13: 879–893. [DOI] [PubMed] [Google Scholar]


