Summary
Natriuretic peptides (NPs) are essential for the maintenance of volume homeostasis, and can be of myocardial, renal, and endothelial origin. Advances in peptide engineering have enabled the design of innovative designer NPs that go beyond native peptides in efficacy, specificity, and resistance to enzymatic degradation. Therefore, designer NPs provide an unparalleled opportunity for the treatment of cardiovascular disease. In this review, we report the conceptual framework of peptide engineering of the NPs that resulted in designer peptides for cardiovascular disease. We specifically provide an update on those currently in clinical trials for heart failure and hypertension.
Key Words: designer natriuretic peptide, drug development, heart failure, hypertension, natriuretic peptide
Abbreviations and Acronyms: ANP, A-type natriuretic peptide; AS-BNP, alternatively spliced variant of BNP; BNP, B-type natriuretic peptide; BP, blood pressure; CD-NP, cenderitide; cGMP, cyclic guanosine monophosphate; CNP, C-type natriuretic peptide; CV, cardiovascular; DNP, D-type natriuretic peptide; FDA, Food and Drug Administration; GFR, glomerular filtration rate; HF, heart failure; HTN, hypertension; NEP, neprilysin; NP, natriuretic peptide; NPR, natriuretic peptide receptor; pGC, particulate guanylyl cyclase; RAAS, renin-angiotensin-aldosterone system; SQ, subcutaneous; URO, urodilatin; VNP, ventricular natriuretic peptide; ZD100, MANP
Central Illustration
The development of new drugs for cardiovascular (CV) disease continues to rapidly expand in recognition of the growing burden of CV disease worldwide (1). The approval of a small molecule, Entresto (Novartis, East Hanover, New Jersey), for heart failure (HF) has provided momentum for drug discovery in CV and related fields (2). The identification of paracrine-acting peptides released from cardiac cell-based therapies, and the role of peptides in the beneficial actions of Entresto via inhibition of neprilysin (NEP), have renewed interest in peptide therapeutics for disease syndromes such as HF and hypertension (HTN) 3, 4. As stated by Jay and Lee (5), peptide therapeutics may permit more targeted approaches through well-characterized receptors and molecular pathways, as well as avoiding the off-target actions associated with small molecules. Furthermore, peptides possess larger surface areas than small molecules, which may optimize receptor activation. However, a major limitation to peptide therapies is rapid degradation, because >600 molecularly different proteases exist in humans, which limit the bioavailability of peptides compared with small molecules (6).
Breakthrough technologies in peptide engineering have markedly accelerated peptide therapeutics in disease areas such as diabetes with novel glucagon-like peptide 1 receptor activators, in HIV with therapeutics that target novel molecular markers, and even more recently in CV disease with the use of peptides such as seralaxin, and as discussed in this review, designer natriuretic peptides (NPs) 7, 8, 9. Insights into peptide and receptor biology have led to peptide modifications that have resulted in innovative analogues with enhanced activity. Thus, state-of-the-art peptide engineering holds the promise to create a wide variety of truly innovative therapies for CV disease that may have a high impact on reducing the burden of this growing area of human disease.
We review the current clinical use and trials of native NPs such as nesiritide (B-type natriuretic peptide [BNP]) in the United States, carperitide (A-type natriuretic peptide [ANP]) in Japan, and the recently completed international trial of ularitide (urodilatin [URO]). We focus most on the rapidly developing area of designer NPs that may go beyond the native NPs in the treatment of CV disease. We discuss innovative peptide modification either based on rational design or genomic medicine that may impart enhanced receptor activation and/or reduced enzymatic degradation. We provide insights into the latest generation of designer NPs now being tested in models of CV disease and in clinical trials that may lead to a new generation of peptide therapeutics.
Natriuretic Peptides
Peptides are biomolecules that consist of amino acids monomers and peptide (acid) bonds. The amino acid composition of these biomolecules is variable, and is considered an important factor that determines unique chemical and physical properties. The number of bound amino acids dictates the length of the peptides: dipeptides are the shortest peptides (2 amino acids and 1 single peptide bond), whereas polypeptides are long, continuous peptide chains. In contrast to biologically complex proteins, peptides have a rather simple biological composition and generally consist of ≤50 amino acids.
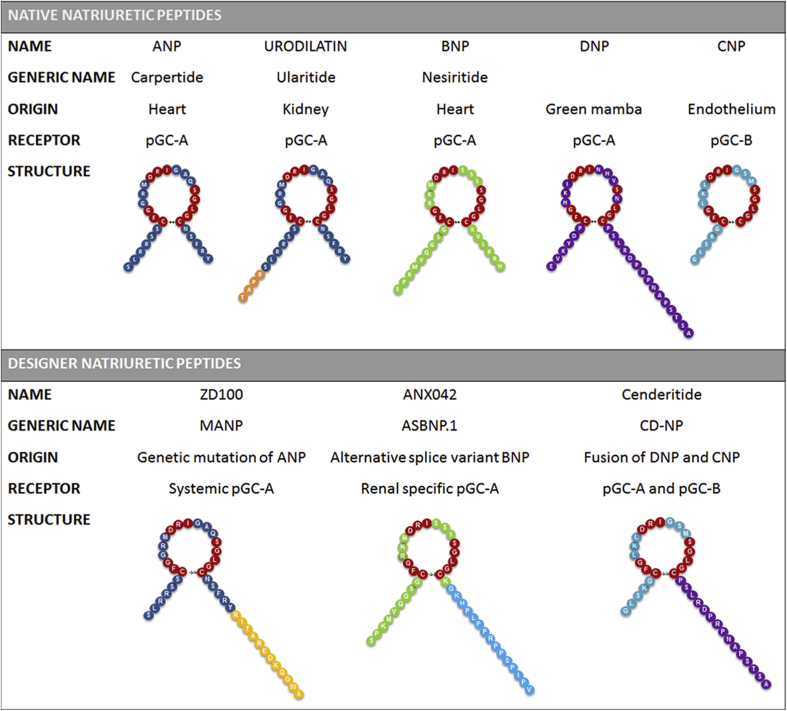
The family of NPs is a group of polypeptides that plays a pivotal role in maintaining the fluid homeostasis of the body by regulating intravascular volume, vascular homeostasis, and arterial pressure (Figure 1) (10). Recently, a role for the NPs in metabolic homeostasis has also been advanced 11, 12, 13. The NP system is highly preserved across species, and currently, the following 6 different NPs have been identified: ANP, BNP, C-type (CNP), D-type (DNP), ventricular NP (VNP), and the renal peptide, URO (14). NPs function as ligands for a set of transmembrane NP receptors (NPRs): CNP, evolutionarily the oldest of the NPs, mainly binds to the extracellular domain of the particulate guanylyl cyclase B receptor (pGC-B, NPR-B), whereas all other NPs bind to the transmembrane pGC-A (NPR-A) receptor (15). pGC-A receptors are expressed in various tissues, including heart, kidney, brain, adrenals, adipocytes, and vasculature (both arteries and veins) (16). pGC-B receptors are expressed in kidney, brain and veins, but less so in arteries (17).
Figure 1.
Overview of Available Native and Designer NPs
ANP = A-type natriuretic peptide; BNP = B-type natriuretic peptide; CNP = C-type natriuretic peptide; DNP = D-type natriuretic peptide; NP = natriuretic peptide; pGC = particular guanylyl cyclase.
A third NPR, called NPR-C, or the clearance receptor (18), actively eliminates endogenous NPs from the circulation using hydrolysis (ranked from the greatest to the lowest degradation rate: VNP = ANP ≥ CNP > BNP = DNP). Studies also suggest a signaling role for NPR-C via modulation of cyclic adenosine monophosphate 19, 20, 21. Clearance of NPs is furthermore regulated by the enzyme NEP, which is widely expressed in endothelium and lung with the highest abundance in the kidney. CNP is the least resistant to NEP-mediated hydrolysis (ranked from greatest to lowest degradation rate: CNP > ANP > BNP > DNP) 3, 10, 15, 16. The differences in local NPR expression, degradation and clearance rates, and NP-binding affinity cause all 6 NPs to have unique and NP-specific properties (15).
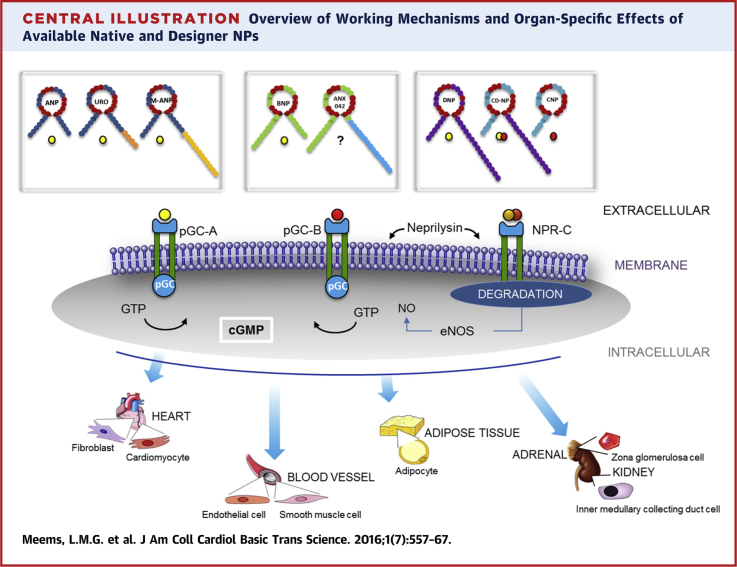
Importantly, binding of a NP to a NPR activates the membrane-bound pGC-A and pGC-B receptors, and induces a variety of autocrine, paracrine, and endocrine effects. Activated pGC receptors produce the second messenger cyclic guanosine monophosphate (cGMP) that in turn activates protein kinase G. cGMP can also be produced in a nitric oxide–dependent manner; its production is then regulated via activation of the soluble guanylyl cyclase pathway (22).
ANP and BNP are believed to be the most important in controlling body fluid and blood pressure homeostasis 23, 24. ANP has renin-inhibiting properties, is a potent aldosterone inhibitor, and is an antagonist to the mineralocorticoid receptor. In addition, via alternative processing of the ANP precursor (pro-ANP) it also contributes to renal sodium and water handling via generation of URO 25, 26, 27. BNP has been identified as an NP highly relevant to HF, which is due to its natriuretic, renin-angiotensin-aldosterone system (RAAS) inhibitory, vasodilating, and lusitropic properties 28, 29, 30, as well as its robust performance as a HF diagnostic and prognostic biomarker 31, 32, 33. CNP is an autocrine and paracrine factor that currently has limited use as a therapeutic for HF, particularly because of its rapid enzymatic degradation and paucity of renal protective actions (29), although its potent antifibrotic actions provide a therapeutic opportunity (34). Moyes et al. (21) elegantly demonstrated a role for CNP in vascular homeostasis that may involve binding and activation of NPR-C (21). DNP is a unique NP that has only been isolated from the venom gland of the Green Mamba snake (35). Its function has not been entirely clarified. Currently, VNP expression has only been confirmed in the hearts of primitive ray-finned bony fish, in which it is responsible for the maintenance of fluid and salt homeostasis (17).
Overall, NPs possess a wide variety of properties that are of value in diagnosis, prognosis, and treatment of CV disease, especially HF and HTN. Despite current optimal therapies, the prognosis for HF remains poor, and 50% of patients die within 5 years after first hospitalization (36). The report of a relative deficiency or low bioavailability of NPs in HTN has also provided a therapeutic opportunity for use of designer NPs, especially in special populations, such as those with resistant HTN, in whom there remains a huge unmet therapeutic need (37). Development of new treatment regimens, with novel modes of action is therefore a high priority, and in this light, the concept of targeting (native) NPs for HF and HTN treatment strategies has attracted increased attention 15, 38.
Therapeutic Use of Native NPs: A Focus on HF
Nesiritide
Nesiritide is a recombinant version of endogenous BNP. In the early 1990s, Hobbs et al. (39) reported early results of nesiritide in human HF. This synthetic peptide was a potent vasodilator and had rapid dose-dependent hemodynamic effects, including reduction of pulmonary wedge pressure, systemic vascular resistance, and mean arterial pressure (40). In 2001, nesiritide received approval from the U.S. Food and Drug Administration (FDA) for the treatment of acute HF, and reported original sales up to $400 million per year. However, initial enthusiasm dampened considerably after pooled data analysis from several small randomized controlled trials showed an association with worsening kidney function and increased mortality 41, 42. Subsequently, the efficacy and safety of nesiritide was re-evaluated in 7,141 patients with acute HF in the ASCEND-HF (Acute Study of Clinical Effectiveness of Nesiritide and Decompensated Heart Failure) trial. Based on outcomes from this large, multicenter randomized controlled trial, it was concluded that nesiritide could no longer be recommended for routine use for treatment of acute HF because it did not improve (or worsen) clinical outcomes or renal function 43, 44. Also, addition of low-dose nesiritide to conventional diuretic therapy in patients hospitalized with acute HF was not effective in improving renal function, decongestion, or clinical outcomes (45). Furthermore, the investigators reported that treatment with low-dose nesiritide resulted in more outspoken vasodilator effects in subjects with HF with reduced ejection fraction compared with subjects with HF with preserved ejection fraction. They therefore suggested that HF etiology may be an important underlying factor in acute HF therapy and response to nesiritide, although they also underscored the need for future studies that would further assess this interesting observation (45).
All of the previously mentioned studies focused on nesiritide as a potential therapeutic for treatment of acute HF. Importantly, an emerging high priority is the need to develop novel therapeutic strategies to prevent the progression of HF. Despite the lack of major efficacy over conventional therapies in acute HF, the compelling biology of chronic activation of pGC-A in pre-clinical studies has demonstrated cardiorenal protection (46). Three recent double-blinded, placebo-controlled studies on the use of long-term subcutaneous (SQ) BNP injections (daily, for 8 to 12 weeks) supported a therapeutic role for NP/pGC-A therapy for treatment of chronic HF. Specifically, nesiritide administration improved cardiorenal function in stable symptomatic HF and in 2 subsequent studies in patients with asymptomatic HF with systolic dysfunction or diastolic dysfunction 47, 48, 49. Major findings included improved left ventricular function and/or structure, enhanced renal handling of acute volume overload, and overall clinical improvement. These 3 seminal studies support the continued development of long-term NP therapeutics to delay HF progression and the safety and efficacy of subcutaneously delivered NPs as a novel delivery platform.
Carperitide
Carperitide is the recombinant formulation of endogenous ANP that has been approved in Japan for the treatment of acute HF (50). Both before and after the clinical launch of carperitide in Japan, there has been no large-scale randomized, double-blind study to confirm whether carperitide improves cardiac function, clinical symptoms, or prognosis in acute HF. To date, however, 2 large-scale prospective open-label observational studies of this peptide have been reported 51, 52. First, a post-marketing surveillance study assessed the efficacy and safety of carperitide in 3,777 patients with acute HF, who were administered carperitide at a median dosage of 0.085 μg/kg/min for a median duration of approximately 2 to 3 days (51). During the infusion, 82% of subjects improved. Adverse events were 16.9%; hypotension (9.5%) was the most frequent. The COMPASS (Carperitide Effects Observed Through Monitoring Dyspnea in Acute Decompensated Heart Failure Study) trial was performed to evaluate carperitide efficacy and safety as monotherapy in acute HF in 1,832 patients (52). Carperitide was administered at an initial dose of <0.05 μg/kg/min in 82.2% of all patients for a mean duration of ∼5 days. Subjective symptoms improved within 2 h after starting infusion of this peptide. Adverse effects were observed in 5% of the subjects; hypotension was the most frequently reported adverse effect (4%).
Beyond the acute action of carperitide on relief of symptoms, the PROTECT trial investigated the effect of carperitide on long-term prognosis after acute HF (53). The PROTECT study enrolled 49 patients who were randomly assigned to a group that received low-dose carperitide (0.01 to 0.05 μg/kg/min) for 72 h or to a standard medical treatment group. During infusion, the cardiac free-fatty acid–binding protein/serum creatinine ratio was reduced, which is consistent with inhibition of myocardial cell membrane damage. There was no significant difference in serum troponin T and creatinine levels between groups. During the 18-month follow-up, the investigators reported that the incidence of death and rehospitalization were significantly lower with carperitide. These studies therefore demonstrated that low-dose ANP infusion in acute HF improved long-term prognosis. Although the mechanism for long-term beneficial effect of carperitide is not clear, inhibition of the RAAS may be involved.
Ularitide
Ularitide is a synthesized version of the endogenous NP URO. Ularitide is a 32-residue ANP that activates cGMP production by binding to the pGC-A receptor (26). It has a fast track designation for treatment of acute HF from the FDA (54).
Endogenous URO is believed to be produced by the kidney through local synthesis and/or processing of renal or circulating pro-ANP. URO plays a pivotal role in regulation of urinary sodium excretion. In rats with HF, URO significantly increased urinary flow, glomerular filtration rate (GFR), sodium excretion, and urinary cGMP excretion in a dose-dependent manner (55). However, in anesthetized dogs with HF that received continuous URO infusion of 2 pmol/kg/min, the increase in urinary sodium excretion was less than in dogs who received BNP infusion (56).
Data from Phase I to IIB clinical phase trials suggest that ularitide may be effective in treatment of acute HF. Ularitide was shown to have vasodilating and renoprotective actions, as well as cardiac unloading properties, including significant improvement of the clinical parameter of dyspnea. Treatment with ularitide further reduced mortality and length of hospital stay, without changing serum creatinine levels. Reported adverse effects were hypotension, cardiac failure, sweating, dizziness, and asthenia 54, 57, 58, 59.
Ularitide was recently tested in a randomized, double-blind, placebo-controlled Phase III study in patients hospitalized with an episode of acute HF (TRUE-HF [Efficacy and Safety of Ularitide for the Treatment of Acute Decompensated Heart Failure]; NCT01661634). Primary outcomes were safety and short- and long-term efficacy of 48 h of continuous intravenous infusion of 15 ng/kg body weight per min, as assessed by patients’ symptoms and persistence of worsening of HF for 48 h as well as clinical status and CV outcome during 180 days of follow-up. The findings of TRUE-HF have yet to be presented.
Designer NPs: For CV Disease
Strategic approach to the engineering of designer NPs
As described in Figure 2, there are 5 goals of designer NP engineering. First, safety of a designer peptide is of highest priority, especially with regard to immunogenicity. Second, enhanced receptor activation remains a major goal that may be achieved through defining which amino acids in a structure are either key mediators of receptor binding and/or limit full receptor activation. Third, as enzymatic degradation may be the major mechanisms limiting bioavailability may probably be enzymatic degradation, the addition of unique amino acids or amino acid substitutions may render a designer peptide immune to degradation and could potentially also markedly enhance efficacy. Fourth, as we have gained insights into peptide design and are able to add or preserve unique elements of a peptide, a major goal is also to design peptides that have properties unique to a specific syndrome (e.g., limiting hypotension in HF or augmenting adipocyte activation in obesity). Fifth, with the progress in sustained delivery systems, developing delivery platforms that permit daily, weekly, or even monthly sustained delivery of a peptide also enhances efficacy and compliance, and has emerged as a key component in peptide engineering.
Figure 2.
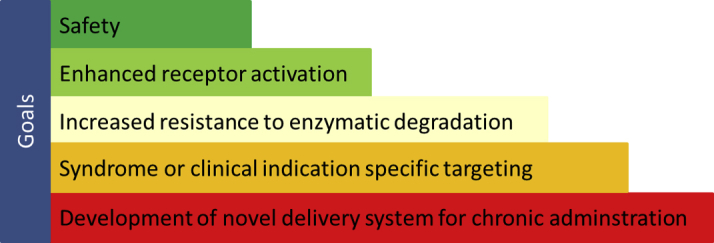
Overview of the 5 Major Goals of Designer Natriuretic Peptide Engineering
Advances in peptide engineering has opened the field of designer NPs, resulting in therapeutic drugs that go beyond native endogenous NPs in actions, efficacy, and safety for the treatment of CV disease. Designer NPs transcend structural, biological, functional, and pharmacological properties of endogenous NPs (60) (Central Illustration). The biochemical design of a designer NP can be a de novo creation or based on selective native NP sequences. Specifically, they may be the result of modification and/or addition of amino acid sequences, or genetic modification of the native forms of NPs. The chemical properties of designer NPs can be easily changed, and this significantly contributes to increased overall applicability of synthetic designer peptides. Although our goal has been to use such engineered peptides for CV disease, synthetic peptides are also used for multiple purposes, including antibody production and polypeptide structure and/or functional studies, as well as for development of new enzymes, vaccines, and drugs. Altogether, these novel chimeras play an important role in advancing the panoply of means available for treatment of HF and HTN.
Central Illustration.
Overview of Working Mechanisms and Organ-Specific Effects of Available Native and Designer NPs
cGMP = cyclic guanosine monophosphate; eNOS = endothelial nitric oxide synthase; GTP = guanosine-5′-triphosphate; NO = nitric oxide; NPR = NP receptor; other abbreviations as in Figure 1.
Our approach to novel peptide design is highly unique and does not use traditional computer modeling or use of high-throughput bioinformatics. In contrast, our strategic engineering of designer peptides use a rational drug discovery approach in which we integrate a set of inputs. Specifically, we use a knowledge-based amino acid mutation approach: the ever-increasing synthesis of selective mutations, such as was reported recently by Lee et al. (61) for the designer NP cenderitide (CD-NP), continuously improving our knowledge and information of properties of key amino acids that may enhance or reduce receptor activation of a native peptide. Furthermore, engineering of designer NPs that are highly resistant to degradation relies upon ours and others (62) laboratory-based knowledge, which provides information about sites of enzyme degradation by degrading enzymes, including NEP. In addition, using novel amino acid sequences from venomous snakes such as the Green Mamba, which was used with CD-NP, results in chimeric peptides with highly unique properties and therapeutic benefits that go beyond mammalian peptides (63). In our designer peptide design, we also use a genomic medicine approach: identification of spontaneous or hereditary NP-coding genetic alterations that can then be used to synthesize novel peptides with unique features. For example, this strategy has resulted in engineering of the unique peptides MANP (ZD100) and ASBNP.1 (ANX042) 64, 65. Finally, we also rely on careful review of public domain reports from biotechnology and medicinal chemistry that provide key insights that may further guide our strategic designs. Altogether, this unique strategy has created a broad assortment of designer NPs, each with unique and rather disease-specific actions.
Vasonatrin
Vasonatrin (VNP) represented our first attempt at a designer NP in 1993 (66). VNP is a 27 amino acid peptide chimera of the full-length 22 amino acid structure of human CNP and the 5-amino acid carboxyl-terminus of human ANP. In vitro and in vivo, VNP has both the venodilating properties of CNP and the natriuretic properties of ANP. It also possesses arterial vasodilating and antiproliferative effects that are unique for this first-in-class chimera that defined the concept of designer NPs going beyond native peptides. The vasorelaxant effects of VNP are dose-dependent, endothelium-independent, and stronger than the effects of ANP and CNP alone 67, 68, 69. However, in healthy rats, the natriuretic and diuretic effects of a single bolus of 50 μg/kg VNP were inferior to ANP (66), which reduced enthusiasm for its clinical development.
CD-NP for HF
CD-NP, developed in 2008, was a major advance in peptide engineering that now has proven efficacy in humans (Table 1). CD-NP is a 37 amino acid hybrid NP that fuses the mature form of native CNP and the 15 amino acid C-terminus of DNP (61). The rationale for the design of CD-NP was in part motivated from results from the ASCEND-HF and ROSE-AHF (Renal Optimization Strategies Evaluation in Acute Heart Failure) trials, which demonstrated excessive hypotension after treatment with the pGC-A activator, nesiritide. During development of CD-NP, the main goal was to design a NP that still possessed the beneficial renal actions of pGC-A and pGC-B activation, but without the unwanted hypotensive properties. We therefore engineered a hybrid that consisted of CNP (pGC-B activator) and the C-terminus of DNP that recognized DNP (pGC-A activator). The C-terminus of DNP itself has less hypotensive actions than DNP but still possesses natriuretic properties. CD-NP is the first designer NP that co-targets both pGC-A and pGC-B receptors, and the biological effects of this designer NP have been a significant advance in NP design.
Table 1.
Highlights of Designer Natriuretic Peptide Particulate Guanylyl Cyclase Activators in Clinical Trials
| Cenderitide |
| Generic name: CD-NP |
| Indication: Post-acute heart failure |
| Current status: 2 Phase II studies of 8-day treatment with continuous subcutaneous administration by Insulet OmniPod (Insulet Corp., Billerica, Massachusetts) delivery system in stable heart failure patients has been completed. |
| ANX042 |
| Generic name: ASBNP.1 |
| Indication: Cardiorenal syndrome in heart failure |
| Current status: Phase I study of acute intravenous administration in normal human volunteers has been completed. |
| ZD100 |
| Generic name: MANP |
| Indication: Hypertension |
| Current status: Phase I study of 3-day once daily subcutaneous injection of subjects with resistant-like hypertension has been completed. |
First, we reported the successful synthesis of the C-terminus of DNP and then of CD-NP (63). Consistent with our goal of a dual pGC-A/pGC-B activator that does not exist in nature, Lee et al. (61), Dickey et al. (70), and Martin et al. (71) elegantly demonstrated the unique ability of CD-NP to co-activate both pGC-A and pGC-B in HEK293 cells, selectively overexpressing each receptor type, which was recently reconfirmed. In vitro, in human cardiac fibroblasts, CD-NP was a stronger cGMP-activator than equimolar doses of BNP, DNP, or CNP 63, 72. This first-in-class designer NP also had antifibrotic, antiproliferative, and antihypertrophic properties that make this drug a promising candidate for HF (29). In vivo, in normal canines, CD-NP was a potent cGMP stimulator that had venodilating and renal enhancing effects, with less effects on systemic blood pressure (BP) (63). At natriuretic doses, only infusion with nesiritide, and not CD-NP, reduced BP (63). Also, CD-NP, compared with CNP alone, possessed unique and additional renal actions, such as cGMP activation in isolated glomeruli, plasma aldosterone suppression, and potent GFR-enhancing and natriuretic effects (61).
Infusion of CD-NP in healthy volunteers stimulated increases in urinary and plasma cGMP levels, induced a significant diuretic and natriuretic response, and resulted in a minimal, but significant, decrease in systemic BP (Table 1) (73).
CD-NP received fast track designation from the FDA in 2011 for the treatment of post-acute HF. Proof-of-concept studies are underway to reduce post–myocardial infarction HF and enhance outcomes in patients with left ventricular assistant device therapy. A Phase Ib/II study in with chronic HF stable chronic HF patients with impaired renal function was initiated in 2015 with the goal of enhancing renal function (NCT02603614). This study will evaluate the safety, tolerability, pharmacokinetics, and pharmacodynamics of CD-NP.
Recently, studies have defined the unique role of key structural components of CD-NP with the ultimate goal of engineering a newer generation CD-NP. Extensive modeling and production of in vitro CD-NP mutants demonstrated and validated the superiority of the structure of CD-NPs to other novel CD-NP–like peptides, establishing the unique structural requirement of the full mature CNP with the 15 amino acid sequence of DNP as a prerequisite for successful co-receptor activation (61).
Originally, CD-NP was developed as a novel therapeutic to enhance renal function with limited effects on BP. However, a more contemporary additional goal in HF treatment is to prevent and or reverse myocardial remodeling, especially cardiac fibrosis. In this light, CD-NP may also appear as an attractive and important therapeutic for HF. This rationale is strengthened by a recent study of Ichiki et al. (72), who demonstrated up-regulation of the pGC-B receptor together with reduced production of CNP in experimental studies in human failing myocardium. In human cardiac fibroblasts, CD-NP was furthermore a stronger inhibitor of collagen production than either BNP or CNP (72). In a model of mild left ventricular diastolic dysfunction with cardiac fibrosis, long-term SQ CD-NP, administered by pump infusion, suppressed the development of both cardiac fibrosis and diastolic dysfunction (71). Altogether, these and ongoing studies suggest that CD-NP is a unique and potent designer NP that has cardiorenal protective properties and antifibrotic actions.
A key strategy in peptide therapeutics is the development of innovative delivery platforms. In current clinical trials, the OmniPod (Insulet Corporation, Billerica, Massachusetts) insulin delivery system is being used to administer long-term SQ CD-NP in patients with HF. In experimental models of CV disease, novel nanoparticle gel polymer strategies are being tested (74). Furthermore, a novel film delivery system in which CD-NP is released from a patch-like device around the heart is also being tested (75). With the advent of highly innovative delivery systems, long-term delivery of CD-NP or designer NPs will continue to emerge.
ANX-042 (ASBNP.1) for HF
Advances in genome-wide investigations have revealed alternative splicing of multiexonic genes. The complexity of the human proteome may be accounted for by alternative splicing of messenger RNA. This provides an opportunity in drug discovery (76). We recently focused on the cardiac peptide BNP, which is encoded by a small multiexonic gene. Its therapeutic use with recombinant BNP (nesiritide) has demonstrated efficacy in HF, but this has been limited by excessive hypotension. In our efforts to identify novel endogenous variants of BNP, we identified an alternative spliced transcript of BNP that resulted from intron retention in failing human hearts. We then used the unique sequence of this alternative spliced BNP (AS-BNP) to design a peptide with unique renal protective actions without associated hypotension (65). Specifically, the designer peptide ANX-042 is a truncated peptide form of an AS-BNP (65). From a structural perspective, this transcript resembles native mature BNP, whereas a unique and distinct longer (34 amino acid) C-terminus is a component due to intron retention. Based on multiple designs, we used the first 16 amino acids of this C-terminal to generate the designer NP ANX-042. ANX-042, based on genomic medicine, presents with highly unique properties that also support its development as a renal-enhancing peptide for HF with limited BP-lowering actions (Central Illustration).
Initial investigations of AS-BNP and ANX-042 demonstrated they lacked the ability to activate cGMP in vascular smooth muscle cells and endothelial cells that are known to possess pGC-A receptors and are responsive to native BNP. Furthermore, in isolated arterial vascular rings, BNP, but not ANX-042, relaxed pre-constricted vessels. Surprisingly, in renal mesangial cells, ANX-042 markedly increased cGMP production greater than BNP, which may be a pGC-B phenomenon rather than pGC-A activation. In addition, in freshly isolated glomeruli, ANX-042 also activated cGMP production. Thus, ANX-042, engineered from the discovery of an alternatively spliced variant of BNP appears to be a selective renal-acting, BNP-like peptide in vitro, but may involve activation of pGC-B and/or yet undefined pGC receptor subtypes in the kidney but not in the systemic vasculature.
In vivo, intravenous administration of ANX-042 in a canine model of pacing-induced HF significantly enhanced diuresis, natriuresis, and GFR without reducing mean arterial pressure (65). Data from a Healthy Volunteer Dose Escalation Study (NCT01638104) furthermore showed that infusion with ANX-042 is safe and results in cGMP-activation (Table 1). ANX-042 obtained FDA approval in 2012 as an investigational new drug and is currently being tested as a potential novel nonhypotensive and renal-enhancing treatment for HF.
MANP for HTN
HTN remains the leading cause of HF and strategies to reduce HF are a high health care priority. The need for more effective BP-lowering agents was recently underscored by the SPRINT (Systolic Blood Pressure Intervention Trial), which reported that intensive control of systolic BP to <120 mm Hg in high-risk HTN subjects reduced mortality and adverse CV outcomes, including HF (77). Furthermore, population studies in Olmsted County (Minnesota) as part of the Rochester Epidemiology Project demonstrated that there is a relative deficiency or low bioavailability of NPs in human HTN and a pathophysiological inverse relationship between an increasing aldosterone and decreasing NP in HTN and metabolic disease (78). Such observations from clinical studies and the known BP-lowering, aldosterone-suppressing, and natriuretic actions of ANP via pGC-A have laid the foundation for the development of designer ANPs for HTN.
The newest designer ANP-like peptide currently entering clinical trials for resistant HTN is MANP (ZD100), which is a best-in-class pGC-A activator. ZD100 is currently in clinical development for resistant HTN. Subjects with resistant HTN do not respond to current BP-lowering agents and are known to have a markedly increased risk of adverse CV outcomes, including HF, stroke, myocardial infarction, and end-stage kidney disease (38). MANP recently completed a first-in-human study in stable HTN and in resistant-like subjects with HTN.
Based upon genomic insights, MANP was engineered as a 40 amino acid peptide with the structure of a mature 28 amino acid ANP fused to a novel 12 amino acid carboxyl terminal extension (Central Illustration) (64). This novel C-terminal extension renders MANP highly resistant to degradation by NEP and thus represents an alternative to a nonspecific NEP enzyme inhibitor strategy (79). Ongoing studies support possible enhanced activation of pGC-A and reduced binding to NPR-C. Thus, MANP has the attractive biological properties of both resistance to NEP degradation with specific and direct ligand activation of pGC-A.
In vivo, MANP had more sustained natriuretic, aldosterone-suppressing and BP-lowering actions than native ANP (64). MANP markedly increased circulating and urinary cGMP and is highly effective in reducing BP in experimental HTN (80). Both experimental and first-in-human studies suggested that MANP is a potent aldosterone inhibitor that reduces the secretion of aldosterone (Table 1). Experimental studies with native ANP and its interaction with the mineralocorticoid receptor also suggested that MANP might have direct actions to inhibit the mineralocorticoid receptor (25). Furthermore, in a model of acute HF in the setting of HTN, MANP was more effective than nitroglycerine in augmenting sodium excretion and preserving GFR, unloading the heart, and suppressing aldosterone (81).
Studies are underway to develop novel strategies to deliver MANP long-term in a formulation that results in sustained delivery. Similar to antidiabetic drugs such as glucagon-like peptide 1 analogs, fatty acids can be linked to MANP to sustain delivery and prolong activation of cGMP. Preliminary studies have demonstrated the feasibility of sustained release formulation of ZD100, and preclinical studies are underway in experimental HTN. Ongoing studies also suggest that advanced peptide engineering can result in next generation ZD100 analogues with gain of function in the activation of pGC-A (82). Recently, we reported data from a first in human Phase I trial, in which single ascending doses of ZD100 were administered to HTN subjects followed by once daily injection for 3 days by SQ administration. Treatment with ZD100 was safe and well-tolerated, and was associated with cGMP generation, natriuresis, enhanced GFR, and suppression of aldosterone (Table 1) (83). Thus, in these exciting days of biotechnology, engineering peptides such as ZD100 provide potentially greater clinical efficacy in the treatment of human disease and yet retain highly specific receptor–mediated actions that contribute to both safety and enhanced efficacy.
Future Directions
NPs have always been regarded as attractive targets for the treatment of HF and HTN. Although use of NPs was initially hampered by limited clinical efficacy, which was mainly the result of limitations of endogenous NPs, such as rapid enzyme degradation or unwanted properties such as excessive hypotension, therapeutic use of NPs remains a highly sought after goal. Progress in this field was made by the introduction of recombinant NPs and was further advanced by the concept of designer NPs. As we presented in this review, several first-in-class designer NPs are being tested in clinical studies. These novel chimeras reflect technological advances that have been made in drug development over the years, incorporating new and innovative engineering strategies, including novel delivery platforms. Overall, current designer NPs are more efficacious in their actions than their (recombinant) predecessors, and because of their specific targeting, advance the novel concept of precision medicine.
Following the successful introduction of Entresto, a first-in-class angiotensin receptor NEP inhibitor, we believe that the concept of a drug with simultaneous NP-augmenting and RAAS-counteracting properties is highly attractive and deserves further attention. The morbidity and mortality of HF and the difficulty of controlling HTN, nevertheless, remains high, and we hypothesize that the engineering of future designer NPs should incorporate a multivalent strategy in an attempt to develop dual-receptor designer NPs. These NPs may even target receptor pathways beyond the pGC receptor family, so as to optimize organ protective properties. The era of the designer NP continues to evolve with the promise of exciting therapeutics for CV disease and beyond.
Footnotes
Mayo Clinic has licensed Cenderitide to Capricor Therapeutics, ANX042 to Anexon, and ZD100 to Zumbro Discovery. Funding from the National Institutes of Health (RO1 HL36634-28 and RO1 DK103850) also supports this work.
Dr. Meems has received a grant from ICIN, the Netherlands Heart Foundation; and Dr. Burnett is the Co-Founder of Zumbro Discovery and holds stock in the company.
References
- 1.Sidney S., Quesenberry C.P., Jr., Jaffe M.G. Recent trends in cardiovascular mortality in the United States and public health goals. JAMA Cardiol. 2016;1:594–599. doi: 10.1001/jamacardio.2016.1326. [DOI] [PubMed] [Google Scholar]
- 2.McMurray J.J., Packer M., Desai A.S. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993–1004. doi: 10.1056/NEJMoa1409077. [DOI] [PubMed] [Google Scholar]
- 3.Mangiafico S., Costello-Boerrigter L.C., Andersen I.A., Cataliotti A., Burnett J.C., Jr. Neutral endopeptidase inhibition and the natriuretic peptide system: an evolving strategy in cardiovascular therapeutics. Eur Heart J. 2013;34:886–893c. doi: 10.1093/eurheartj/ehs262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ruilope L.M., Dukat A., Bohm M., Lacourciere Y., Gong J., Lefkowitz M.P. Blood-pressure reduction with LCZ696, a novel dual-acting inhibitor of the angiotensin II receptor and neprilysin: a randomised, double-blind, placebo-controlled, active comparator study. Lancet. 2010;375:1255–1266. doi: 10.1016/S0140-6736(09)61966-8. [DOI] [PubMed] [Google Scholar]
- 5.Jay S.M., Lee R.T. Protein engineering for cardiovascular therapeutics: untapped potential for cardiac repair. Circ Res. 2013;113:933–943. doi: 10.1161/CIRCRESAHA.113.300215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fosgerau K., Hoffmann T. Peptide therapeutics: current status and future directions. Drug Discov Today. 2015;20:122–128. doi: 10.1016/j.drudis.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 7.Marso S.P., Daniels G.H., Brown-Frandsen K. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311–322. doi: 10.1056/NEJMoa1603827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rizzuti M., Nizzardo M., Zanetta C., Ramirez A., Corti S. Therapeutic applications of the cell-penetrating HIV-1 Tat peptide. Drug Discov Today. 2015;20:76–85. doi: 10.1016/j.drudis.2014.09.017. [DOI] [PubMed] [Google Scholar]
- 9.Teerlink J.R., Cotter G., Davison B.A. Serelaxin, recombinant human relaxin-2, for treatment of acute heart failure (RELAX-AHF): a randomised, placebo-controlled trial. Lancet. 2013;381:29–39. doi: 10.1016/S0140-6736(12)61855-8. [DOI] [PubMed] [Google Scholar]
- 10.Levin E.R., Gardner D.G., Samson W.K. Natriuretic peptides. N Engl J Med. 1998;339:321–328. doi: 10.1056/NEJM199807303390507. [DOI] [PubMed] [Google Scholar]
- 11.Bordicchia M., Liu D., Amri E.Z. Cardiac natriuretic peptides act via p38 MAPK to induce the brown fat thermogenic program in mouse and human adipocytes. J Clin Invest. 2012;122:1022–1036. doi: 10.1172/JCI59701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cannone V., Boerrigter G., Cataliotti A. A genetic variant of the atrial natriuretic peptide gene is associated with cardiometabolic protection in the general community. J Am Coll Cardiol. 2011;58:629–636. doi: 10.1016/j.jacc.2011.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang T.J. The natriuretic peptides and fat metabolism. N Engl J Med. 2012;367:377–378. doi: 10.1056/NEJMcibr1204796. [DOI] [PubMed] [Google Scholar]
- 14.Martinez-Rumayor A., Richards A.M., Burnett J.C., Januzzi J.L., Jr. Biology of the natriuretic peptides. Am J Cardiol. 2008;101:3–8. doi: 10.1016/j.amjcard.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 15.van Kimmenade R.R., Januzzi J.L., Jr. The evolution of the natriuretic peptides - current applications in human and animal medicine. J Vet Cardiol. 2009;11(Suppl 1):S9–S21. doi: 10.1016/j.jvc.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 16.Potter L.R., Abbey-Hosch S., Dickey D.M. Natriuretic peptides, their receptors, and cyclic guanosine monophosphate-dependent signaling functions. Endocr Rev. 2006;27:47–72. doi: 10.1210/er.2005-0014. [DOI] [PubMed] [Google Scholar]
- 17.Takei Y., Ogoshi M., Inoue K. A 'reverse' phylogenetic approach for identification of novel osmoregulatory and cardiovascular hormones in vertebrates. Front Neuroendocrinol. 2007;28:143–160. doi: 10.1016/j.yfrne.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 18.Fuller F., Porter J.G., Arfsten A.E. Atrial natriuretic peptide clearance receptor. Complete sequence and functional expression of cDNA clones. J Biol Chem. 1988;263:9395–9401. [PubMed] [Google Scholar]
- 19.Anand-Srivastava M.B. Natriuretic peptide receptor-C signaling and regulation. Peptides. 2005;26:1044–1059. doi: 10.1016/j.peptides.2004.09.023. [DOI] [PubMed] [Google Scholar]
- 20.Becker J.R., Chatterjee S., Robinson T.Y. Differential activation of natriuretic peptide receptors modulates cardiomyocyte proliferation during development. Development. 2014;141:335–345. doi: 10.1242/dev.100370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moyes A.J., Khambata R.S., Villar I. Endothelial C-type natriuretic peptide maintains vascular homeostasis. J Clin Invest. 2014;124:4039–4051. doi: 10.1172/JCI74281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuhn M. Structure, regulation, and function of mammalian membrane guanylyl cyclase receptors, with a focus on guanylyl cyclase-A. Circ Res. 2003;93:700–709. doi: 10.1161/01.RES.0000094745.28948.4D. [DOI] [PubMed] [Google Scholar]
- 23.Burnett J.C., Jr., Granger J.P., Opgenorth T.J. Effects of synthetic atrial natriuretic factor on renal function and renin release. Am J Physiol. 1984;247:F863–F866. doi: 10.1152/ajprenal.1984.247.5.F863. [DOI] [PubMed] [Google Scholar]
- 24.Cataliotti A., Chen H.H., Schirger J.A. Chronic actions of a novel oral B-type natriuretic peptide conjugate in normal dogs and acute actions in angiotensin II-mediated hypertension. Circulation. 2008;118:1729–1736. doi: 10.1161/CIRCULATIONAHA.107.759241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakagawa H., Oberwinkler H., Nikolaev V.O. Atrial natriuretic peptide locally counteracts the deleterious effects of cardiomyocyte mineralocorticoid receptor activation. Circ Heart Fail. 2014;7:814–821. doi: 10.1161/CIRCHEARTFAILURE.113.000885. [DOI] [PubMed] [Google Scholar]
- 26.Schulz-Knappe P., Forssmann K., Herbst F., Hock D., Pipkorn R., Forssmann W.G. Isolation and structural analysis of “urodilatin”, a new peptide of the cardiodilatin-(ANP)-family, extracted from human urine. Klinische Wochenschrift. 1988;66:752–759. doi: 10.1007/BF01726570. [DOI] [PubMed] [Google Scholar]
- 27.Ichiki T., Huntley B.K., Sangaralingham S.J., Burnett J.C., Jr. Pro-atrial natriuretic peptide: a novel guanylyl cyclase-A receptor activator that goes beyond atrial and B-type natriuretic peptides. J Am Coll Cardiol HF. 2015;3:715–723. doi: 10.1016/j.jchf.2015.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mills R.M., Hobbs R.E., Young J.B. “BNP” for heart failure: role of nesiritide in cardiovascular therapeutics. Congest Heart Fail. 2002;8:270–273. doi: 10.1111/j.1527-5299.2002.01154.x. [DOI] [PubMed] [Google Scholar]
- 29.von Lueder T.G., Sangaralingham S.J., Wang B.H. Renin-angiotensin blockade combined with natriuretic peptide system augmentation: novel therapeutic concepts to combat heart failure. Circ Heart Fail. 2013;6:594–605. doi: 10.1161/CIRCHEARTFAILURE.112.000289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huntley B.K., Sandberg S.M., Heublein D.M., Sangaralingham S.J., Burnett J.C., Jr., Ichiki T. Pro-B-type natriuretic peptide-1-108 processing and degradation in human heart failure. Circ Heart Fail. 2015;8:89–97. doi: 10.1161/CIRCHEARTFAILURE.114.001174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maisel A.S., Krishnaswamy P., Nowak R.M. Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. N Engl J Med. 2002;347:161–167. doi: 10.1056/NEJMoa020233. [DOI] [PubMed] [Google Scholar]
- 32.Januzzi J.L., van Kimmenade R., Lainchbury J. NT-proBNP testing for diagnosis and short-term prognosis in acute destabilized heart failure: an international pooled analysis of 1256 patients: the International Collaborative of NT-proBNP Study. Eur Heart J. 2006;27:330–337. doi: 10.1093/eurheartj/ehi631. [DOI] [PubMed] [Google Scholar]
- 33.McKie P.M., Cataliotti A., Lahr B.D. The prognostic value of N-terminal pro-B-type natriuretic peptide for death and cardiovascular events in healthy normal and stage A/B heart failure subjects. J Am Coll Cardiol. 2010;55:2140–2147. doi: 10.1016/j.jacc.2010.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sangaralingham S.J., Wang B.H., Huang L. Cardiorenal fibrosis and dysfunction in aging: imbalance in mediators and regulators of collagen. Peptides. 2016;76:108–114. doi: 10.1016/j.peptides.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schweitz H., Vigne P., Moinier D., Frelin C., Lazdunski M. A new member of the natriuretic peptide family is present in the venom of the green mamba (Dendroaspis angusticeps) J Biol Chem. 1992;267:13928–13932. [PubMed] [Google Scholar]
- 36.van Jaarsveld C.H., Ranchor A.V., Kempen G.I., Coyne J.C., van Veldhuisen D.J., Sanderman R. Epidemiology of heart failure in a community-based study of subjects aged > or = 57 years: incidence and long-term survival. Eur J Heart Fail. 2006;8:23–30. doi: 10.1016/j.ejheart.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 37.Macheret F., Heublein D., Costello-Boerrigter L.C. Human hypertension is characterized by a lack of activation of the antihypertensive cardiac hormones ANP and BNP. J Am Coll Cardiol. 2012;60:1558–1565. doi: 10.1016/j.jacc.2012.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McKie P.M., Ichiki T., Burnett J.C., Jr. M-atrial natriuretic peptide: a novel antihypertensive protein therapy. Curr Hyperten Rep. 2012;14:62–69. doi: 10.1007/s11906-011-0244-5. [DOI] [PubMed] [Google Scholar]
- 39.Hobbs R.E., Miller L.W., Bott-Silverman C., James K.B., Rincon G., Grossbard E.B. Hemodynamic effects of a single intravenous injection of synthetic human brain natriuretic peptide in patients with heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol. 1996;78:896–901. doi: 10.1016/s0002-9149(96)00464-x. [DOI] [PubMed] [Google Scholar]
- 40.Colucci W.S., Elkayam U., Horton D.P. Intravenous nesiritide, a natriuretic peptide, in the treatment of decompensated congestive heart failure. Nesiritide Study Group. N Engl J Med. 2000;343:246–253. doi: 10.1056/NEJM200007273430403. [DOI] [PubMed] [Google Scholar]
- 41.Sackner-Bernstein J.D., Kowalski M., Fox M., Aaronson K. Short-term risk of death after treatment with nesiritide for decompensated heart failure: a pooled analysis of randomized controlled trials. JAMA. 2005;293:1900–1905. doi: 10.1001/jama.293.15.1900. [DOI] [PubMed] [Google Scholar]
- 42.Sackner-Bernstein J.D., Skopicki H.A., Aaronson K.D. Risk of worsening renal function with nesiritide in patients with acutely decompensated heart failure. Circulation. 2005;111:1487–1491. doi: 10.1161/01.CIR.0000159340.93220.E4. [DOI] [PubMed] [Google Scholar]
- 43.O'Connor C.M., Starling R.C., Hernandez A.F. Effect of nesiritide in patients with acute decompensated heart failure. N Engl J Med. 2011;365:32–43. doi: 10.1056/NEJMoa1100171. [DOI] [PubMed] [Google Scholar]
- 44.van Deursen V.M., Hernandez A.F., Stebbins A. Nesiritide, renal function, and associated outcomes during hospitalization for acute decompensated heart failure: results from the Acute Study of Clinical Effectiveness of Nesiritide and Decompensated Heart Failure (ASCEND-HF) Circulation. 2014;130:958–965. doi: 10.1161/CIRCULATIONAHA.113.003046. [DOI] [PubMed] [Google Scholar]
- 45.Chen H.H., Anstrom K.J., Givertz M.M. Low-dose dopamine or low-dose nesiritide in acute heart failure with renal dysfunction: the ROSE acute heart failure randomized trial. JAMA. 2013;310:2533–2543. doi: 10.1001/jama.2013.282190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cataliotti A., Tonne J.M., Bellavia D. Long-term cardiac pro-B-type natriuretic peptide gene delivery prevents the development of hypertensive heart disease in spontaneously hypertensive rats. Circulation. 2011;123:1297–1305. doi: 10.1161/CIRCULATIONAHA.110.981720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McKie P.M., Schirger J.A., Benike S.L. Chronic subcutaneous brain natriuretic peptide therapy in asymptomatic systolic heart failure. Eur J Heart Fail. 2016;18:433–441. doi: 10.1002/ejhf.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wan S.H., McKie P.M., Schirger J.A. Chronic peptide therapy with B-Type Natriuretic peptide in patients with pre-clinical diastolic dysfunction (stage B heart failure) J Am Coll Cardiol HF. 2016;4:539–547. doi: 10.1016/j.jchf.2015.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen H.H., Glockner J.F., Schirger J.A., Cataliotti A., Redfield M.M., Burnett J.C., Jr. Novel protein therapeutics for systolic heart failure: chronic subcutaneous B-type natriuretic peptide. J Am Coll Cardiol. 2012;60:2305–2312. doi: 10.1016/j.jacc.2012.07.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saito Y. Roles of atrial natriuretic peptide and its therapeutic use. J Cardiol. 2010;56:262–270. doi: 10.1016/j.jjcc.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 51.Suwa M., Seino Y., Nomachi Y., Matsuki S., Funahashi K. Multicenter prospective investigation on efficacy and safety of carperitide for acute heart failure in the 'real world' of therapy. Circ J. 2005;69:283–290. doi: 10.1253/circj.69.283. [DOI] [PubMed] [Google Scholar]
- 52.Nomura F., Kurobe N., Mori Y. Multicenter prospective investigation on efficacy and safety of carperitide as a first-line drug for acute heart failure syndrome with preserved blood pressure: COMPASS: Carperitide Effects Observed Through Monitoring Dyspnea in Acute Decompensated Heart Failure Study. Circ J. 2008;72:1777–1786. doi: 10.1253/circj.cj-07-0760. [DOI] [PubMed] [Google Scholar]
- 53.Hata N., Seino Y., Tsutamoto T. Effects of carperitide on the long-term prognosis of patients with acute decompensated chronic heart failure: the PROTECT multicenter randomized controlled study. Circ J. 2008;72:1787–1793. doi: 10.1253/circj.cj-08-0130. [DOI] [PubMed] [Google Scholar]
- 54.Anker S.D., Ponikowski P., Mitrovic V., Peacock W.F., Filippatos G. Ularitide for the treatment of acute decompensated heart failure: from preclinical to clinical studies. Eur Heart J. 2015;36:715–723. doi: 10.1093/eurheartj/ehu484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Abassi Z.A., Powell J.R., Golomb E., Keiser H.R. Renal and systemic effects of urodilatin in rats with high-output heart failure. Am J Physiol. 1992;262:F615–F621. doi: 10.1152/ajprenal.1992.262.4.F615. [DOI] [PubMed] [Google Scholar]
- 56.Chen H.H., Cataliotti A., Schirger J.A., Martin F.L., Burnett J.C., Jr. Equimolar doses of atrial and brain natriuretic peptides and urodilatin have differential renal actions in overt experimental heart failure. Am J Physiol Regul, Integ Comp Physiol. 2005;288:R1093–R1097. doi: 10.1152/ajpregu.00682.2004. [DOI] [PubMed] [Google Scholar]
- 57.Kentsch M., Ludwig D., Drummer C., Gerzer R., Muller-Esch G. Haemodynamic and renal effects of urodilatin bolus injections in patients with congestive heart failure. Eur J Clin Invest. 1992;22:66–69. doi: 10.1111/j.1365-2362.1992.tb01427.x. [DOI] [PubMed] [Google Scholar]
- 58.Mitrovic V., Luss H., Nitsche K. Effects of the renal natriuretic peptide urodilatin (ularitide) in patients with decompensated chronic heart failure: a double-blind, placebo-controlled, ascending-dose trial. Am Heart J. 2005;150:1239. doi: 10.1016/j.ahj.2005.01.022. [DOI] [PubMed] [Google Scholar]
- 59.Luss H., Mitrovic V., Seferovic P.M. Renal effects of ularitide in patients with decompensated heart failure. Am Heart J. 2008;155:1012.e1–1012.e8. doi: 10.1016/j.ahj.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 60.Patel J.B., Valencik M.L., Pritchett A.M., Burnett J.C., Jr., McDonald J.A., Redfield M.M. Cardiac-specific attenuation of natriuretic peptide A receptor activity accentuates adverse cardiac remodeling and mortality in response to pressure overload. Am J Physiol Heart Circ Physiol. 2005;289:H777–H784. doi: 10.1152/ajpheart.00117.2005. [DOI] [PubMed] [Google Scholar]
- 61.Lee C.Y., Huntley B.K., McCormick D.J. Cenderitide: structural requirements for the creation of a novel dual particulate guanylyl cyclase receptor agonist with renal-enhancing in vivo and ex vivo actions. Eur Heart J Cardiovasc Pharm. 2016;2:98–105. doi: 10.1093/ehjcvp/pvv040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dickey D.M., Yoder A.R., Potter L.R. A familial mutation renders atrial natriuretic peptide resistant to proteolytic degradation. J Biol Chem. 2009;284:19196–19202. doi: 10.1074/jbc.M109.010777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lisy O., Huntley B.K., McCormick D.J., Kurlansky P.A., Burnett J.C., Jr. Design, synthesis, and actions of a novel chimeric natriuretic peptide: CD-NP. J Am Coll Cardiol. 2008;52:60–68. doi: 10.1016/j.jacc.2008.02.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.McKie P.M., Cataliotti A., Huntley B.K., Martin F.L., Olson T.M., Burnett J.C., Jr. A human atrial natriuretic peptide gene mutation reveals a novel peptide with enhanced blood pressure-lowering, renal-enhancing, and aldosterone-suppressing actions. J Am Coll Cardiol. 2009;54:1024–1032. doi: 10.1016/j.jacc.2009.04.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pan S., Chen H.H., Dickey D.M. Biodesign of a renal-protective peptide based on alternative splicing of B-type natriuretic peptide. Proc Natl Acad Sci U S A. 2009;106:11282–11287. doi: 10.1073/pnas.0811851106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wei C.M., Kim C.H., Miller V.M., Burnett J.C., Jr. Vasonatrin peptide: a unique synthetic natriuretic and vasorelaxing peptide. J Clin Invest. 1993;92:2048–2052. doi: 10.1172/JCI116800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Feng H.S., Zang Y.M., Zhu M.Z. [Comparison of vasorelaxing actions of vasonatrin peptide, C-type natriuretic peptide and atrial natriuretic peptide] Sheng li xue bao [Acta Physiol Sinica] 1999;51:515–520. [PubMed] [Google Scholar]
- 68.Yu J., Zhu M.Z., Wei G.Z. [Vasorelaxing role of vasonatrin peptide in human intramammary artery in vitro] Sheng li xue bao [Acta Physiol Sinica] 2003;55:187–190. [PubMed] [Google Scholar]
- 69.Dong M.Q., Zhu M.Z., Yu J., Shang L.J., Feng H.S. [Comparison of inhibitory effects of three natriuretic peptides on the proliferation of pulmonary artery smooth muscle cells of rats] Sheng li xue bao [Acta Physiol Sinica] 2000;52:252–254. [PubMed] [Google Scholar]
- 70.Dickey D.M., Burnett J.C., Jr., Potter L.R. Novel bifunctional natriuretic peptides as potential therapeutics. J Biol Chem. 2008;283:35003–35009. doi: 10.1074/jbc.M804538200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Martin F.L., Sangaralingham S.J., Huntley B.K. CD-NP: a novel engineered dual guanylyl cyclase activator with anti-fibrotic actions in the heart. PloS One. 2012;7:e52422. doi: 10.1371/journal.pone.0052422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ichiki T., Schirger J.A., Huntley B.K. Cardiac fibrosis in end-stage human heart failure and the cardiac natriuretic peptide guanylyl cyclase system: regulation and therapeutic implications. J Mol Cell Cardiol. 2014;75:199–205. doi: 10.1016/j.yjmcc.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lee C.Y., Chen H.H., Lisy O. Pharmacodynamics of a novel designer natriuretic peptide, CD-NP, in a first-in-human clinical trial in healthy subjects. J Clin Pharmacol. 2009;49:668–673. doi: 10.1177/0091270009336233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lim J., Clements M.A., Dobson J. Delivery of short interfering ribonucleic acid-complexed magnetic nanoparticles in an oscillating field occurs via caveolae-mediated endocytosis. PloS One. 2012;7:e51350. doi: 10.1371/journal.pone.0051350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ng X.W., Huang Y., Chen H.H., Burnett J.C., Jr., Boey F.Y., Venkatraman S.S. Cenderitide-eluting film for potential cardiac patch applications. PloS One. 2013;8:e68346. doi: 10.1371/journal.pone.0068346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Le K.Q., Prabhakar B.S., Hong W.J., Li L.C. Alternative splicing as a biomarker and potential target for drug discovery. Acta Pharmacol Sinica. 2015;36:1212–1218. doi: 10.1038/aps.2015.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wright J.T., Jr., Williamson J.D., Whelton P.K. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103–2116. doi: 10.1056/NEJMoa1511939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Buglioni A., Cannone V., Cataliotti A. Circulating aldosterone and natriuretic peptides in the general community: relationship to cardiorenal and metabolic disease. Hypertension. 2015;65:45–53. doi: 10.1161/HYPERTENSIONAHA.114.03936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dickey D.M., Potter L.R. Dendroaspis natriuretic peptide and the designer natriuretic peptide, CD-NP, are resistant to proteolytic inactivation. J Mol Cell Cardiol. 2011;51:67–71. doi: 10.1016/j.yjmcc.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.McKie P.M., Cataliotti A., Boerrigter G. A novel atrial natriuretic peptide based therapeutic in experimental angiotensin II mediated acute hypertension. Hypertension. 2010;56:1152–1159. doi: 10.1161/HYPERTENSIONAHA.110.159210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.McKie P.M., Cataliotti A., Ichiki T., Sangaralingham S.J., Chen H.H., Burnett J.C., Jr. M-atrial natriuretic peptide and nitroglycerin in a canine model of experimental acute hypertensive heart failure: differential actions of 2 cGMP activating therapeutics. J Am Heart Assoc. 2014;3:e000206. doi: 10.1161/JAHA.113.000206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Buglioni A., Scott C.G., Bailey K.R., Rodeheffer R.J., Sarzani R., Burnett J.C. Aldosterone levels, atrial natriuretic peptide and resistant hypertension in the general community. J Am Soc Hyperten. 2016;10:e40. [Google Scholar]
- 83.Chen H.H., Neutel J., Smith D., Heublein D., Burnett J. A first-in-human trial of a novel designer natriuretic peptide ZD100 in human hypertension. J Am Soc Hyperten. 2016;10:e23. [Google Scholar]