SUMMARY
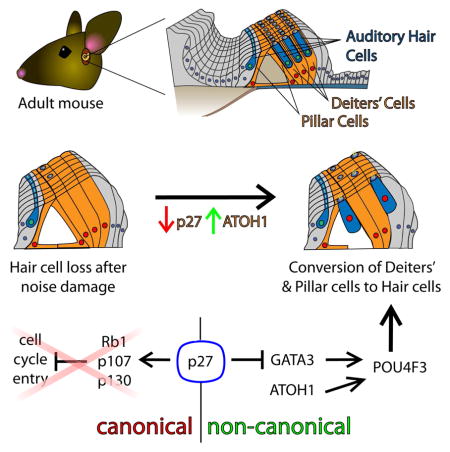
Hearing loss is widespread and persistent because mature mammalian auditory hair cells (HCs) are nonregenerative. In mice, the ability to regenerate HCs from surrounding supporting cells (SCs) declines abruptly after postnatal maturation. We find that combining p27Kip1 deletion with ectopic ATOH1 expression surmounts this age-related decline, leading to conversion of SCs to HCs in mature mouse cochleae and after noise damage. p27Kip1 deletion, independent of canonical effects on Rb-family proteins, upregulated GATA3, a co-factor for ATOH1 that is lost from SCs with age. Co-activation of GATA3 or POU4F3 and ATOH1 promoted conversion of SCs to HCs in adult mice. Remarkably, activation of POU4F3 alone also converted mature SCs to HCs in vivo. These data illuminate a genetic pathway that initiates auditory HC regeneration and suggest p27Kip1, GATA3, and POU4F3 as additional therapeutic targets for Atoh1-mediated HC regeneration.
Keywords: Regeneration, aging, differentiation, proliferation, development, cancer, sensory, cochlea, hearing
Graphical Abstract
INTRODUCTION
Sensorineural hearing loss (SNHL) is one of the most common long term disabilities in humans (Blackwell et al., 2014; Timmer et al., 2015). The vast majority of SNHL results from the loss of sensory hair cells (HCs), which, in mature mammalian cochleae, do not regenerate (McGill and Schuknecht, 1976; Soucek et al., 1986). Recently, research aimed at regenerating HCs has focused on ATOH1, a basic helix-loop-helix (bHLH) transcription factor that represents a therapeutic target for converting nonsensory supporting cells (SCs) into sensory HCs (Atkinson et al., 2014; Chen et al., 2013; Izumikawa et al., 2008; Izumikawa et al., 2005; Kawamoto et al., 2003; Kelly et al., 2012; Kraft et al., 2013; Kuo et al., 2015; Liu et al., 2012; Liu et al., 2014; Ouji et al., 2013; Pan et al., 2013; Parker et al., 2014; Wu et al., 2013; Yang et al., 2012; Yang et al., 2013; Zhao et al., 2011; Zheng and Gao, 2000). Indeed, clinical trials are being conducted to test whether an ATOH1 gene therapy can rehabilitate hearing in SNHL patients (Novartis-Pharmaceuticals). However, research in animal models suggests that ectopic Atoh1 may be insufficient because (1) a limited number of SCs respond by upregulating HC-specific genes (Izumikawa et al., 2005; Kawamoto et al., 2003; Liu et al., 2012; Liu et al., 2014); (2) those cells that do respond lack markers of terminal differentiation and the physiological properties of mature cochlear HCs (Atkinson et al., 2014; Liu et al., 2012; Liu et al., 2014; Yang et al., 2012); and (3) in genetic mouse models, it has yet to be demonstrated that ATOH1 can convert SCs into HCs in the adult cochlea (Kelly et al., 2012; Liu et al., 2012). As human cochleae become functionally mature neonatally (Hepper and Shahidullah, 1994), and as SNHL is most prevalent in older adults (Blackwell et al., 2014), overcoming the limits that aging imposes on reprogramming cochlear cells is of paramount importance.
Reprogramming of cells is rarely accomplished by manipulating a single factor. Rather, the coordinated manipulation of several factors is needed for reprogramming and regeneration of cardiac, neural, intestinal, hepatic, and pancreatic tissues (Lalit et al., 2016; Niu et al., 2013; Shaffiey et al., 2016; Song et al., 2016; Wei and Hong, 2016). Similarly, recent in vitro evidence suggests that co-expression of several factors (POU4F3, GFI1, ATOH1) is more effective than ectopic expression of ATOH1 alone in converting embryonic stem cells into HC-like cells (Costa et al., 2015). In embryonic and neonatal mouse cochlear tissue, ectopic expression of TCF3, GATA3, ETV4, NMYC, or ETS2 in combination with ATOH1 yielded more HC-like cells than did overexpression of ATOH1 alone (Ikeda et al., 2015; Masuda et al., 2012).
In non-mammalian models of HC regeneration, p27Kip1 (p27) expression is significantly decreased at early timepoints following HC loss (Hawkins et al., 2007; Jiang et al., 2016; Jiang et al., 2014). Indeed p27 is a known regulator of quiescence in HCs and SCs in the inner ear (Chen and Segil, 1999; Lowenheim et al., 1999), and it is negatively correlated with regenerative potential in other tissues as well (Georgia and Bhushan, 2006; Minamishima et al., 2002; Wang et al., 2002; Yoshida et al., 2002). As such, the targeting of p27 has been suggested as a means for increasing the number of HCs that can be generated by ectopic ATOH1 via a proliferative increase in the pool of SCs to be targeted (Minoda et al., 2007). However, p27 has not been investigated for any direct role in cellular transdifferentiation or more specifically the loss of reprogramming efficiency with age.
Here we show that, unlike ectopic ATOH1 alone, concomitant p27 deletion and ectopic ATOH1 expression results in a significant number of SCs converting to HCs in adult mice, in vivo. However, in contrast to known functions of p27, we did not observe proliferation of SCs or the converted HCs (cHCs). Furthermore, when cell-cycle regulators downstream of p27 were similarly deleted in conjunction with ectopic ATOH1 there was no increase in SC to HC conversion. These results suggest a role for p27 in ATOH1 function and cell differentiation that is cell-cycle-independent. Our data support this, revealing a previously uncharacterized role for p27 in repressing GATA3 expression in mature SCs. GATA3, in turn, facilitates ATOH1-mediated conversion of mature SCs to HCs. Also, we demonstrate that POU4F3 upregulation is a critical event in the conversion of SCs by ATOH1. Indeed, ectopic expression of human POU4F3 (hPOU4F3) is, by itself, sufficient to upregulate HC-specific genes in mature SCs, and ectopic hPOU4F3 in conjunction with ATOH1 converts even more SCs into HCs than either ectopic hPOU4F3 or ectopic ATOH1 individually. In sum, p27 plays a cell-cycle independent role in preventing ATOH1-mediated conversion of adult SCs to HCs by repressing GATA3 expression; GATA3 and POU4F3 promote ATOH1-mediated HC regeneration in the mature cochlea; and ATOH1-based gene therapies for auditory HC regeneration may benefit from the additional targeting of one or more of the gene products described (i.e. p27, GATA3, or POU4F3).
RESULTS
Co-manipulation of p27 and ATOH1 results in the phenotypic conversion of adult cochlear SCs to HCs
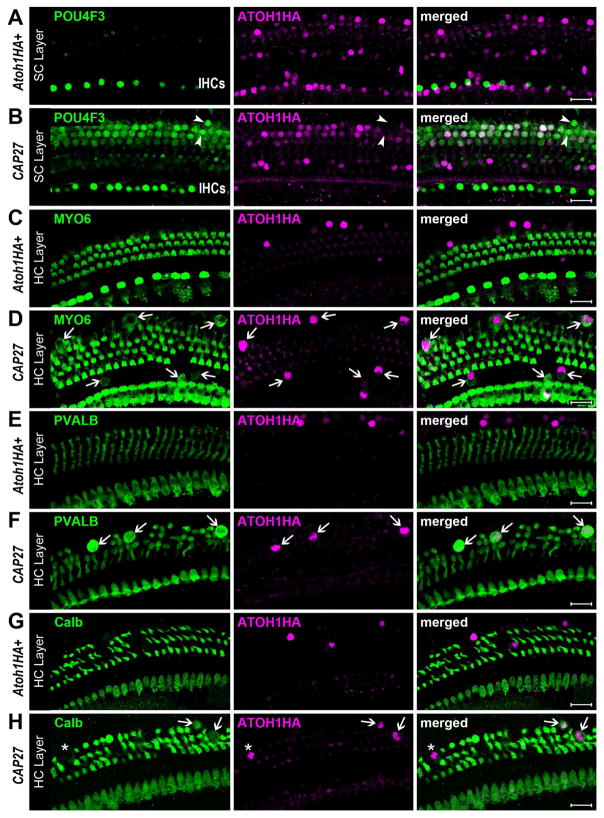
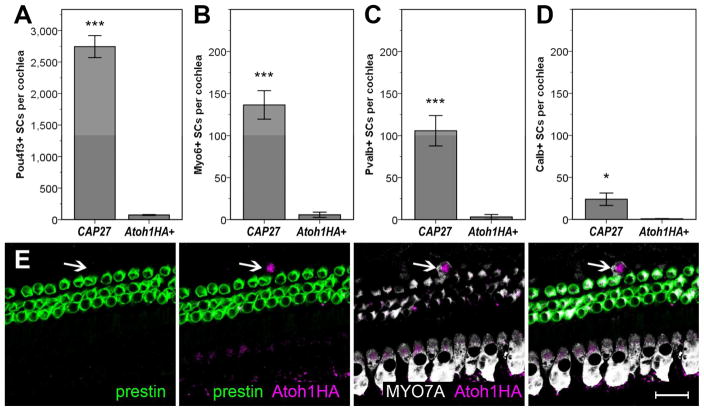
We have previously demonstrated that an Fgfr3-iCreER mouse line (Young et al., 2010) can be used to overexpress an Atoh1HA transgene in cochlear pillar cells (PCs) and Deiters cells (DCs), but not HCs, when tamoxifen (Tamox) is administered to mice at postnatal day (P) 12 or later (Liu et al., 2012). Furthermore, a hemagglutinin (HA) tag on the Atoh1HA transgene allowed us to fate map PCs and DCs that ectopically express the transgene and distinguish those cells from endogenous HCs. Ectopic expression of ATOH1HA at P12–13 resulted in phenotypic conversion of 11.7% of the HA-positive PCs and DCs, with 13 different HC-specific markers, hair bundles, and innervating fibers being observed (Liu et al., 2012). However, when ectopic ATOH1HA was induced in mature cochleae (P30), no PCs or DCs exhibited HC-specific markers, even though most of them were HA-positive. To test whether the conditional knockout (CKO) of p27 could overcome this limitation and facilitate the phenotypic conversion of PCs and DCs by ATOH1HA in mature cochleae, in vivo, we bred p27loxP/loxP mice (Chien et al., 2006) with Fgfr3iCreER+;Atoh1HA+ mice and induced them with Tamox at P28 (hereafter, CAP27 mice, for co-manipulation of ATOH1HA and p27). Three weeks after induction (P49), cochleae were collected and immunostained for the HC-specific proteins POU4F3, myosin VI (MYO6), myosin VIIa (MYO7A), parvalbumin (PVALB), calbindin (Calb), and prestin. Similar to previous observations, very few HA-positive SCs expressed HC-specific markers in the Fgfr3iCreER+;Atoh1HA+ mice (Figure 1). In contrast, CAP27 littermates demonstrated a robust upregulation of several HC-specific markers (Figures 1, S1), specifically MYO6 (12.1 ± 2.4% of HA+ cells), MYO7A (not quantified), PVALB (9.3 ± 1.9% of HA+ cells), Calb (2.1 ± 0.7% of HA+ cells), and POU4F3 (> 100% of HA+ cells). We did not detect any cells that had upregulated prestin, a marker of HC maturation (Figure 2). Cochlear cells that were immunopositive for HA exhibited several different phenotypes suggestive of different stages of maturation or conversion (Figure S2). While many of the cHCs exhibited apical migration of the nucleus from to the HC layer, a hallmark of HC regeneration in non-mammals (Stone and Cotanche, 2007), some of the PVALB or MYO7A positive cells retained morphologies consistent with that of SCs and exhibited no nuclear migration. Some cHCs exhibited nuclear and somal sizes larger than that of endogenous outer HCs (OHCs), while nuclear diameter in others were more consistent with that of SCs, and endogenous OHCs (Figures S1, S2). This is consistent with the fact that we observed markers typical of early HC development (e.g. POU4F3, MYO6) more often than markers that are typical of later maturation (e.g. Calb, prestin) (Figure 2). This pattern suggests that SCs were converted to HCs with varying latencies, leading to cHCs that resembled diverse stages of normal HC development.
Figure 1.
Co-manipulation of p27 and ATOH1 converts SCs to HCs. Little to no co-expression of HA and POU4F3 was observed in Fgfr3iCreER+;Atoh1HA+ (Atoh1HA+) samples (A), while numerous HA+ cells were POU4F3+ in CAP27 mice (B). Some HA-negative SCs were also seen to upregulate POU4F3 (arrowheads). Little to no co-expression of HA and MYO6 was observed in Atoh1HA+ samples (C), but many HA+ cells were MYO6+ (arrows) in CAP27 samples (D). Little to no co-expression of HA and PVALB was observed in Atoh1HA+ samples (E), while several HA+ cells were PVALB+ (arrows) in CAP27 samples (F). Little to no co-expression of HA and Calb was observed in Fgfr3iCreER+;Atoh1HA+ samples (G), but HA and Calb double-positive cells (arrows), as well as Calb-negative cells (asterisk) were readily observed in CAP27 samples (H). Scale bars = 20 μm
Figure 2.
Converted cells represent different stages of HC maturation, but fail to terminally differentiate. Significantly more POU4F3+ PCs & DCs were observed in CAP27 mice as compared to Fgfr3iCreER+;Atoh1HA+ (Atoh1HA+) littermates (A). Significantly more MYO6+ PCs & DCs were observed in CAP27 mice than in Atoh1HA+ littermates (B). Significantly more PVALB+ PCs & DCs were observed in CAP27 mice than in Atoh1HA+ littermates (C). Significantly more Calb+ PCs & DCs were observed in CAP27 mice than in Atoh1HA+ littermates (D). Co-expression of HA and the mature HC marker prestin was not observed in any of the CAP27 mice, not even in cells that were co-labeled for ATOH1HA and MYO7A (arrow). Data are presented as mean ± 1 S.E.M. ***p ≤ 0.001, *p < 0.05, scale bar = 20 μm.
As enlarged nuclei were observed in several of the cHCs, we sought to investigate whether those cells might be dying, or if enlarged nuclei and somal size were merely part of the conversion process. Measures of the density of PCs and DCs are consistent with similar measures from wild type mice (Mizutari et al., 2013), and did not reveal any significant loss of SCs: 70.91 ± 0.64 SCs per 100 μm in Fgfr3iCreER+;Atoh1HA+ samples versus 70.64 ± 0.45 SCs per 100 μm in CAP27 mice. However, immunostaining for cleaved caspase 3, a marker of apoptotic cell death, suggested that a small portion of the cHCs (< 6 per cochlea) were undergoing apoptosis three weeks after Tamox injections (Figure S2). Examination PVALB and Atoh1HA double-positive cells in CAP27 cochleae 6 weeks after Tamox revealed a possible loss of some cHCs between 3 and 6 weeks after Tamox (105.8 ± 18.1 vs. 69.3 ± 5.6 PVALB+ cHCs), though this difference was not statistically significant (Figure S2). Thus, co-manipulation of p27 and ATOH1 appears to overcome the limitation of SC to HC conversion in the adult mouse cochlea, but induces cell death in some of the affected cells, and fails to elicit further maturation of cHCs beyond what ATOH1 alone accomplishes in neonatal mice.
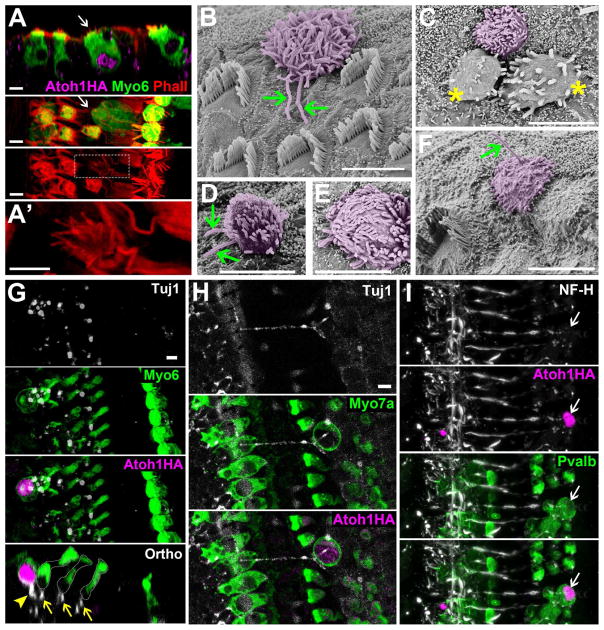
Consistent with what has been observed in neonatal models (Liu et al. 2012) we did observe PVALB-positive cHCs with hair bundles that stained readily with phalloidin, and attracted beta-3-tubulin (Tuj1) or neurofilament (NF-H) positive nerve terminals (Figure 3), suggesting that a portion of the converted cells had differentiated at least to a state where they exhibited morphological attributes of functional HCs. However, future work will be needed to determine whether the apposition of neurites is reflective of actual synaptic communication. Scanning electron microscopy (SEM) also revealed a number of hair bundles projecting out from under the reticular lamina which were consistent with previous reports of SC to HC conversion (Kawamoto et al., 2003; Minoda et al., 2007). Many of these cells had a kinocilium and/or disorganized bundles, again suggesting incomplete maturation. A few cells presented with 2 kinocilia (Figure 3), which is not typical of a mammalian cochlea. Rather, previous EM studies have shown HCs with 2 kinocilia in the balance organs of fish and amphibians (Flock, 1964; Hudspeth and Jacobs, 1979). This observation suggests that SCs converted to HCs by ATOH1 may represent a more primitive type of HC, as suggested previously (Yang et al., 2012).
Figure 3.
Converted cells have hair bundles and recruit neuronal fibers. Co-labeling of HA, MYO6, and phalloidin (Phall) demonstrates a converted SC that has actin bundles (A, A′). The first panel provides an orthogonal view (Ortho), while the second and third panels show a top view. A′ is an enlarged image of the white square in the third panel of A. SEM images reveal ectopic hair bundles (artificially colored magenta) with atypical morphologies (B–F). Some cells exhibited one or even two kinocilia (arrows) (F and B &D, respectively). Some HCs appeared to be dying and possibly extruded from the membrane (asterisks, C) though whether these were converted cells or existing HCs was indeterminate. (G) Tuj1+ terminals were readily observed underneath endogenous HCs (arrows), and seen to contact HA & MYO6 double positive cells (arrowhead). (H) A partial projection image revealed a Tuj1+ nerve fiber stretching across the tunnel of Corti to contact an HA and MYO7A double-positive cell. (I) An HA+ cell that is also PVALB+ is contacted by a NF-H+ neurite. Scale bars = 5 μm
There has been much speculation, yet no conclusive answer, as to whether Notch signaling, or other factors that might hinder or foster a regenerative response in the cochlea are changed in response to noise damage (Mizutari et al., 2013; Maass et al., 2015). To determine whether the context of noise damage would enhance or inhibit the effect of p27 and ATOH1 co-manipulation in reprogramming PCs and DCs, we exposed Fgfr3iCreER+;Atoh1HA+ mice and CAP27 littermates to octave-band noise (8–16 kHz) at 120 dB SPL for 2 h at P30 (2 days after Tamox). Immunostaining for HA and PVALB at P49 revealed a similar number of co-labeled cells in the noise damaged CAP27 mice as what had been observed in undamaged CAP27 mice (Figure S3). Again, we did not observe any cells that were positive for both HA and prestin. In noise damaged Fgfr3iCreER+;Atoh1HA+ cochleae we did not observe any cHCs that co-expressed either PVALB or prestin. These data reproduce the finding that p27CKO enhances ATOH1-mediated conversion of mature PCs and DCs and suggest that co-manipulation of p27 and ATOH1 is a viable strategy for initiating a regenerative response in mature mammalian cochleae. In addition, the results provide some insight into the question of whether noise damage results in the up-or down-regulation of factors that affect the phenotypic conversion of SCs to HCs. In the context of ATOH1-mediated conversion as studied here, there appear to be no dramatic changes resulting from noise trauma that prevent (or promote) the conversion of PCs or DCs to HCs, though this does not necessarily exclude possible changes in the expression of Notch-related, or other pertinent factors.
Conversion of PCs and DCs in CAP27 mice involves a non-canonical role of p27
The canonical function of p27 is to halt cell division by inhibiting cyclin/CDK complexes that would otherwise phosphorylate the Rb (Rb1), p107 (Rbl1), or p130 (Rbl2) proteins (Ezhevsky et al., 1997). To determine whether this canonical pathway is pertinent in the SC to HC conversion in CAP27 mice, we examined cell proliferation in this model, and also looked for SC to HC conversion in mice where ATOH1 and Rb-family proteins were co-manipulated. To assay proliferation, we added 5-bromo-2′-deoxyuridine (BrdU) to the drinking water of experimental and control mice for 2 weeks after Tamox induction at P28. Neither Fgfr3iCreER+;p27CKO nor CAP27 mice (N = 4 for each) exhibited SCs or HCs that were positive for BrdU when examined at P42 (Figure S4). Cells in the tympanic border and in the modiolus were positive for BrdU, revealing that it entered the peripheral auditory system and was detectable. We next tested whether genetic ablation of Rb-family proteins, concomitant with ectopic ATOH1HA, could recapitulate the CAP27 phenotype. The co-manipulation of Rb1 and ATOH1 did not result in an increase in PVALB-positive cHCs as compared to ectopic expression of ATOH1HA alone (Figure S4). Similarly, neither the co-manipulation of p130 and ATOH1HA (Fgfr3iCreER+;Atoh1HA+;p130CKO), nor of p107 and ATOH1HA (Fgfr3Cre; Atoh1HA; p107KO) resulted in an increase in the numbers of PVALB-positive cHCs as compared to Fgfr3iCreER+;Atoh1HA+ mice. In double knockout models (Fgfr3iCreER+;Atoh1HA+;RbCKO;p130CKO and Fgfr3iCreER+;Atoh1HA+;RbCKO;p107KO) we also saw no significant increase in the numbers of PVALB-expressing cHCs beyond what was seen in Fgfr3iCreER+;Atoh1HA+ mice. Finally, we bred triple CKO mice that would also express ATOH1HA (Fgfr3iCreER;Atoh1HA;RbCKO;p130CKO;p107KO), but none of the mice that were homozygous for all 3 knockouts and positive for Fgfr3iCreER and ATOH1HA survived more than 4 days after Tamox injection. Despite this limitation, the data demonstrate that deletion of any 2 Rb-family proteins does not facilitate conversion of PCs and DCs by ATOH1 in mature mouse cochleae. These findings suggest that the conversion in CAP27 mice is independent of an effect on Rb-family proteins, and that, in mature PCs and DCs, inhibition of ATOH1 function by p27 is non-canonical.
p27CKO results in an upregulation of GATA3 in mature PCs and DCs
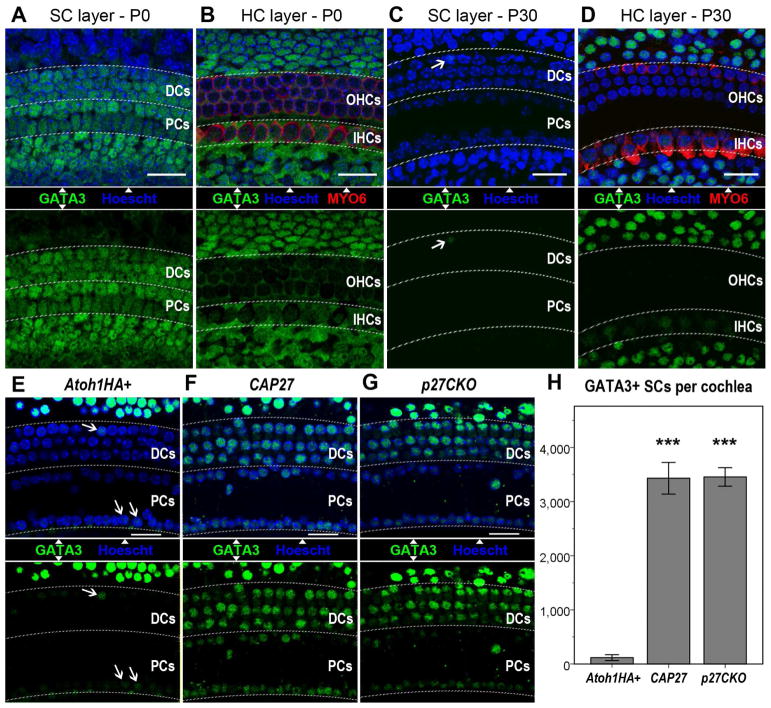
To investigate why ATOH1-mediated conversion of PCs and DCs declines after P12–P13, we examined the expression of 2 known cofactors for ATOH1: GATA3 and TCF3 (Masuda et al., 2012). Immunostaining for these factors revealed prevalent expression of both GATA3 and TCF3 in neonatal SCs, including PCs and DCs (Figures 4, S5). At P30, TCF3 was still present in the majority of SCs, whereas GATA3 was undetectable in PCs and DCs (Figures 4, S5). This inverse correlation of GATA3 expression with age, and its role in facilitating the upregulation of POU4F3 and MYO7A by ATOH1 (Masuda et al., 2012), implicated GATA3 as a critical factor in ATOH1HA-mediated conversion of SCs. To test whether GATA3 was mediating the effect of p27CKO on ATOH1 function in mature PCs and DCs, we first examined whether p27CKO could affect GATA3 expression. Fgfr3iCreER+;Atoh1HA+ mice, Fgfr3iCreER+;p27CKO mice, and CAP27 mice were induced with Tamox at P28, and stained for MYO6, and GATA3 at P49. Cell counts revealed many more GATA3-positive PCs and DCs in the CAP27 and the Fgfr3iCreER+;p27CKO mice as compared to Fgfr3iCreER+;Atoh1HA+ mice (Figure 4). These data suggest that p27CKO in adult PCs and DCs reinstates GATA3 expression to a neonatal-like pattern.
Figure 4.
GATA3 expression positively correlates with SC responsiveness to ATOH1. GATA3 is widely distributed in neonatal cochlear SCs (A & B), but is selectively lost from PCs & DCs by P30 (C & D). IPh/BCs and Hensen cells remain positive for GATA3 at P30 (D). Ectopic ATOH1 in Fgfr3iCreER+;Atoh1+ (Atoh1+) mice does not alter GATA3 expression (E). p27CKO, either in conjunction with ATOH1HA (F), or independently (G), causes a significant increase in GATA3+ PCs & DCs as compared to ectopic ATOH1HA alone (H). Data are presented as mean ± 1 S.E.M. *** p < 0.01. Scale bars = 20 μm
GATA3 facilitates ATOH1-mediated conversion of SCs to HCs in the mature mouse cochlea
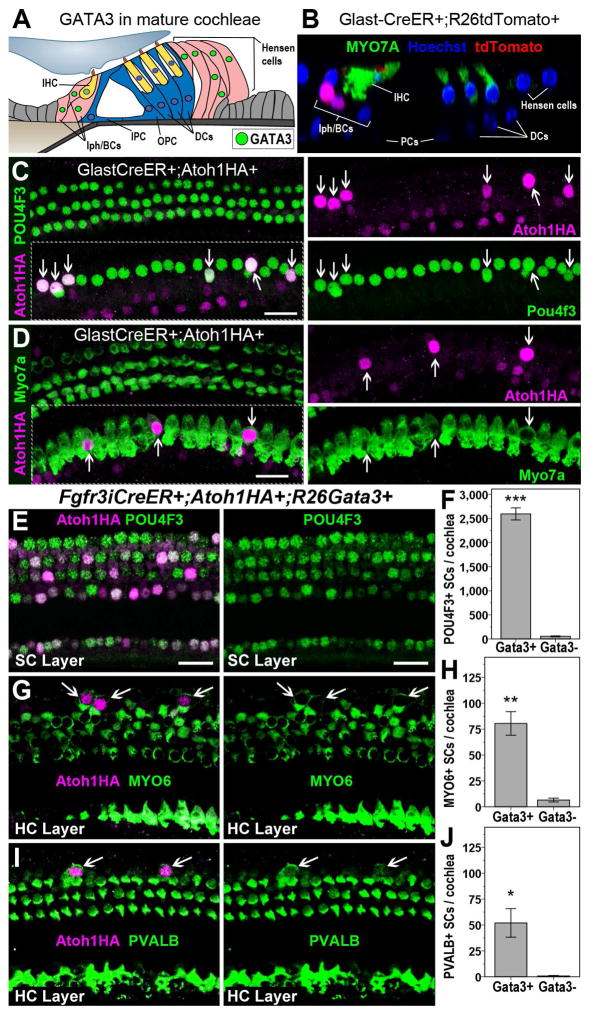
While PCs and DCs lost GATA3 expression with age, inner phalangeal and border cells (IPh/BCs) (Figure 5A) remained GATA3 immunopositive in adult cochleae. To test whether mature IPh/BCs could respond to ATOH1HA, we bred GLAST-CreER mice, where Cre is active in IPh/BCs (Mellado Lagarde et al., 2014), with Atoh1HA+ mice, and induced with Tamox at P28. Ectopic expression of ATOH1HA by itself resulted in the upregulation of POU4F3 (not quantified) and MYO7A (50.75 ± 6.1 cHCs/cochlea) in IPh/BCs (Figures 5, S5). This positive correlation of GATA3 expression and ATOH1 function in converting IPh/BCs suggests that the lack of a similar response to ATOH1HA in mature PCs and DCs is due to loss of GATA3 in those cells, and that p27CKO confers responsiveness to mature PCs and DCs by reinstating GATA3 expression. To test this, we attempted to conditionally delete GATA3 from IPh/BCs while ectopically expressing ATOH1HA (Glast-CreER; Atoh1HA; GATA3loxP/loxP), but these animals died within days of Tamox administration. As a second approach, we bred Rosa26-GATA3 mice (Nguyen et al., 2013) with Fgfr3iCreER+; Atoh1HA+ mice (Fgfr3iCreER+; Atoh1HA+;GATA3+) to ectopically express both GATA3 and ATOH1HA in mature PCs and DCs. Similar to CAP27 mice, significantly more PCs and DCs were converted to HCs by GATA3 and ATOH1HA co-expression than ATOH1HA expression alone (Figure 5E–J). This suggests that GATA3 is a critical cofactor for ATOH1 in the initial regenerative conversion of SCs to HCs and that the age-related decline of GATA3 in PCs and DCs prevents ATOH1-mediated conversion.
Figure 5.
GATA3 promotes ATOH1-mediated conversion of SCs to HCs. A diagram of the typical pattern of GATA3 expression in the mature organ of Corti (A). IPC = inner pillar cell, OPC = outer pillar cell. A representative image of GLAST-CreER+;R26tdTomato+ activity in IPh/IB cells one week after Tamox induction at P28 (B). Ectopic ATOH1HA results in the upregulation of POU4F3 (C) and MYO7A (D) in IPh/IB cells (arrows). POU4F3 (E), MYO6 (G), and PVALB (I) are upregulated in PCs & DCs (arrows) of Fgfr3iCreER+;Atoh1HA+;GATA3+ mice. Co-manipulation of GATA3 and ATOH1HA (GATA3+) resulted in significantly more POU4F3+ (F), MYO6+ (H), and PVALB+ (J) SCs than ectopic ATOH1HA alone (GATA3−). Data are presented as mean ± 1 S.E.M, *p < 0.05, **p < 0.01, ***p < 0.001. Scale bars = 20 μm
Ectopic expression of hPOU4F3 promotes the conversion of adult cochlear SCs to HCs
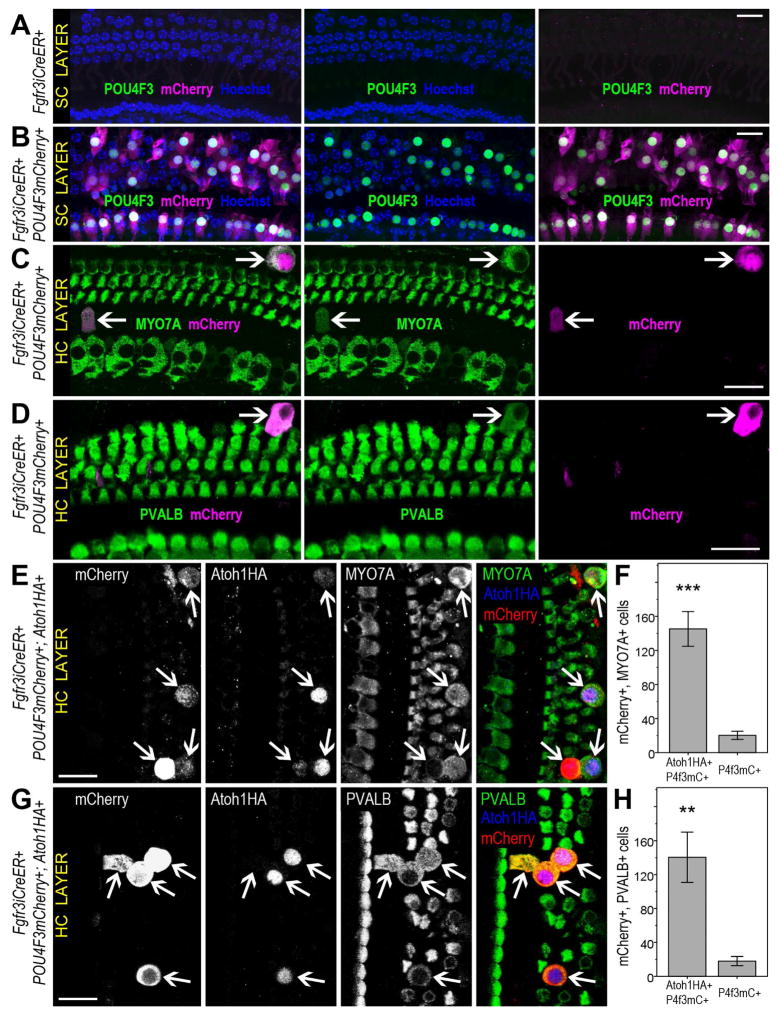
To further elucidate the role of GATA3 in converting mature PCs and DCs to HCs, we sought to test the involvement of POU4F3, a known target of both ATOH1 and GATA3 (Masuda et al., 2012). In both CAP27 and Fgfr3iCreER+;Atoh1HA+;GATA3+ mice, a majority of PCs and DCs upregulated POU4F3, as compared to only a minority of cells that upregulated other HC markers. To test the role of POU4F3 directly, we generated a mouse line that simultaneously overexpresses hPOU4F3 and the fluorescent marker mCherry upon Cre recombination (Figure S6). By breeding these mice with Fgfr3iCreER+ mice, we were able to ectopically express hPOU4F3 in mature PCs and DCs and fate map the affected mCherry-positive cells. Remarkably, a number of cells were positive for both mCherry and either PVALB (1.9 ± 0.8% of mCherry+ cells) or MYO7A (2.1 ± 0.8% of mCherry+ cells), demonstrating that ectopic hPOU4F3 can upregulate HC-specific markers in mature SCs (Figures 6, S6). Although more PCs and DCs could be induced to express PVALB or MYO7A by ectopic POU4F3-mCherry than by ATOH1HA, the phenotype for ectopic hPOU4F3 was not as robust as the CAP27 phenotype. This suggests that both ATOH1 and POU4F3 are needed to elicit the upregulation of HC markers in a greater number of PCs and DCs. To test this, we bred POU4F3-mCherry mice with Fgfr3iCreER+;Atoh1HA mice and ectopically expressed both hPOU4F3 and ATOH1 in mature PCs and DCs (Fgfr3iCreER+;Atoh1HA+;POU4F3-mCherry+). This combined expression resulted in even more PVALB+ (16.5 ± 1.7% of mCherry+ cells) and MYO7A+ (20.7 ± 3.3% of mCherry+ cells) cHCs than in either Fgfr3iCreER+;Atoh1HA+ or Fgfr3iCreER+;POU4F3mCherry+ mice (Figures 6, S6). These data demonstrate that POU4F3 alone can upregulate HC-specific markers in mature PCs and DCs, and that POU4F3 when combined with ATOH1 causes the conversion of greater numbers of PCs and DCs than either ATOH1 or POU4F3 alone.
Figure 6.
Ectopic hPOU4F3 causes upregulation of HC-specific markers in mature PCs & DCs. Tamox induction at P12–P13 did not result in any mCherry or hPOU4F3 expression in PCs or DCs of control mice (Fgfr3iCreER+) at P35 (A). Fgfr3iCreER+;POU4F3-mCherry mice exhibited robust expression of mCherry and POU4F3 in PCs & DCs (B). When Fgfr3iCreER+;POU4F3-mCherry mice were induced with Tamox at P28, several mCherry+ cells upregulated the HC markers MYO7A (C) and PVALB (D) by P49. Combined POU4F3-mCherry and ATOH1HA expression also resulted in MYO7A (E,F) and PVALB (G,H) expression in a number of PCs & DCs. The number of MYO7A and mCherry double-positive cells per cochlea was significantly higher in Fgfr3iCreER+;Atoh1HA+;POU4F3-mCherry+ samples than in Fgfr3iCreER+;POU4F3− mCherry+ samples ***p = 0.001 (F). The number of PVALB and mCherry double positive cells per cochlea was significantly higher in Fgfr3iCreER+;Atoh1HA+;POU4F3-mCherry+ samples than in Fgfr3iCreER+;POU4F3mCherry+ samples, **p < 0.01 (H). Data are presented as mean ± 1 S.E.M. Scale bars = 20 μm
DISCUSSION
Sensorineural hearing loss is one of the most common and costly long term disabilities as rehabilitation is limited by a lack of cochlear HC regeneration. Much attention has focused on ectopic ATOH1 as a potential gene therapy for HC regeneration, however, studies from our lab and others suggest that ATOH1 is inefficient in converting SCs to HCs in adult cochleae. Here we show that p27CKO potentiates the effect of ectopic ATOH1 in adult mice, causing upregulation of HC-specific markers in mature PCs and DCs in both the undamaged and damaged context. This finding reveals key players in the initiation of regeneration in a model that is highly resistant to reprogramming, becomes even more resistant with age, and is directly linked to a widespread human health condition. Also, the notion that ATOH1 can generate new HCs in adult cochleae has been controversial. Several studies (Izumikawa et al., 2008; Kelly et al., 2012; Liu et al., 2012) suggest that ATOH1 is ineffectual in converting SCs to HCs in mature or damaged ears, particularly in genetic mouse models. Other reports suggest that virally transduced ATOH1 (Izumikawa et al., 2005; Kawamoto et al., 2003; Kraft et al., 2013) can convert SCs to HCs in mature rodent cochleae. Our data suggest that responsiveness to ATOH1 varies across SC subtypes and that mature PCs and DCs specifically are unresponsive to ATOH1. As viral transduction of ATOH1 affects SCs other than PCs and DCs (Atkinson et al., 2014; Kawamoto et al., 2003; Kraft et al., 2013), the difference in target cells may explain the paradox. Importantly, the data here show that adult PCs and DCs can be made to respond to ectopic ATOH1 via additional manipulations of p27, GATA3, or hPOU4F3. This is significant, not only because it helps to reconcile the controversy concerning ATOH1 activity in adult SCs, but also because PCs and DCs represent highly attractive targets for HC regeneration. Location is critical to HC function, therefore the optimal approach to HC regeneration would be one where newly generated HCs not only replace endogenous OHCs in number, but positionally as well. PCs and DCs are intercalated between and beneath the existing OHCs, and exhibit nuclear migration into the HC-layer upon conversion. In contrast, other SCs would have to be induced to migrate along the mediolateral axis in order to replace endogenous OHCs. Furthermore, in cell populations that do respond to ATOH1, the number of cells that convert is low. Targeting p27, GATA3, or POU4F3 in conjunction with ATOH1, can therefore lead to increased numbers of converted cells by expanding the pool of responsive cells to include PCs and DCs. Such an increase could prove beneficial to Atoh1 gene therapies that are currently being developed.
In addition to its usefulness for regenerative strategies, the findings pertaining to p27CKO provide insight into uncharacterized roles of p27. Specifically, the data suggest that the conversion of mature PCs and DCs by ATOH1HA and p27CKO is independent of Rb-family inactivation. While cyclin/CDK complexes could still be involved in the observed phenotypes, the apparent lack of involvement of Rb, p107, or p130 suggests a non-canonical response to p27CKO. Indeed, loss of p27 resulted in GATA3 upregulation in PCs and DCs. To our knowledge, there is no precedented role for p27 in the regulation of GATA3 expression, or in HC differentiation. These findings have numerous implications since p27 is present in many different cell and tissue types and important in development, aging, and cancer, where cell differentiation is an important process (Chu et al., 2008; Pruitt et al., 2013; Teratake et al., 2016). Similarly, GATA3 is a critical transcription factor in many cell types and cell processes including T-cell differentiation, neurosensory development, and tumor progression and metastasis (Du et al., 2015; Duncan and Fritzsch, 2013; Si et al., 2015; Van de Walle et al., 2016). If, and how, p27 regulates GATA3 expression in these and other cells, tissues, and processes therefore remain intriguing and open questions.
The fact that GATA3 expression declines in cochlear SCs after gestation has been previously demonstrated (Rivolta and Holley, 1998). The data here suggest a refinement of that observation in that GATA3 was most prominently lost from PCs and DCs, but remained detectable in IPh/IB cells, Hensen cells, and Claudius cells. In addition, our data suggest that GATA3 is sufficient to restore neonatal-like responsiveness of PCs and DCs to ATOH1. These findings suggest an important role for GATA3 in regeneration and aging where loss of GATA3 correlates with senescence, and persistence of GATA3 correlates with greater regenerative potential. Indeed, it has been suggested that GATA3 is universally important in tissue regeneration, playing roles in neural, cardiac, and fin regeneration in zebrafish (Strahle and Schmidt, 2012). In the cochlea, SCs of the types shown here to be GATA3+ are generally more proliferative, more regenerative, and more responsive to manipulations intended to induce a cell fate change (Kamiya et al., 2001; Kawamoto et al., 2003; Kelly et al., 2012; Kuo et al., 2015; Mellado Lagarde et al., 2014). Since human cochleae become mature and functional during fetal development (Clark-Gambelunghe and Clark, 2015), the current murine data suggest that human GATA3 expression may already be lost from PCs and DCs by birth. Therefore it may be of profound importance to determine whether GATA3 is similarly lost from SCs in humans as a result of development or aging. If GATA3 does decline with age in humans, the success of ATOH1-mediated therapies may be hindered. However, the findings here suggest that simultaneous ectopic expression of GATA3 and ATOH1 could bolster the effectiveness of such an approach.
In both CAP27 and Fgfr3iCreER+;Atoh1HA+;GATA3+ strains, the increased expression of POU4F3 was particularly robust. Additionally, ectopic hPOU4F3 caused the upregulation of HC-specific markers in mature PCs and DCs, and co-manipulation of hPOU4F3 and ATOH1 led to increased conversion of PCs and DCs compared to the manipulation of either gene individually. POU4F3, therefore, appears to be an important factor in HC regeneration. This is consistent with previous findings that suggested a synergistic role for POU4F3 in helping ATOH1 to upregulate MYO7A in embryonic, nonsensory progenitor cells (Ikeda et al., 2015; Masuda et al., 2012). Our data extend these findings to demonstrate a direct role for POU4F3 in the conversion of adult cochlear SCs to HCs, in vivo. However, it is interesting to note that despite robust upregulation of POU4F3 in the vast majority of PCs and DCs in CAP27, Fgfr3iCreER+;Atoh1HA+;GATA3+, and Fgfr3iCreER+;Atoh1HA+;POU4F3mCherry+ models, only a limited number of cells upregulated PVALB or MYO7A. This is consistent with the low numbers of cHCs that have been generated in previous models of ATOH1 overexpression (where it is presumed POU4F3 was also upregulated), and in our current model where POU4F3 was directly, ectopically expressed. These findings suggest that the low efficiency of conversion in ectopic Atoh1 models may not be due to an inability of Atoh1 to bind to and activate its target genes, but rather an inability of POU4F3 to bind to and activate its target genes. It is therefore likely that factors that improve accessibility to, or activation of, POU4F3 target genes (e.g. epigenetic factors or transcriptional co-factors) would be useful in increasing the numbers of cHCs in ATOH1 models. Indeed, the data strongly suggest that POU4F3 is critical in ATOH1-mediated SC to HC conversion, particularly in the mature cochlea, and also that POU4f3 represents a therapeutic target, the activity of which could be enhanced independently or in conjunction with ATOH1 to initiate auditory HC regeneration.
Finally, we provide here a more detailed description of a genetic pathway in the initial events of regenerating HCs from SCs. However, the regenerative phenotypes described are clearly incomplete. While p27CKO, or ectopic expression of GATA3 or hPOU4F3, are able to overcome the age limitation of ATOH1-mediated SC conversion, the converted cells are still immature, lacking appropriate bundle morphology and failing to express the mature HC marker prestin. In addition, only a limited number of cHCs could be produced in the models presented, and some of the cHCs exhibited clear signs of apoptotic cell death. In order to have regeneration that can lead to functional recovery, additional targets need to be identified that can increase the numbers of new HCs generated and promote the maturation and survival of the cHCs. Multiple genes in addition to those identified here will most likely need to be manipulated. For example, ectopic espin1, concomitant to p27 and ATOH1 or hPOU4F3 and ATOH1 manipulations, may be useful in promoting normal bundle morphology (Taura et al., 2016). Also, the enhancement of Wnt/β-catenin signaling in addition to the manipulations described above could increase the numbers of cells that convert to HCs (Kuo et al., 2015; Ni et al., 2016).
In summary we show the substantive conversion of adult PCs and DCs specifically into HCs using three different combinatorial genetic approaches, thus establishing a genetic pathway for the initiation of HC regeneration in the mature mammalian cochlea. This identification of several key molecular players in the initial steps of HC regeneration in the mature cochlea will hopefully act as a foundation for improving genetic and small molecule approaches to auditory HC regeneration and the rehabilitation of hearing following SNHL. It should also serve to improve our understanding of the interactions and functions of several factors (p27, GATA3, POU4F3, ATOH1) that are critical to numerous biological processes.
EXPERIMENTAL PROCEDURES
Animals and housing
Animals were housed in the animal resources center at St. Jude Children’s Research Hospital according to approved IACUC protocols and guidelines. Mice were provided food and water ad libitum and maintained in a 12:12 light:dark cycle. Generation of the genetic mouse models ATOH1HA, Fgfr3iCreER, GlastCre-ER, p27loxP/loxP, GATA3loxP/loxP, Rb1loxP/loxP, p130loxP/loxP, p107KO, and Rosa26-GATA3 have been described previously (See Table S1). hPOU4F3 overexpressing mice (POU4F3-mCherry) were generated by pronuclear injection of a purified bicistronic transgene containing a CAG promoter followed by the ORF of hPOU4F3, an IRES2 internal ribosome entry site, and the coding sequence for the mCherry fluorescent protein, followed by a woodchuck hepatitis virus posttranscriptional response element and an SV40 polyA terminator sequence (Figure S6). While four potential founders genotyped positive for the POU4F3-mCherry transgene, mCherry fluorescence upon Cre recombination was only observed in offspring from one founder, and this line was used for the experiments described.
For all experiments, sample sizes were N = 4 experimental animals and N = 4 controls, except for the noise damage experiment where we used 3 experimental mice and 3 controls, and the cleaved caspase-3 staining, where we used N = 2 and N = 2. Experiments used balanced male:female ratios, except for the POU4F3-mCherry experiments where only females were used due to a much lower rate of recombination and mCherry expression observed in males from this strain. For all experiments, mice were injected with Tamox (Sigma, 250 mg/kg ip) at P28 and euthanized at P49, except in 3 cases: (1) the initial characterization of the POU4F3-mCherry mice (Figure 6A–B, Tamox at 75mg/kg ip on P12 and P13, euthanasia at P35), (2) where mice were provided BrdU (Sigma) at 2mg/mL in the drinking water for 14 days following Tamox (250mg/kg ip on P28, euthanasia at P42), and (3) where CAP27 mice were analyzed at a 6-week survival timepoint (Figure S2). Upon euthanasia, temporal bones were quickly removed and immersed overnight in 2% paraformaldehyde in PBS.
Scanning Electron Microscopy
Anesthetized mice were perfused with fixative (2.5% Glutaraldehyde and 2% paraformaldehyde in 0.1M CaCO4, pH 7.4). The inner ears were further perfused via the round window using the same fixative solution and decalcified in fixative containing 150mM EDTA using a Pelco Biowave microwave tissue processor (Ted Pella). Samples were critical point dried (Tousimis Sandai 790) and mounted with conductive silver paint. Samples were then coated using horizontal placed Stubs in a Denton Desk II Sputter Coater and coated with less than ~20nm of Iridium. All Samples were examined in an FEI TeneoVS Scanning Electron Microscope (FEI, Eindhoven, the Netherlands).
Immunostaining
Temporal bones were decalcified in 0.125M EDTA in PBS for 48 hours prior to microdissection of the organ of Corti. Tissues were immunostained using antibodies and dilutions listed in Table 1. Samples were imaged using either a Zeiss LSM700 or LSM710 point-scanning confocal microscope. Where possible, samples were counterstained with Hoechst nuclear dye (Life technologies) or with Alexa conjugated phalloidin (Life technologies) to label hair bundles and other actin-rich structures. Where necessary, TSA amplification kits (Life technologies) were used to amplify signal according to the manufacturer’s protocol.
Table 1.
Primary Antibodies
| Antigen | Host | Concentration | Supplier | Catalog# |
|---|---|---|---|---|
| HA | Rat | 1:100 | Roche | 11 867 431 001 |
| MYO6 | Rabbit | 1:200 | Proteus Bioscience | 25–6791 |
| MYO7A | Mouse | 1:80 | Santa Cruz | sc-74516 |
| PVALB | Mouse | 1:1000 | Sigma | P3088 |
| POU4F3 | Rabbit | 1:2500 + TSA | Novus | NBP1–88349 |
| Calbindin | Rabbit | 1:500 | Millipore | AB1778 |
| Prestin | Goat | 1:500 | Santa Cruz | sc-22692 |
| SOX2 | Rabbit | 1:500 | Millipore | AB5603 |
| GATA3 | Mouse | 1:200 + AR | BD Biosciences | 558686 |
| TCF3 | Goat | 1:100 + AR | Novus | NB100–57092 |
| Cleaved caspase 3 | Rabbit | 1:50 | Cell Signaling | 9669 |
KEY: TSA = TSA amplification kit from Invitrogen/Life Technologies
AR = low pH antigen retrieval from Vector Labs (1 hour at 98°C)
Data Analysis
Immunopositive cells were counted manually from images of whole mounted cochleae using Zen and LSM browser software packages (Carl Zeiss). For PCs and DCs that were positive for HA, GATA3, and/or POU4F3, counts were obtained from 9 representative images per cochlea (avg. 268 ± 3 μm in length per image) separated by roughly equal distances along the tonotopic axis and covering apical, middle and basal turns. The densities of positive cells/μm were then extrapolated to a 6 mm total cochlear length. For the remaining HC markers that were labeled (PVALB, MYO6, Calb, prestin), counts were taken from the entire cochlear sample. For all images, cochlear length was measured as described previously (Liu et al., 2013). For all of the experiments, two-tailed t-tests were conducted, and where applicable, Bonferroni corrections were used. All statistical analyses were conducted using SPSS 17.0 (IBM).
Supplementary Material
Acknowledgments
The authors would like to thank Maxime Bouchard for providing the Rosa26-GATA3 mice that were generated by Jody Haigh. We would also like to thank Sharon Frase, Richard Gursky, Julie Justice (Cellular Imaging Shared Resources at St. Jude), Lou Boykins (Univ of Memphis), and Jie Fang (St. Jude Children’s Research Hospital) for their technical assistance, and Keith Laycock for editing assistance. This research was supported by funding from the National Institutes of Health (grants 2 R01DC006471 (J.Z.), 1 R01DC015010 (J.Z.), 1R01DC015444 (J.Z.), 1R21DC013879 (J.Z.), P30CA21765 (St. Jude)), ALSAC, the Office of Naval Research (grants N000140911014, N000141210191, N000141210775, N000141612315 (J.Z.)), the National Organization for Hearing Research (NOHR) Foundation (B.W.), the Hearing Health Foundation (Emerging Research Grant, B.W.), and The Hartwell Foundation (Individual Biomedical Research Award, J.Z.).
Footnotes
Author Contributions
B.W. designed the experiments, conducted experiments, collected and analyzed data, and wrote the manuscript. E.C., J.D., T.Y., G.B. and B.K. conducted experiments and collected data. J.Z. assisted with experimental design and writing of the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Atkinson PJ, Wise AK, Flynn BO, Nayagam BA, Richardson RT. Hair cell regeneration after ATOH1 gene therapy in the cochlea of profoundly deaf adult guinea pigs. PloS one. 2014;9:e102077. doi: 10.1371/journal.pone.0102077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell DL, Lucas JW, Clarke TC. Summary health statistics for U.S. adults: national health interview survey, 2012. Vital Health Stat. 2014;10:1–161. [PubMed] [Google Scholar]
- Chen P, Segil N. p27(Kip1) links cell proliferation to morphogenesis in the developing organ of Corti. Development. 1999;126:1581–1590. doi: 10.1242/dev.126.8.1581. [DOI] [PubMed] [Google Scholar]
- Chen Y, Yu H, Zhang Y, Li W, Lu N, Ni W, He Y, Li J, Sun S, Wang Z, et al. Cotransfection of Pax2 and Math1 promote in situ cochlear hair cell regeneration after neomycin insult. Sci Rep. 2013;3:2996. doi: 10.1038/srep02996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien WM, Rabin S, Macias E, Miliani de Marval PL, Garrison K, Orthel J, Rodriguez-Puebla M, Fero ML. Genetic mosaics reveal both cell-autonomous and cell-nonautonomous function of murine p27Kip1. Proc Natl Acad Sci USA. 2006;103:4122–4127. doi: 10.1073/pnas.0509514103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu IM, Hengst L, Slingerland JM. The Cdk inhibitor p27 in human cancer: prognostic potential and relevance to anticancer therapy. Nat Rev Cancer. 2008;8:253–267. doi: 10.1038/nrc2347. [DOI] [PubMed] [Google Scholar]
- Clark-Gambelunghe MB, Clark DA. Sensory development. Pediatr Clin North Am. 2015;62:367–384. doi: 10.1016/j.pcl.2014.11.003. [DOI] [PubMed] [Google Scholar]
- Costa A, Sanchez-Guardado L, Juniat S, Gale JE, Daudet N, Henrique D. Generation of sensory hair cells by genetic programming with a combination of transcription factors. Development. 2015;142:1948–1959. doi: 10.1242/dev.119149. [DOI] [PubMed] [Google Scholar]
- Du F, Yuan P, Wang T, Zhao J, Zhao Z, Luo Y, Xu B. The Significance and Therapeutic Potential of GATA3 Expression and Mutation in Breast Cancer: A Systematic Review. Med Res Rev. 2015;35:1300–1315. doi: 10.1002/med.21362. [DOI] [PubMed] [Google Scholar]
- Duncan JS, Fritzsch B. Continued expression of GATA3 is necessary for cochlear neurosensory development. PloS one. 2013;8:e62046. doi: 10.1371/journal.pone.0062046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezhevsky SA, Nagahara H, Vocero-Akbani AM, Gius DR, Wei MC, Dowdy SF. Hypo-phosphorylation of the retinoblastoma protein (pRb) by cyclin D:Cdk4/6 complexes results in active pRb. Proc Natl Acad Sci USA. 1997;94:10699–10704. doi: 10.1073/pnas.94.20.10699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flock A. Structure of the Macula Utriculi with Special Reference to Directional Interplay of Sensory Responses as Revealed by Morphological Polarization. J Cell Biol. 1964;22:413–431. doi: 10.1083/jcb.22.2.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgia S, Bhushan A. p27 Regulates the transition of beta-cells from quiescence to proliferation. Diabetes. 2006;55:2950–2956. doi: 10.2337/db06-0249. [DOI] [PubMed] [Google Scholar]
- Hawkins RD, Bashiardes S, Powder KE, Sajan SA, Bhonagiri V, Alvarado DM, Speck J, Warchol ME, Lovett M. Large scale gene expression profiles of regenerating inner ear sensory epithelia. PloS one. 2007;2:e525. doi: 10.1371/journal.pone.0000525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepper PG, Shahidullah BS. Development of fetal hearing. Arch Dis Child. 1994;71:F81–87. doi: 10.1136/fn.71.2.f81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudspeth AJ, Jacobs R. Stereocilia mediate transduction in vertebrate hair cells (auditory system/cilium/vestibular system) Proc Natl Acad Sci U S A. 1979;76:1506–1509. doi: 10.1073/pnas.76.3.1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda R, Pak K, Chavez E, Ryan AF. Transcription factors with conserved binding sites near ATOH1 on the POU4F3 gene enhance the induction of cochlear hair cells. Mol Neurobiol. 2015;51:672–684. doi: 10.1007/s12035-014-8801-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumikawa M, Batts SA, Miyazawa T, Swiderski DL, Raphael Y. Response of the flat cochlear epithelium to forced expression of Atoh1. Hear Res. 2008;240:52–56. doi: 10.1016/j.heares.2008.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumikawa M, Minoda R, Kawamoto K, Abrashkin KA, Swiderski DL, Dolan DF, Brough DE, Raphael Y. Auditory hair cell replacement and hearing improvement by Atoh1 gene therapy in deaf mammals. Nat Med. 2005;11:271–276. doi: 10.1038/nm1193. [DOI] [PubMed] [Google Scholar]
- Jiang L, Jin R, Xu J, Ji Y, Zhang M, Zhang X, Zhang X, Han Z, Zeng S. Hair cell regeneration or the expression of related factors that regulate the fate specification of supporting cells in the cochlear ducts of embryonic and posthatch chickens. Hear Res. 2016;332:17–28. doi: 10.1016/j.heares.2015.12.001. [DOI] [PubMed] [Google Scholar]
- Jiang L, Romero-Carvajal A, Haug JS, Seidel CW, Piotrowski T. Gene-expression analysis of hair cell regeneration in the zebrafish lateral line. Proc Natl Acad Sci U S A. 2014;111:E1383–1392. doi: 10.1073/pnas.1402898111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya K, Takahashi K, Kitamura K, Momoi T, Yoshikawa Y. Mitosis and apoptosis in postnatal auditory system of the C3H/He strain. Brain Res. 2001;901:296–302. doi: 10.1016/s0006-8993(01)02300-9. [DOI] [PubMed] [Google Scholar]
- Kawamoto K, Ishimoto S, Minoda R, Brough DE, Raphael Y. Math1 gene transfer generates new cochlear hair cells in mature guinea pigs in vivo. J Neurosci. 2003;23:4395–4400. doi: 10.1523/JNEUROSCI.23-11-04395.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly MC, Chang Q, Pan A, Lin X, Chen P. Atoh1 directs the formation of sensory mosaics and induces cell proliferation in the postnatal mammalian cochlea in vivo. J Neurosci. 2012;32:6699–6710. doi: 10.1523/JNEUROSCI.5420-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraft S, Hsu C, Brough DE, Staecker H. Atoh1 induces auditory hair cell recovery in mice after ototoxic injury. Laryngoscope. 2013;123:992–999. doi: 10.1002/lary.22171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo BR, Baldwin EM, Layman WS, Taketo MM, Zuo J. In Vivo Cochlear Hair Cell Generation and Survival by Coactivation of beta-Catenin and Atoh1. J Neurosci. 2015;35:10786–10798. doi: 10.1523/JNEUROSCI.0967-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalit PA, Salick MR, Nelson DO, Squirrell JM, Shafer CM, Patel NG, Saeed I, Schmuck EG, Markandeya YS, Wong R, et al. Lineage Reprogramming of Fibroblasts into Proliferative Induced Cardiac Progenitor Cells by Defined Factors. Cell Stem Cell. 2016;18:354–367. doi: 10.1016/j.stem.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Dearman JA, Cox BC, Walters BJ, Zhang L, Ayrault O, Zindy F, Gan L, Roussel MF, Zuo J. Age-dependent in vivo conversion of mouse cochlear pillar and Deiters’ cells to immature hair cells by Atoh1 ectopic expression. J Neurosci. 2012;32:6600–6610. doi: 10.1523/JNEUROSCI.0818-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Fang J, Dearman J, Zhang L, Zuo J. In Vivo Generation of Immature Inner Hair Cells in Neonatal Mouse Cochleae by Ectopic Atoh1 Expression. PloS one. 2014;9:e89377. doi: 10.1371/journal.pone.0089377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Liu Z, Walters BJ, Owen T, Kopan R, Zuo J. In vivo visualization of Notch1 proteolysis reveals the heterogeneity of Notch1 signaling activity in the mouse cochlea. PloS one. 2013;8:e64903. doi: 10.1371/journal.pone.0064903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowenheim H, Furness DN, Kil J, Zinn C, Gultig K, Fero ML, Frost D, Gummer AW, Roberts JM, Rubel EW, et al. Gene disruption of p27(Kip1) allows cell proliferation in the postnatal and adult organ of corti. Proc Natl Acad Sci U S A. 1999;96:4084–4088. doi: 10.1073/pnas.96.7.4084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maass JC, Gu R, Basch ML, Waldhaus J, Lopez EM, Xia A, Oghalai JS, Heller S, Groves AK. Changes in the regulation of the Notch signaling pathway are temporally correlated with regenerative failure in the mouse cochlea. Front Cell Neurosci. 2015;9:110. doi: 10.3389/fncel.2015.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda M, Pak K, Chavez E, Ryan AF. TFE2 and GATA3 enhance induction of POU4F3 and myosin VIIa positive cells in nonsensory cochlear epithelium by ATOH1. Dev Biol. 2012;372:68–80. doi: 10.1016/j.ydbio.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill TJ, Schuknecht HF. Human cochlear changes in noise induced hearing loss. Laryngoscope. 1976;86:1293–1302. doi: 10.1288/00005537-197609000-00001. [DOI] [PubMed] [Google Scholar]
- Mellado Lagarde MM, Wan G, Zhang L, Gigliello AR, McInnis JJ, Zhang Y, Bergles D, Zuo J, Corfas G. Spontaneous regeneration of cochlear supporting cells after neonatal ablation ensures hearing in the adult mouse. Proc Natl Acad Sci U S A. 2014;111:16919–16924. doi: 10.1073/pnas.1408064111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minamishima YA, Nakayama K, Nakayama K. Recovery of liver mass without proliferation of hepatocytes after partial hepatectomy in Skp2-deficient mice. Cancer Res. 2002;62:995–999. [PubMed] [Google Scholar]
- Minoda R, Izumikawa M, Kawamoto K, Zhang H, Raphael Y. Manipulating cell cycle regulation in the mature cochlea. Hear Res. 2007;232:44–51. doi: 10.1016/j.heares.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen AH, Tremblay M, Haigh K, Koumakpayi IH, Paquet M, Pandolfi PP, Mes-Masson AM, Saad F, Haigh JJ, Bouchard M. Gata3 antagonizes cancer progression in Pten-deficient prostates. Hum Mol Gen. 2013;22:2400–2410. doi: 10.1093/hmg/ddt088. [DOI] [PubMed] [Google Scholar]
- Mizutari K, Fujioka M, Hosoya M, Bramhall N, Okano HJ, Okano H, Edge AS. Notch inhibition induces cochlear hair cell regeneration and recovery of hearing after acoustic trauma. Neuron. 2013;77:58–69. doi: 10.1016/j.neuron.2012.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni W, Lin C, Guo L, Wu J, Chen Y, Chai R, Li W, Li H. Extensive Supporting Cell Proliferation and Mitotic Hair Cell Generation by In Vivo Genetic Reprogramming in the Neonatal Mouse Cochlea. J Neurosci. 2016;36:8734–45. doi: 10.1523/JNEUROSCI.0060-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu W, Zang T, Zou Y, Fang S, Smith DK, Bachoo R, Zhang CL. In vivo reprogramming of astrocytes to neuroblasts in the adult brain. Nat Cell Biol. 2013;15:1164–1175. doi: 10.1038/ncb2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novartis-Pharmaceuticals. ClinicalTrialsgov [Internet] Bethesda (MD): National Library of Medicine (US); 2000. A Three-part, Multicenter, Open Label, Single Dose Study to Assess the Safety, Tolerability, and Efficacy of Intra Labyrinthine (IL) CGF166 in Patients With Severe-to-profound Hearing Loss. [cited 2015 Nov 02] Available from: https://clinicaltrialsgov/ct2/show/NCT02132130 NLM Identifier: NCT02132130. [Google Scholar]
- Ouji Y, Ishizaka S, Nakamura-Uchiyama F, Wanaka A, Yoshikawa M. Induction of inner ear hair cell-like cells from Math1-transfected mouse ES cells. Cell Death Dis. 2013;4:e700. doi: 10.1038/cddis.2013.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan S, Wan J, Liu S, Zhang S, Xiong H, Zhou J, Xiong W, Yu K, Fu Y. Lentivirus carrying the Atoh1 gene infects normal rat cochlea. Neural Regen Res. 2013;8:1551–1559. doi: 10.3969/j.issn.1673-5374.2013.17.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker MA, Cheng YF, Kinouchi H, Bieber R, Edge AS. An independent construct for conditional expression of atonal homolog-1. Hum Gene Ther Methods. 2014;25:1–13. doi: 10.1089/hgtb.2013.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruitt SC, Freeland A, Rusiniak ME, Kunnev D, Cady GK. Cdkn1b overexpression in adult mice alters the balance between genome and tissue ageing. Nat Commun. 2013;4:2626. doi: 10.1038/ncomms3626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivolta MN, Holley MC. GATA3 is downregulated during hair cell differentiation in the mouse cochlea. J Neurocytol. 1998;27:637–647. doi: 10.1023/a:1006951813063. [DOI] [PubMed] [Google Scholar]
- Shaffiey SA, Jia H, Keane T, Costello C, Wasserman D, Quidgley M, Dziki J, Badylak S, Sodhi CP, March JC, et al. Intestinal stem cell growth and differentiation on a tubular scaffold with evaluation in small and large animals. Regen Med. 2016;11:45–61. doi: 10.2217/rme.15.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si W, Huang W, Zheng Y, Yang Y, Liu X, Shan L, Zhou X, Wang Y, Su D, Gao J, et al. Dysfunction of the Reciprocal Feedback Loop between GATA3- and ZEB2-Nucleated Repression Programs Contributes to Breast Cancer Metastasis. Cancer Cell. 2015;27:822–836. doi: 10.1016/j.ccell.2015.04.011. [DOI] [PubMed] [Google Scholar]
- Song G, Pacher M, Balakrishnan A, Yuan Q, Tsay HC, Yang D, Reetz J, Brandes S, Dai Z, Putzer BM, et al. Direct Reprogramming of Hepatic Myofibroblasts into Hepatocytes In Vivo Attenuates Liver Fibrosis. Cell Stem Cell. 2016;18:797–808. doi: 10.1016/j.stem.2016.01.010. [DOI] [PubMed] [Google Scholar]
- Soucek S, Michaels L, Frohlich A. Evidence for hair cell degeneration as the primary lesion in hearing loss of the elderly. J Otolaryngol. 1986;15:175–183. [PubMed] [Google Scholar]
- Strahle U, Schmidt R. A universal program for tissue regeneration? Dev Cell. 2012;23:1123–1124. doi: 10.1016/j.devcel.2012.11.009. [DOI] [PubMed] [Google Scholar]
- Taura A, Taura K, Koyama Y, Yamamoto N, Nakagawa T, Ito J, Ryan AF. Hair cell stereociliary bundle regeneration by espin gene transduction after aminoglycoside damage and hair cell induction by Notch inhibition. Gene Ther. 2016;23:415–423. doi: 10.1038/gt.2016.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teratake Y, Kuga C, Hasegawa Y, Sato Y, Kitahashi M, Fujimura L, Watanabe-Takano H, Sakamoto A, Arima M, Tokuhisa T, et al. Transcriptional repression of p27 is essential for murine embryonic development. Sci Rep. 2016;6:26244. doi: 10.1038/srep26244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone JS, Cotanche DA. Hair cell regeneration in the avian auditory epithelium. Int J Dev Biol. 2007;51:633–647. doi: 10.1387/ijdb.072408js. [DOI] [PubMed] [Google Scholar]
- Timmer BH, Hickson L, Launer S. Adults with mild hearing impairment: Are we meeting the challenge? Int J Audiol. 2015;54:786–795. doi: 10.3109/14992027.2015.1046504. [DOI] [PubMed] [Google Scholar]
- Van de Walle I, Dolens AC, Durinck K, De Mulder K, Van Loocke W, Damle S, Waegemans E, De Medts J, Velghe I, De Smedt M, et al. GATA3 induces human T-cell commitment by restraining Notch activity and repressing NK-cell fate. Nat Commun. 2016;7:11171. doi: 10.1038/ncomms11171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters BJ, Lin W, Diao S, Brimble M, Iconaru LI, Dearman J, Goktug A, Chen T, Zuo J. High-throughput screening reveals alsterpaullone, 2-cyanoethyl as a potent p27Kip1 transcriptional inhibitor. PloS one. 2014a;9:e91173. doi: 10.1371/journal.pone.0091173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters BJ, Liu Z, Crabtree M, Coak E, Cox BC, Zuo J. Auditory hair cell-specific deletion of p27Kip1 in postnatal mice promotes cell-autonomous generation of new hair cells and normal hearing. J Neurosci. 2014b;34:15751–15763. doi: 10.1523/JNEUROSCI.3200-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters BJ, Zuo J. Postnatal development, maturation and aging in the mouse cochlea and their effects on hair cell regeneration. Hear Res. 2013;297:68–83. doi: 10.1016/j.heares.2012.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Krupczak-Hollis K, Tan Y, Dennewitz MB, Adami GR, Costa RH. Increased hepatic Forkhead Box M1B (FoxM1B) levels in old-aged mice stimulated liver regeneration through diminished p27Kip1 protein levels and increased Cdc25B expression. J Biol Chem. 2002;277:44310–44316. doi: 10.1074/jbc.M207510200. [DOI] [PubMed] [Google Scholar]
- Wei R, Hong T. Lineage Reprogramming: A Promising Road for Pancreatic beta Cell Regeneration. Trends Endocrinol Metab. 2016;27:163–176. doi: 10.1016/j.tem.2016.01.002. [DOI] [PubMed] [Google Scholar]
- Wu N, Li M, Chen ZT, Zhang XB, Liu HZ, Li Z, Guo WW, Zhao LD, Ren LL, Li JN, et al. In vivo delivery of Atoh1 gene to rat cochlea using a dendrimer-based nanocarrier. J Biomed Nanotechnol. 2013;9:1736–1745. doi: 10.1166/jbn.2013.1684. [DOI] [PubMed] [Google Scholar]
- Yang J, Bouvron S, Lv P, Chi F, Yamoah EN. Functional features of trans-differentiated hair cells mediated by Atoh1 reveals a primordial mechanism. J Neurosci. 2012;32:3712–3725. doi: 10.1523/JNEUROSCI.6093-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Cong N, Han Z, Huang Y, Chi F. Ectopic hair cell-like cell induction by Math1 mainly involves direct transdifferentiation in neonatal mammalian cochlea. Neurosci Lett. 2013;549:7–11. doi: 10.1016/j.neulet.2013.04.053. [DOI] [PubMed] [Google Scholar]
- Yoshida K, Nakayama K, Nagahama H, Harada T, Harada C, Imaki J, Matsuda A, Yamamoto K, Ito M, Ohno S, et al. Involvement of p27(KIP1) degradation by Skp2 in the regulation of proliferation in response to wounding of corneal epithelium. Invest Ophthalmol Vis Sci. 2002;43:364–370. [PubMed] [Google Scholar]
- Young KM, Mitsumori T, Pringle N, Grist M, Kessaris N, Richardson WD. An Fgfr3-iCreER(T2) transgenic mouse line for studies of neural stem cells and astrocytes. Glia. 2010;58:943–953. doi: 10.1002/glia.20976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao LD, Guo WW, Lin C, Li LX, Sun JH, Wu N, Ren LL, Li XX, Liu HZ, Young WY, et al. Effects of DAPT and Atoh1 overexpression on hair cell production and hair bundle orientation in cultured Organ of Corti from neonatal rats. PloS one. 2011;6:e23729. doi: 10.1371/journal.pone.0023729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng JL, Gao WQ. Overexpression of Math1 induces robust production of extra hair cells in postnatal rat inner ears. Nat Neurosci. 2000;3:580–586. doi: 10.1038/75753. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.