Abstract
Disparities in women’s reproductive health outcomes across the life course have been well-documented. Endocrine disrupting chemicals may be one factor driving disparities, as studies suggest exposure to certain environmental endocrine disrupting chemicals, such as certain phthalates, bisphenol A, parabens and polybrominated diphenyl ethers are higher in non-whites. Yet, a limited amount of research has focused on these chemical exposures as a potential mediator of racial/ethnic differences in women’s reproductive health outcomes, such as pubertal development, fibroids, infertility, and pregnancy complications. Given that race/ethnicity is a social construct, the purpose of this review was to present the current state of the literature on racial/ethnic disparities in both environmental endocrine disrupting chemicals, as well as associations between these chemicals and selected women’s reproductive health outcomes. Our goal was to evaluate literature from populations based in the United States to: 1) characterize racial/ethnic differences in environmental endocrine disrupting chemicals and 2) systematically review literature on environmental endocrine disrupting chemicals and selected women’s health outcomes in populations containing more than one racial/ethnic group. This review highlights the need for future work in determining whether higher exposures to some environmental endocrine disrupting chemicals might partly explain differences in women’s reproductive health outcomes in these higher-exposure and high-risk groups.
Keywords: Race, ethnicity, endocrine disruptors, and women’s health
Introduction
Health disparities are defined as differences that systematically and negatively impact less-advantaged subgroups of the population [1]. Within the U.S. race/ethnicity is a determinant of health [2–4]. Much research has documented racial/ethnic disparities in women’s reproductive health outcomes [5–7], noting both social and biological determinants. While geographic region of one’s ancestral origin may explain some genetic or underlying biological differences, race is predominately a social construct related to societal structure, behaviors, cultural traditions, and environmental factors [8, 1]; the latter has received limited attention in disparities research.
When considering environmental factors, environmental endocrine disrupting chemicals (EDCs) warrant attention in racial/ethnic disparities research because many sources of EDCs such as consumer product use [9–12], diet [13, 14], and the built environment [15–17] are socially patterned [18, 19]. Consequently, non-whites have been found to have higher concentrations of many of these chemicals in numerous epidemiologic studies. Higher exposures in non-whites may contribute to increasing disparities in the incidence of adverse health outcomes due to the unequal distribution of both chronic exposures to non-persistent and persistent chemicals, along with an unequal distribution of protective factors (Figure 1). These differences could lead to disparate health outcomes, which may become magnified across the life course due to weathering or cumulative wear and tear. Yet, the link between racial/ethnic disparities in exposure patterns and their impact on disparities in adverse health outcomes is not well understood. Of interest, is that EDC exposure can be modifiable with implications for identification of and interventions for reducing disparities in exposures and associated outcomes.
Figure 1.
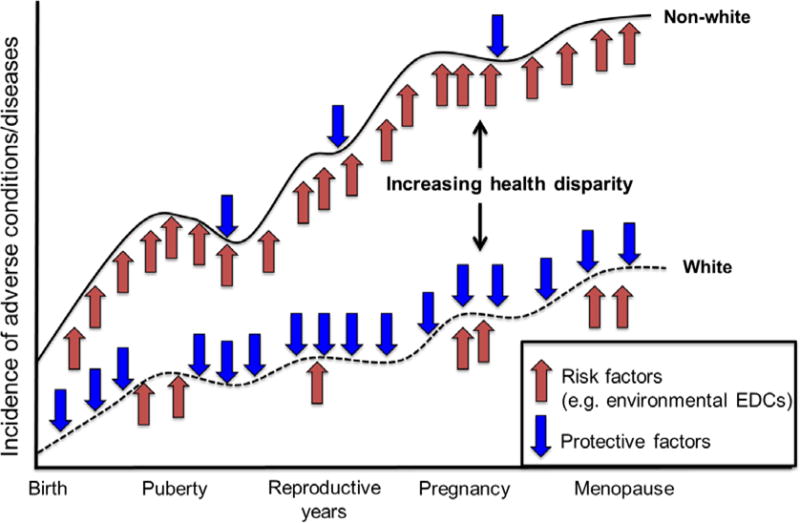
The contribution of unequal environmental exposures to increasing risk of adverse women’s health disparities (adapted from Lu and Haflon)5
While studies have presented racial/ethnic differences in EDC exposures (i.e. phthalates, bisphenol A, parabens, and polybrominated diphenyl ethers), as well as racial/ethnic differences in women’s health outcomes, few studies have evaluated whether racial/ethnic differences in EDC exposures contributes to differences we see in these outcomes. The purpose of this paper is to call attention to this important gap in disparities research. Here, we will 1) review the current state of the literature for racial/ethnic disparities in women’s exposures to 4 types of environmental EDCs – phthalates, bisphenol A (BPA), parabens, and polybrominated diphenyl ethers (PBDEs); and 2) summarize the existing literature on associations between EDCs and selected and disparate women’s reproductive health outcomes—puberty, fibroids, infertility, and pregnancy complications. Given that one of the 2012–2017 strategic goals for the National Institute of Environmental Health Sciences is to reduce health disparities, a better understanding for how these chemicals affect racial/ethnic health disparities is a critical public health question [20].
Disparities in environmental EDC exposures among women
Phthalic acid esters, also known as phthalates, are a class of industrial chemicals that are ubiquitously used in commercial products. Low molecular weight phthalates, such as diethyl phthalate (DEP), di-n-butyl phthalate (DnBP), and di-iso-butyl phthalate (DiBP), are used in personal care products, solvents, adhesives, and medications [21–23]. High molecular weight phthalates, such as butylbenzyl phthalate (BBzP), di(2-ethylhexyl) phthalate (DEHP), di-iso-nonyl phthalate (DiNP), and di-iso-decyl phthalate (DiDP) are primarily used as plasticizers in polyvinyl chloride (PVC) applications found in building materials, medical equipment, and food packaging [21, 24, 25]. Phthalates are non-persistent chemicals in humans, with half-lives of about 12–24 hours, so measured levels of metabolites in urine reflect recent exposures. Virtually all U.S. women are exposed to multiple phthalates [26]. Among reproductive-aged women in the U.S. general population, non-Hispanic black and Mexican American women have higher metabolite concentrations of the low-molecular weight phthalates (e.g. DEP, DnBP) than non-Hispanic white women[27, 12, 28]. Similar exposure disparities between non-white and white subpopulations have also been observed in pregnant women [29, 30] and girls aged 6–8 years [31, 32]. While socio-cultural differences in personal care product use have been hypothesized as a possible driver of disparities in low-molecular weight phthalate exposure, only one study has attempted to characterize this relationship. Branch et al [12] found that differences in vaginal douching practices may contribute to black/white disparities in DEP exposure. Racial/ethnic patterns in DEHP or other high molecular weight phthalates among women are less consistent; most studies report that levels of DEHP metabolites are similar across race/ethnicity [27, 29, 32, 31], with a few studies reporting higher exposures among white compared to non-white women [33•, 28].
Phenols are used in consumer products, including food can linings, plastic bottles, thermal receipt paper, antimicrobial agents, and preservatives [34, 35]. Phenols, including bisphenol A (BPA) and parabens, are non-persistent chemicals that are rapidly metabolized and eliminated, with half-lives in the human body between 6 and 30 hours [36]. Most U.S. women are exposed to BPA, and levels among women are higher than men [37]. Among studies that have reported BPA levels by race in female populations, approximately one-third report no racial/ethnic differences [31, 38–40], one-third report higher levels among black women compared to white women [41–43], and the remainder report significant racial/ethnic differences; but the groups with elevated levels are heterogeneous (e.g. non-Hispanic, non-Asian or other) making it difficult to compare across studies [44–46].
Methyl paraben (MP) and propyl paraben (PP) are two of the most used parabens with detection frequencies above 90% in the U.S., while butyl paraben (BP) and ethyl paraben (EP) are detected less frequently. Women have higher levels of MP and PP than men [47]. Racial/ethnic differences in paraben levels have been less studied; however, most studies find that black women have significantly higher total paraben burden than white women [31, 48] Personal care product use and diet have been hypothesized as sources of variability for BPA and parabens but have not been confirmed by empirical studies [49].
PBDE flame retardants are commonly found in consumer products such as upholstered furniture, electronics, and textile products [34]. Most PBDE flame retardants are no longer used in the U.S., and replacement flame retardants have been developed as alternatives. Consequently, PBDE exposures appear to be declining in some U.S. populations [50]; however, because PBDEs are lipophilic, persistent chemicals similar to PCBs, with half-lives between a few months to over 10 years in adult human adipose tissue, they will likely persist for decades [50].
The majority of PBDE body burden studies find higher levels among non-white women compared to white women. For example, among adolescent girls in the U.S., whites had lower total PBDE concentrations than Mexican Americans and others [51]. Furthermore, higher total PBDE levels have been observed in blacks compared to whites among pre-adolescent girls [52] as well as pregnant women [53]. Similarly, a study of mostly post-menopausal women from California found that non-white women consisting of blacks, Asians, and Hispanics, had higher levels of PBDE-47, -100, and -153 than white women [54]. In contrast, another study of California women found that non-Hispanic women (white, Asian, or Native American) had higher levels of BDE-100 and -153 than Hispanic women although the differences in ethnicity were largely driven by one Native American women with very high levels [55]. Since an important source of human exposure to PBDEs is household dust, exposure disparities in PBDEs by race/ethnicity are hypothesized to stem from differences in housing stock and furniture quality [56] although more work is needed in this area.
Potential mechanisms for action
Phthalates, phenols, and PBDEs are suspected EDCs because they can interfere with hormone regulation and action [57]. In vitro and animal studies suggest that certain phthalates, such as DEHP and DnBP, exert their toxicity primarily through disruption of androgen production [58]. Phthalates may also interact with peroxisome proliferator–activated receptor γ, estrogen, and thyroid receptors [59, 60]. Animal studies demonstrate that exposure to phthalate mixtures results in greater risk than exposure to individual phthalates [61, 58]. Consequently, the National Academy of Sciences has recommended that simultaneous exposure to phthalates be examined using a cumulative risk assessment framework [58]. BPA is a reproductive toxicant that can act through physiological receptors, such as genomic estrogen receptors 1 and 2, membrane-bound estrogen receptors, androgen receptor, peroxisome proliferator–activated receptor γ, and thyroid hormone receptor [62]. Parabens exhibit weak estrogenic activity and can bind to both the estrogen receptor-α and estrogen receptor-β [60, 59]. PBDEs can affect thyroid homeostasis through multiple pathways. They can alter the binding of thyroid hormones to thyroid transport proteins as well as thyroid hormone receptors [63, 64]. PBDEs can also exhibit weak estrogenic and anti-androgenic activity [65, 66]. These chemicals can also affect other biological pathways. For example, there is increasing attention on the ability of chemicals such as phthalates and BPA to induce oxidative stress and inflammation as well as epigenetic modifications such as DNA methylation and microRNA expression [67–69].
Methods for systematic review of the literature
To conduct a literature review of racial/ethnic disparities in environmental chemicals and the effects on women’s health outcomes in the U.S., we searched all English articles in PubMed and EMBASE from the inception of all databases to Jan 15, 2016. We pre-specified four major EDCs (phthalates, BPA, parabens and PBDEs) and specific women’s reproductive health outcomes (i.e. puberty, fibroids, pregnancy, and pregnancy complications). In article searching for chemical exposures from Pubmed, we combined the Medical Subject Headings (MeSH) terms and key words as follows: “phthalic acids,” “bisphenol A-glycidyl methacrylate,” “parabens,” or “halogenated diphenyl ethers,” as MeSH terms, and phthalic acid, phthalate, bisphenol A, methylparaben, butylparaben, propylparaben, polybrominated diphenyl ether, and organobromine compound as specific key words in texts.
For women’s health outcomes, the MeSH terms included “puberty,” “puberty, delayed,” “puberty, precocious,” “pregnancy,” “infertility, female,” “ovarian reserve,” “ovarian follicle,” “pregnancy complications,” “premature birth,” and “leiomyoma,” key words included menarche, thelarche, breast development, antral follicle count, preeclampsia, gestational diabetes and preterm.
Similarly, in our EMBASE search for chemical exposure, we combined Emtree terms and key words as follows: “phthalic acid derivative,” “phthalate,” “4,4 isopropylidenediphenol,” “4 hydroxybenzoic acid ester,” “propyl paraben,” “methyl paraben,” “ethyl paraben,” “butyl paraben,” “benzyl paraben,” and “polybrominated diphenyl ether” searched as Emtree terms; phthalate, BPA, paraben, polybrominated diphenyl ethers, and PBDE as key words in text.
For women’s health outcomes, we used all the Emtree terms including “puberty,” “delayed puberty,” “precocious puberty,” “adrenarche,” “breast development,” “pregnancy diabetes mellitus,” “preeclampsia,” “premature labor,” “pregnancy complication,” “pregnancy rate,” “uterus myoma,” and “leiomyoma. After excluding in vitro studies, animal studies, studies conducted outside of the U.S., as well as studies that did not assess the outcomes of interests, the searching strategies yielded a total of 612 articles in Pubmed and EMBASE.
We reviewed these articles and identified 46 discrete studies examining the association between environmental EDCs and women’s reproductive health outcomes among women living in the U.S. We also documented whether race-specific measures of association were reported in the main findings.
Epidemiologic examples across the life course
Environmental EDCs and puberty
Racial/ethnic differences in puberty have been well-documented [70]. For example, black girls are more likely to reach menarche earlier, with 62% of black girls reaching menarche by age 12 compared to 35% of white girls. These age differences can be seen for breast and pubic hair development, as well. Environmental factors are thought to contribute to this difference.
Ten studies that met our criteria evaluated the association between EDCs and indicators of pubertal development [48, 51, 71, 41, 72–75•, 32, 31]. Specifically, the studies assessed a range of outcomes, from central precocious puberty to hormone levels and self-reported age at menarche (See Table 1). Most studies adjusted for race/ethnicity, with a few presenting stratified analyses.
Table 1.
Summary of epidemiologic studies examining the association between environmental chemicals and pubertal development.
| Author and year | Number of participants | Chemical tested | Time of exposure | Study design | Age range or mean±SD | Outcomes of interest | Main findings |
|---|---|---|---|---|---|---|---|
| Rais-Bahrami 2004[72] | Total: 19 teens Female: 32% (unknown race) |
Phthalates | Subjects undergone ECMO as neonates were considered as exposed | P, adolescents undergone ECMO as neonates at Children’s National Medical Center, Washington, DC | 14–16 | Tanner stage, testicular volume, LH, FSH, estradiol, testosterone |
|
| Wolff 2008[74] | Total: 192 girls -34% white -28% black -38% Hispanic |
Urinary BPA | CS | CS, Mount Sinai Hospital in NY city and in a nearby pediatric private practice | 9.5 ± 0.3 | Pubertal stages (breast and pubic hair development) |
|
| Lomenick 2010[71] | Total: 56 girls Cases (n=28) -70% Cau -30% AA Control (n=28) -71% Cau -30% AA |
Urinary phthalates | CS | CC, pediatric endocrinology clinics at three centers in Lexington, Cincinnati and Louisville. | 6–8 | Central precocious puberty (CPP) |
|
| Wolff 2010[31] | Total:1151 girls -34% white -31% black -30% Hispanic -5% Asian -1% other |
Urinary phthalates, BPA, and parabens | At visit 1 (majority were pre-pubertal) | P, BCERC: New York City, greater Cincinnati and the San Francisco Bay area | 6–8 year followed through puberty | Pubertal stages (breast and pubic hair development) |
|
| Chen 2011[51] | Total: 271 girls -28% white -35% black -37% MA and other |
Blood PBDEs | CS (majority had reached menarche) | CS, NHANES 2003–2004 | 12–19 | Age of menarche |
|
| Buttke 2012[48] | Total: 440 girls -28% NHW -31% NHB -31% MA -6% other Hispanic -5% other |
Urinary phthalates, BPA and parabens | CS | CS, NHANES 2003–2008 | 12–16 | Age of menarche |
|
| Wolff 2014[32] | Total: 1239 girls -31% white -30% black -29% Hispanic - 5% Asian |
Urinary phthalates | At enrollment (>80% were pre-pubertal) | P, BCERC | 6–8 year at enrollmentand followed for 6 years | Pubertal stages (breast and pubic hair development) |
|
| Wolff 2015 [75•] | Total: 1239 girls -31% white -30% black -29% Hispanic - 5% Asian |
Urinary BPA and parabens | At enrollment (>80% were pre-pubertal) | P, BCERC | 6–8 year at enrollment and followed for 7 years | Breast and pubic hair development |
|
| McGuinn 2015[41] | Total: 987 girls -63% NHW -15% AA -11% MA -12% others |
Urinary BPA | CS | CS, NHANES 2003–2010 | 12–19 | Age at menarche |
|
| Windham 2015 [73•] | Total: 645 girls -52% white -26% black -15% Hispanic -7% Asian |
Blood PBDEs | The earliest visit for each girl | P, BCERC | 6–8 at enrollment and followed for 7 years | Pubertal onset for breast and pubic hair development. |
|
Abbreviations: AA, African Americans; CC, case control; Cau: Caucasian; CS, cross-sectional; HMWP, high molecular weight phthalate; LMWP, low molecular weight phthalate; MA, Mexican American; NHB, non-Hispanic Black; NHW, non-Hispanic White; P, prospective cohort study; R: retrospective cohort study; •of importance to the field
Phthalates
In general, sample sizes ranged from 56 to 1239; however, almost all of the studies found no association between urinary phthalate metabolite concentrations measured at different time points in early life/childhood and any of the pubertal outcomes. The one study by Wolff et al showed that higher concentrations of high molecular weight phthalates, including DEHP metabolites were associated with later age of pubic hair development [32]. There was signal of this finding in an earlier evaluation of the same study population [31].
Bisphenol A
A number of these same studies also evaluated BPA. Two cross-sectional studies using data from the National Health and Nutrition Examination Survey (NHANES) found discordant results for the BPA and age at menarche, with an earlier study finding no association and a later study finding a slightly reduced risk for earlier age at menarche with higher BPA concentrations [48, 41]. When looking longitudinally with a different pubertal outcome, data from the Breast Cancer and Environment Research Program (BCERP) found no association between BPA and age at pubertal staging [75•].
Parabens
In the 3 studies that evaluated parabens, two came from the BCERP study population at different time points. In the first study of 1,151 girls, no association was found [31]. However, the later study of 1,239 girls found that higher paraben concentrations were associated with earlier age at stage B2 for breast development [75•]. Interestingly, after adjustment for race/ethnicity and caregiver education, the association no longer existed. The third study, which was a cross-sectional study of the NHANES population, found no association between parabens and age at menarche [48].
Polybrominated diphenyl ethers
Only two studies have evaluated PBDEs and pubertal outcomes, and the findings were not entirely consistent. Specifically, the longitudinal study found higher PBDE concentrations to be associated with a delay in breast development [73•], while the cross-sectional study found an association between higher PBDEs and earlier age at menarche [51].
Environmental EDCs and gynecologic conditions—fibroids
When considering other life stages, a number of studies have documented racial/ethnic disparities in the incidence of gynecologic disorders, such as uterine leiomyoma, or uterine fibroids. In fact, compared to white women, black women have a higher incidence of fibroid tumors [77, 78] and have larger and more symptomatic fibroids [78, 77, 79]. While a number of studies have attempted to evaluate reasons for these disparities associations, studies evaluating associations between environmental risk factors and fibroids have only recently emerged (Table 2).
Table 2.
Summary of epidemiologic studies examining the association of environmental chemicals with uterine fibroids
| Author and year | Number of participants | Chemical tested | Time of exposure | Study design | Age range or mean± SD | Outcomes of interest | Main findings |
|---|---|---|---|---|---|---|---|
| Weuve 2010[28] | Total: 1227 women -68% NHW -12% NHB -8% MA -12% other |
Urinary Phthalates | CS | CS, NHANES 1999–2004 | 20–54 | Leiomyoma & Endometriosis |
|
| Pollack 2015[80] | Total: 473 women Cases (n=99) -62% white -15% Hispanic -23% black/Asian/other Controls (n=374) -78% white -13% Hispanic -9% black/Asian/other |
Urinary Phthalates and BPA | Before surgery | Matched cohort design, ENDO study | 18–44 | Postoperative surgical diagnosis of fibroids. |
|
Abbreviations: CS, cross-sectional; MA, Mexican American; NHB, non-Hispanic Black; NHW, non-Hispanic White.
Phthalates
Two papers evaluated associations between phthalates with fibroids [80, 28]. While differences in exposure and outcome patterns existed by race/ethnicity, associations were conflicting. In the Endometriosis, Natural history, Diagnosis, and Outcomes (ENDO) Study, no association was found between higher concentrations of any of the urinary metabolite concentrations and surgically confirmed fibroids [80]. In a cross-sectional study, there were weak positive associations for MBP in relation to self-reported history of fibroids [28]. On the other hand, higher concentrations of MEHP were associated with a reduced odds of fibroids [28].
Bisphenol A
One paper evaluated BPA and fibroids. In this paper, cases had higher BPA levels compared to controls, but these differences were not statistically significant in adjusted models [80].
Based on research conducted in U.S. populations, the association between parabens and fibroids, as well as the association between PBDE concentrations and fibroids has not been explored in published epidemiological papers.
Environmental EDCs and infertility
Infertility is a common disease affecting 15% of couples during reproductive years in the U.S. [81]. It has been reported that black and Hispanic women had higher infertility rates [82] and greater risk of pregnancy loss compared to white women despite non-white women being less likely to utilize assisted reproductive technology (ART) [83]. In addition, black women also experienced lower live birth rates than white women following ART even under the “equal access-to-care” settings from military ART centers [84], suggesting that other important factors are at play. When looking at U.S.-based studies, 18 studies were published evaluating environmental chemicals and infertility and associated pregnancy outcomes (Table 3). One main issue of these studies is that there was a low minority representation in fertility centers, and as a result, these studies were unable to explore whether differences in higher exposure patterns among non-whites might contribute to different patterns of infertility and its related outcomes.
Table 3.
Summary of epidemiologic studies examining the associations of environmental chemicals with female fertility and associated outcomes
| Author and year | Number of participants | Chemical tested | Time of exposure | Study design | Age range or mean± SD | Outcomes of interest | Main findings |
|---|---|---|---|---|---|---|---|
| Mok-Lin 2010[94] | Total: 84 women -88% Cau -4% AA -5% Hispanic -4% other |
Urinary BPA | Preconception | P, Environmental and Reproductive Health study (EARTH study): women presenting to a fertility clinic at Boston MA | 21–44 (35.6 ± 3.9) | IVF intermediate outcomes: serum FSH, estradiol, oocyte retrieval |
|
| Harley 2010[99] | Total: 223 women -2% NHW -96% Hispanic -2% other |
Blood PBDEs | Near the end of the 2nd trimester | P, Center for the Health Assessment of Mothers and Children of Salinas (CHAMACOS study): pregnant women from 6 low-income prenatal care clinics in Salinas Valley, CA | 21.5–27.3 | Fecundability odds ratios (FORs) |
|
| Fujimoto 2011[91] | Total: 58 women -28% Asian -72% not Asian |
Urinary BPA | Preconception | CS, couples undergoing a first IVF cycle at the University of California at San Francisco Center for Reproductive Health | 35.80 ± 4.08 | Oocyte quality during IVF |
|
| Bloom 2011[45] | Total: 44 women -30% Asian -70% not Asian |
Urinary BPA | Preconception | CS, couples undergoing a first IVF cycle at the University of California at San Francisco Center for Reproductive Health | 35.80 ± 4.08 | IVF intermediate outcomes: peak-estradiol level (E2) and the number of oocytes retrieved during IVF. |
|
| Johnson 2012[100] | Total: 65 women -84% Cau -16% other |
Blood and follicular fluid PBDEs | Preconception | P, couples undergoing IVF or ICSI were recruited through three clinics in the Boston area | 36.0±3.8 (27–44) | Odds of failed embryo implantation |
|
| Ehrlich 2012[90] | Total: 137 women -87% white -4% AA -4% Hispanic/other -6% Asian |
Urinary BPA | Preconception | P, EARTH study | 21–44 (35.8 ± 4.0) | IVF treatment outcomes |
|
| Souter 2013[95] | Total: 154 women -82% Cau -4% AA -6% Asian -1% Native Americans/Alaska Native -8% other |
Urinary BPA | Preconception | P, EARTH study | 18–46 (35.7 ± 4.6) | Antral follicle count, day 3 FSH, ovarian volume (OV) |
|
| Smith 2013[96] | Total: 192 women -81% Cau -5% AA -5% Asian -1% Native Americans/Alaska Native -8% other |
Urinary parabens | Preconception | P, EARTH study | 21–46.7 (36.1 ± 4.64) | Antral follicle count |
|
| Messerlian 2015[89] | Total: 215 women -81% Cau -3% AA -7% Asian -8% other |
Urinary phthalates | Preconception | P, EARTH study | 18–46 (35.7 ± 4.6) | Antral follicle count |
|
| Buck Louis 2013[98] | Total: 501 couples -79% NHW |
Blood PBDEs | Preconception | P, the Longitudinal Investigation of Fertility and the Environment (LIFE) Study: couples attempting to become pregnant from 16 targeted counties in Michigan and Texas | 30.0±4.1 | Fecundability odds ratios (FORs) |
|
| Buck Louis 2014[86] | Total: 501 couples -79% NHW |
Urinary Phthalates and BPA | Preconception | P, LIFE Study | 30.0±4.1 | Fecundability odds ratios(FORs), time to pregnancy (TTP) |
|
| Lathi 2014[92] | Total: 115 women (unknown race) | Blood BPA | At the first trimester (around GA 4 week) | R, women who sought treatment for infertility or recurrent pregnancy loss at Stanford Fertility and Reproductive Medicine Clinic | Miscarriage women 36.5 (4.3); Live birth women 35.9 (4.4) | Live birth, miscarriage, and chromosome content of miscarriage. |
|
| Hauser R 2015[87] | Total: 256 women -82% Cau -2% AA -8% Asian -7% other |
Urinary phthalates | Preconception | P, EARTH study | 18–46 | IVF treatment outcomes |
|
| Minguez-Alarcon 2015[93] | Total: 256 women -82% Cau - 2% black -8% Asian -7% other |
Urinary BPA | Preconception | P, EARTH study | 18–45 | IVF treatment outcomes |
|
| Minguez-Alarcon 2015[97] | Total: 245 women -83% white -3% black -8% Asian -7% other |
Urinary parabens | on days 3–9 of the monitoring phase of their cycle (ie. Preconception) | P, EARTH study | 18–45 | IVF treatment outcomes |
|
| Alur 2015[85] | Total: 468 women Women using ART (n=41) -78% white -0% AA -12% Asian -10% other Infertile not using ART (n=25) -96% white -4% AA Comparison (n=402) -76% white -6% AA -9% Asian -9% other |
Urinary phthalates | At the 1st trimester | P, the Infant Development and Environment Study (TIDES): women through prenatal care clinics at University of Minnesota & Rochester &, University of Washington School of Medicine, and UCSF, and Seattle Children’s Hospital. | Women using ART (35.37±4.78), infertility not using ART (33.94±4.46), comparison group (32.00±4.63) | History of infertility and use of assisted reproductive technology (ART) |
|
| Jukic 2015[88] | Total: 221 women -96% white |
Urinary phthalates and BPA | Three urines were from each menstrual cycle until pregnant were pooled | P, North Carolina Early Pregnancy Study (EPS): healthy women with no known fertility problem | 29 (26, 31) | Follicular- and luteal-phase lengths, time to pregnancy, and early pregnancy loss |
|
Abbreviations: AA, African Americans; ART, assisted reproductive technology; Cau: Caucasian; CS, cross-sectional; FSH: follicle stimulating hormones; GA: gestational age; ICSI: intracytoplasmic sperm injection; IVF: in vitro fertilization; LGT, longitudinal study; MA, Mexican American; NHB, non-Hispanic Black; NHW, non-Hispanic White; P, prospective cohort study; R: retrospect cohort study
Phthalates
Five U.S. studies have recently assessed associations between urinary phthalate metabolites and infertility and related outcomes, including results from natural conception and pregnancies following ART treatment [85–89]. For the former, studies evaluated the probability of being pregnant, time to pregnancy, length of follicular and luteal phases, and pregnancy loss. For the evaluation of pregnancies following ART treatment, chronological endpoints ranged from ovarian reserve (e.g., antral follicle count), ovarian stimulation response (e.g., oocyte yields, peak estradiol levels), fertilization rates, embryo quality, implantation, pregnancy to live birth, were evaluated. While consistency across studies was difficult given the multiple endpoints, the research points to both DEHP and its replacement chemicals, DiNP and DIDP as being related to fertility potential. For example, DEHP was associated with reduced antral follicle count [89] and oocyte yield at retrieval [87]. Other studies have shown that metabolites of DiNP was associated with shorter luteal phase [88]. Another study pointed to higher concentrations of DEHP metabolites among women with fertility issues [85]. This same study also found MEP, a metabolite of DEP, to be higher in women with reported infertility that sought ART [85].
Bisphenol A
Nine studies evaluated the association between BPA and fertility outcomes based on studies conducted in the U.S. [45, 86, 90, 91, 88, 92–95]. Of these, two cohorts of healthy women without known fertility problems found that urinary BPA was unrelated to time to pregnancy despite a shorter luteal phase being reported in one study [86, 88]. On the other hand, studies conducted in fertility centers suggested BPA exposure was associated with lower ovarian reserve [95], poor ovarian stimulation responses [94], and higher risk of miscarriage [92]. While BPA was associated with worse earlier endpoints following ART, higher concentrations of this chemical was unrelated to live birth rate [93], the most relevant outcomes for fertility patients and their care providers. These studies suggest that BPA may affect ovarian functions and early process of conception. However, only one study presented strata-specific racial/ethnic differences. They found that Asian women had an increased oocyte maturity rate, but this group had substantially lower BPA concentrations in this study [91].
Parabens
Two studies from the same population evaluated the association between parabens and infertility outcomes [96, 97]. One study found an association between higher propyl paraben and diminished ovarian reserves [96]. The other study found no association between parabens and IVF treatment outcomes [97].
Polybrominated diphenyl ethers
Three U.S. studies have evaluated PBDEs as they relate to reproductive success [98–100]. These studies found an association between certain congeners of PBDEs with pregnancy outcomes. For example, a study conducted among predominantly white women living in Texas and Michigan found a suggestively inverse association between PBDE 183 and fecundity as measured by time to pregnancy [98]. Another study comprised predominantly of Mexican population living in California found an association with higher PBDE153 and PBDE100 concentrations with a decreased fecundity odds ratio, a measure of longer time to pregnancy [99]. In a separate study conducted in the Boston area, which was comprised of over 80% white women, there was an association between PBDE153 and increased odds of failed embryo implantation [100].
Environmental EDCs and pregnancy complications
For the last decade researchers investigated the role of certain EDCs in maternal pregnancy complications. Much of this work has focused on preterm birth, pregnancy hypertensive disorders, gestational diabetes, and related factors known to be more common in racial/ethnic minorities. Below, we describe the associations between the selected EDCs, pregnancy complications and related factors (Table 4). Most studies adjust for race/ethnicity, but do not present stratified analyses or assess whether EDCs explain racial/ethnic disparities in the pregnancy complications under study.
Table 4.
Summary of epidemiologic studies examining the association between environmental chemicals and pregnancy complications and associated outcomes
| Author and year | Number of participants | Chemical tested | Time of exposure | Study design | Age range or mean± SD | Outcomes of interest | Main findings |
|---|---|---|---|---|---|---|---|
| Wolff 2008[108] | Total: 404 women -21% white -28% black -50% Hispanic - 1% other |
Urinary phthalates and BPA | At the 3rd trimester | P, Pregnant women enrolled before delivery at Mount Sinai Medical Center in New York City | 24.0±6.2 | Birth outcomes including gestational age |
|
| Adibi 2009[101] | Total: 283 women -84% white -9% Hispanic -6% other |
Urinary Phthalates | Late pregnancy (mean: 28.3 weeks) | P, Study for Future Families (SEF) study: women with natural conception recruited at prenatal clinics associated with hospital in California, Iowa, Minnesota, and Missouri. | 39.2±1.5 | Timing of parturition |
|
| Whyatt 2009[107] | Total: 331 women -28% black -72% Dominican or other Hispanic |
Urinary phthalates | At the 3rd trimester | P, Columbia Center for Children’s Environmental Health (CCCEH): a birth cohort included either AA or Dominican who had resided northern Manhattan or the South Bronx for ≥1 year before pregnancy | 25.5±4.8 | Length of gestation |
|
| Robledo 2013 [46] | Total: 94 women Cases (n=22) -64% Hispanic Controls (n=72) -28% Hispanic |
Urinary BPA | At 2nd trimester | CC, Medical Center Women’s and High Risk Pregnancy clinics located in Oklahoma City | % ≥ 25 y Case: 77% Controls: 47% |
Blood glucose levels at the time of screening for GDM |
|
| Patel 2014 [110] | Total: 780 women Cases (n=62) -58% white -27% black -7% Mexican -4% other Hispanic -4% other Controls (n=718) -61% white -14% black -17% Mexican -5% other Hispanic -3% other |
Urinary BPA | Unknown temporal relationship between sample collection and outcome | CS, NHANES 1999–2006; participants restricting pregnancy in the last year prior to survey | Case: 27.8±1.1; control: 27.5±0.4 | Preterm birth |
|
| Ferguson 2014 [103•] | Total: 482 women -59% Cau -16% AA -26% other |
Urinary phthalates | At median 10, 18, 26, 35 GA | Nested CC, Lifecodes study: 130 mothers who delivered preterm and 352 who delivered term from a prospective birth cohort at Brigham and Women’s Hospital and Beth Israel Deaconess Medical Center in Boston, MA and the Hospital of the University of Pennsylvania in Philadelphia, PA | Maternal age at visit 1 was 32.0 years on average | Preterm birth |
|
| Ferguson 2014 [115] | Total: 482 women -59% Cau -16% AA -26% other |
Urinary phthalates | At median 10, 18, 26, 35 GA | Nested CC, Lifecodes study | Maternal age at visit 1 was 32.0 years on average | Preterm birth |
|
| Robledo 2015 [105] | Total: 72 women -29% NHW -37% NHB -24% Hispanic -10% other |
Urinary phthalates | At the 1st trimester | P, Pregnant women recruited for a pilot study during their first prenatal care visit at the University of Oklahoma Medical Center Women’s Clinic. | 65% <25 y | Blood glucose levels at the time of screening for GDM |
|
| Ferguson 2015 [109] | Total: 482 women -59% white -16% AA -26% other |
Urinary phthalates and BPA | At median 10, 18, 26, 35 GA | Nested CC, Lifecodes study | Maternal age at visit 1 was 32.0 years on average | Circulating angiogenic biomarkers: Placental growth factor (PlGF) and soluble fms-like tyrosine kinase-1 (sFlt-1) |
|
| Ferguson 2015 [102] | Total: 480 women -59% white -16% AA -26% other |
Urinary phthalates | At median 10, 18, 26, 35 GA | Nested CC, Lifecodes study | 18.3–50.2 (Median age 32.7 yrs) | Inflammatory biomarkers : CRP, IL-1β, IL-6, IL-10, and TNF-α |
|
| Ferguson 2015 [69] | Total: 482 women -59% white -16% AA -26% other |
Urinary phthalates | At median 10, 18, 26, 35 GA | Nested CC, Lifecodes study | Maternal age at visit 1 was 32.0 years on average | Oxidative stress biomarkers in urine (8-OHdG and total 8-isoprostane) |
|
| Cantonwine 2015 [104] | Total: 482 women -59% white -16% AA -26% other |
Urinary BPA | At median 10, 18, 26, 35 GA | Nested CACO, Lifecodes study | Maternal age at visit 1 was 32.0 years on average | Preterm birth |
|
| Werner 2015 [33•] | Total: 369 pregnant women -62% white -38% non-white |
Urinary phthalates | At median 16 and 26 weeks of GA | P, the Health Outcomes and Measures of the Environment (HOME) study: women living in the Cincinnati, OH area in homes built before 1978 | 29.5 ± 5.8 | Systolic blood pressure and diastolic blood pressure at GA < 20 and ≥ 20 weeks |
|
| Smarr 2015 [106] | Total: 233 mothers -84% NHW -1% NHB -9% Hispanic -6% Other |
Urinary phthalates and BPA | Preconception | P, a subset of LIFE study who had a single live birth. | 29.8±3.7 | Birth outcomes including gestational age |
|
| Veiga-Lopez 2015 [44] | Total: 80 women -77% Cau -9% AA -14% other |
Blood BPA | At 1st trimester and at delivery | P, Round Robin study aimed to validated BPA measurement across several labs. Samples were collected at the University of Michigan Von Voiglnder Women’s Hospital. | Most between 30–35 (51.2%) | Birth weight and gestational length |
|
| Peltier 2015 [111] | Total: 279 women Case (n=82) -89% Cau -11% AA Control (n=197) -33% Cau -67% AA |
Blood PBDEs | At the time of admission for labor | CC, patients at Centennial Women’s Hospital in Nashville, TN | 18–43 | Preterm birth |
|
| Smarr 2016 [112] | Total: 258 women -84% white -16% non white |
Blood PBDEs | Preconception | P, a subset of LIFE study who had a single live birth. | 29.8±3.7 | Pregnancy complication: GDM, gestational hypertension, gestational age |
|
Abbreviations: AA, African Americans; CC, case control; Cau: Caucasian; CS, cross-sectional; HR: Hazard ratios; GA: gestational age; LMWP, low molecular weight phthalate; MA, Mexican American; NHB, non-Hispanic Black; NHW, non-Hispanic White; P, prospective cohort study; R: retrospect cohort study; •of importance to the field
Phthalates
Preterm birth is one of the most studied pregnancy complications in terms of EDC exposures [101, 102, 69, 103•, 104, 46, 105, 106, 33•, 107, 108, 115]. Two studies have found an association between higher DEHP concentrations with earlier gestational ages and preterm birth [103•, 107]. Another study found associations with higher maternal concentrations of metabolites of DiNP and DIDP, and dimethyl phthalate with shorter gestation length [106]. However, this study and an earlier study found associations between higher DEHP concentrations and reduced risk of shorter gestation length and preterm birth [101, 106]. These conflicting results may be attributed to differences in timing, adjusted potential confounders, and differences in the distribution of phthalate metabolites across the populations. In fact, of the studies with positive associations with preterm birth, one was in a diverse population [108] and the other was composed of only blacks and Hispanics [107].
To explore potential pathways by which phthalates could be associated with preterm birth, Ferguson et al evaluated risk factors of preterm birth [109, 69, 102]. In these studies, a DEHP replacement was found to be associated with higher levels of a pro-inflammatory marker, interleukin-6 [102]. In another study, DEHP metabolites were found to be associated with decreases in the angiogenic marker, placental growth factor, a marker of good placental vasculature [109]. Likewise, there was an association between higher DEHP metabolites and sFLT-1/PLGF ratio, an indicator of preeclampsia and subsequently preterm birth [109]. Several phthalate metabolites were also found to be associated with elevated markers of oxidative stress in this same population [69].
Bisphenol A
Five studies evaluated BPA with pregnancy related outcomes, albeit the majority evaluated preterm birth [46, 104, 110, 44, 108]. Two studies found no association between BPA and gestation length or preterm birth [106, 108]. However, these studies assessed BPA concentrations at different time points, with one looking at pre-conception concentrations [106], while the other assessed 3rd trimester concentrations [108]. On the other hand, two studies found an association between BPA concentrations and an increased risk of preterm birth [104, 110]; however, one study was cross-sectional making it difficult to determine temporality [110]. Only one study found an association between BPA and longer gestational age, but BPA was measured in serum possible contamination issues present [44]. These contradictory findings could point to the need to replicate the studies and take into account the high variability of BPA with respect to the timing of sample collection, type of study design, and the population under study. Of these studies, only one presented BPA concentrations stratified by race/ethnicity which showed that gestational age was shorter by almost 1 day among African Americans than Dominican women for each logarithmic unit increase in MEHP metabolites [107].
While five studies have examined gestation length, studies of other outcomes are only starting to emerge. Specifically, a small case-control study examined BPA in second trimester and GDM finding no association [46]. Another study evaluated BPA and angiogenic biomarkers, finding that sFLt-1 and the ratio between sFlt-1to PlGF was positively associated [109].
Parabens
To our knowledge, studies have not evaluated the role of parabens and pregnancy complications in populations living in the U.S.
Polybrominated diphenyl ethers
Two U.S. studies have evaluated PBDEs and pregnancy complications. One study used a nested case-control design to evaluate the PBDEs at the time of delivery and risk of preterm birth finding that higher concentrations of PBDE-47 were associated with an increased odds of preterm birth [111]. Another study evaluated pre-conception PBDEs in a prospective cohort study examining several outcomes, including GDM and gestational hypertension [112]. They found associations between PBDE-153 and an increased odds of GDM, but did not find an association with any PBDEs and gestational hypertension. Both studies were predominantly white and adjusted for race/ethnicity in statistical models.
Gaps in the environmental EDC disparities and women’s reproductive health outcomes—a call for further investigation
In this review, we focused on phthalates, phenols, and PBDEs, and their relations to women’s reproductive health outcomes during 3 selected periods across the life course to determine the state of the literature on the role of environmental EDCs as a mediator of racial/ethnic differences in women’s reproductive health outcomes. We presented our argument by first describing racial/ethnic disparities in EDC exposure for these selected chemicals in the U.S. Next, in each section we highlighted disparities in specific women’s reproductive health outcomes. We then evaluated the literature on EDCs and adverse women’s reproductive health outcomes to determine whether the research warrants further evaluation for assessing these chemicals as a partial explanation for racial/ethnic disparities in these health outcomes.
While most studies found no association or inverse associations between the selected EDCs and pubertal outcomes, few studies evaluated the higher exposure/higher risk groups separately to determine if associations may have differed. Studies evaluating fibroids, infertility, and pregnancy complications were similar, in that they either adjusted for race/ethnicity or evaluated predominantly white populations. Several studies were cross-sectional, which limited the ability to evaluate EDCs as potential mediators of the racial/ethnic disparities in women’s reproductive health outcomes due to temporality issues. Finally, certain reproductive time periods were better studied than others. For example, only one cross-sectional study evaluated EDCs and menopause-related outcomes [113]. Therefore, we suggest the following to fill important gaps in epidemiologic research related to racial/ethnic health disparities in women’s reproductive health outcomes:
Evaluation of environmental chemical exposures and women’s health outcomes in more diverse study populations
Examination of individual and contextual determinants of racial/ethnic disparities in EDC exposures
Assessment of whether differences in EDC exposures contribute to disparities in women’s reproductive health outcomes through mediation and stratified analyses
Examination of relatively understudied EDCs that are racially/ethnically patterned, such as certain parabens and metals that are able to affect the endocrine system, such as cadmium.
Increased research in less-studied racial/ethnic minority groups, such as Asian subgroups, as well as more research in areas of women’s reproductive health, including certain pregnancy complications and menopausal outcomes
Several studies have taken initial steps toward evaluating racial/ethnic differences in exposure as a possible explanation for racial/ethnic disparities in outcomes. These studies have prospectively addressed research questions in more diverse study populations, which could facilitate future studies that look at EDCs as a potential contributor to racial/ethnic disparities in outcomes. These studies have also reported out racial/ethnic differences in exposures and/or outcomes, which allow for future work to build on these established findings. Specifically, Wolff [75•] and Windham [73•] provided detailed information about racial/ethnic differences in pubertal outcomes in a racially/ethnically diverse study population. Ferguson [103•] and Werner [33•] evaluated two different racially/ethnically disparate pregnancy outcomes in two racially/ethnically diverse pregnancy cohorts. In the future, these studies may provide needed insight into racial/ethnic disparities in puberty, preterm birth, and pregnancy hypertension, as they relate to EDC exposures.
Conclusion
In 2010, a call was made by the Institute of Medicine’s Committee on Women’s Health Research to identify and better understand the physical and social environmental determinants of women’s health disparities [114]. Data suggest that certain groups have higher exposure to the discussed chemicals across the life course, with implications for a higher incidence for adverse conditions relative to more advantaged groups (Figure 1). Furthermore, chronic and cumulative exposure to these EDCs coupled with a lack of protective factors could lead to cumulative wear and tear thereby increasing disparities in adverse health outcomes across the life course for these high-exposure/high-risk groups.
While the literature is still in its infancy for EDC—women’s reproductive health outcomes, the current research suggests that certain EDCs are associated with a number of adverse women’s reproductive health outcomes. By evaluating EDCs’ contribution to racial/ethnic health disparities and identifying modifiable sources of EDC exposure in these vulnerable populations, this work could provide opportunities for prevention and reduction in racial/ethnic health disparities.
Acknowledgments
We would like to thank Carol Mita, the Reference & Education Services Librarian at Harvard Medical School’s Countway Library of Medicine.
Sources of Financial Support: T.J-T. was supported by the National Institute of Environmental Health Sciences (R01ES026166). A.Z. was supported by the National Institute of Environmental Health Sciences (R00ES019881). Y.-H.C. was supported by the Irene M. and Fredrick J. Stare Nutrition Education Fund.
Footnotes
Disclosures: The authors have no competing financial interests.
References
- 1.Dehlendorf C, Bryant AS, Huddleston HG, Jacoby VL, Fujimoto VY. Health disparities: definitions and measurements. Am J Obstet Gynecol. 2010;202(3):212–3. doi: 10.1016/j.ajog.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Williams DR, Mohammed SA, Leavell J, Collins C. Race, socioeconomic status, and health: complexities, ongoing challenges, and research opportunities. Ann N Y Acad Sci. 2010;1186:69–101. doi: 10.1111/j.1749-6632.2009.05339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williams DR. Race and health: basic questions, emerging directions. Ann Epidemiol. 1997;7(5):322–33. doi: 10.1016/s1047-2797(97)00051-3. [DOI] [PubMed] [Google Scholar]
- 4.Braveman P, Egerter S, Williams DR. The social determinants of health: coming of age. Annu Rev Public Health. 2011;32:381–98. doi: 10.1146/annurev-publhealth-031210-101218. [DOI] [PubMed] [Google Scholar]
- 5.Lu MC, Halfon N. Racial and ethnic disparities in birth outcomes: a life-course perspective. Matern Child Health J. 2003;7(1):13–30. doi: 10.1023/a:1022537516969. [DOI] [PubMed] [Google Scholar]
- 6.Bryant AS, Worjoloh A, Caughey AB, Washington AE. Racial/ethnic disparities in obstetric outcomes and care: prevalence and determinants. Am J Obstet Gynecol. 2010;202(4):335–43. doi: 10.1016/j.ajog.2009.10.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dominguez TP. Race, racism, and racial disparities in adverse birth outcomes. Clin Obstet Gynecol. 2008;51(2):360–70. doi: 10.1097/GRF.0b013e31816f28de. [DOI] [PubMed] [Google Scholar]
- 8.Lin SS, Kelsey JL. Use of race and ethnicity in epidemiologic research: concepts, methodological issues, and suggestions for research. Epidemiol Rev. 2000;22(2):187–202. doi: 10.1093/oxfordjournals.epirev.a018032. [DOI] [PubMed] [Google Scholar]
- 9.Duty SM, Ackerman RM, Calafat AM, Hauser R. Personal care product use predicts urinary concentrations of some phthalate monoesters. Environ Health Perspect. 2005;113(11):1530–5. doi: 10.1289/ehp.8083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.James-Todd T, Senie R, Terry MB. Racial/ethnic differences in hormonally-active hair product use: a plausible risk factor for health disparities. J Immigr Minor Health. 2012;14(3):506–11. doi: 10.1007/s10903-011-9482-5. [DOI] [PubMed] [Google Scholar]
- 11.James-Todd T, Terry MB, Rich-Edwards J, Deierlein A, Senie R. Childhood hair product use and earlier age at menarche in a racially diverse study population: a pilot study. Ann Epidemiol. 2011;21(6):461–5. doi: 10.1016/j.annepidem.2011.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Branch F, Woodruff TJ, Mitro SD, Zota AR. Vaginal douching and racial/ethnic disparities in phthalates exposures among reproductive-aged women: National Health and Nutrition Examination Survey 2001–2004. Environmental health : a global access science source. 2015;14:57. doi: 10.1186/s12940-015-0043-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Block JP, Scribner RA, DeSalvo KB. Fast food, race/ethnicity, and income: a geographic analysis. Am J Prev Med. 2004;27(3):211–7. doi: 10.1016/j.amepre.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 14.Pereira MA, Kartashov AI, Ebbeling CB, Van Horn L, Slattery ML, Jacobs DR, Jr, et al. Fast-food habits, weight gain, and insulin resistance (the CARDIA study): 15-year prospective analysis. Lancet. 2005;365(9453):36–42. doi: 10.1016/S0140-6736(04)17663-0. [DOI] [PubMed] [Google Scholar]
- 15.Nelson MC, Gordon-Larsen P, Song Y, Popkin BM. Built and social environments associations with adolescent overweight and activity. Am J Prev Med. 2006;31(2):109–17. doi: 10.1016/j.amepre.2006.03.026. [DOI] [PubMed] [Google Scholar]
- 16.Ye X, Wong LY, Zhou X, Calafat AM. Urinary concentrations of 2,4-dichlorophenol and 2,5-dichlorophenol in the U.S. population (National Health and Nutrition Examination Survey, 2003–2010): trends and predictors. Environ Health Perspect. 2014;122(4):351–5. doi: 10.1289/ehp.1306816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sjodin A, Wong LY, Jones RS, Park A, Zhang Y, Hodge C, et al. Serum concentrations of polybrominated diphenyl ethers (PBDEs) and polybrominated biphenyl (PBB) in the United States population: 2003–2004. Environmental science & technology. 2008;42(4):1377–84. doi: 10.1021/es702451p. [DOI] [PubMed] [Google Scholar]
- 18.Hicken MT, Gee GC, Morenoff J, Connell CM, Snow RC, Hu H. A novel look at racial health disparities: the interaction between social disadvantage and environmental health. Am J Public Health. 2012;102(12):2344–51. doi: 10.2105/AJPH.2012.300774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gee GC, Payne-Sturges DC. Environmental health disparities: a framework integrating psychosocial and environmental concepts. Environ Health Perspect. 2004;112(17):1645–53. doi: 10.1289/ehp.7074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Institute of Environmental Health Sciences. 2012–2017 Strategic Plan: Advancing Science, Improving Health: A Plan for Environmental Health Research. U.S. Department of Health and Human Services, National Institutes of Health; (NIH No. 12-7935). http://www.niehs.nih.gov/about/strategicplan/strategicplan2012_508.pdf. Accessed February 4, 2016 2016. [Google Scholar]
- 21.US EPA (Environmental Protection Agency) Phthalates Action Plan (Revised) 2012. [Google Scholar]
- 22.Kelley KE, Hernandez-Diaz S, Chaplin EL, Hauser R, Mitchell AA. Identification of phthalates in medications and dietary supplement formulations in the United States and Canada. Environ Health Perspect. 2012;120(3):379–84. doi: 10.1289/ehp.1103998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koniecki D, Wang R, Moody RP, Zhu J. Phthalates in cosmetic and personal care products: concentrations and possible dermal exposure. Environ Res. 2011;111(3):329–36. doi: 10.1016/j.envres.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 24.Stringer R, Labunska I, Santillo D, Johnston P, Siddorn J, Stephenson A. Concentrations of phthalate esters and identification of other additives in PVC children’s toys. Environ Sci Pollut Res Int. 2000;7(1):27–36. doi: 10.1065/espr199910.007. [DOI] [PubMed] [Google Scholar]
- 25.Schecter A, Lorber M, Guo Y, Wu Q, Yun SH, Kannan K, et al. Phthalate concentrations and dietary exposure from food purchased in New York State. Environ Health Perspect. 2013;121(4):473–94. doi: 10.1289/ehp.1206367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zota AR, Calafat AM, Woodruff TJ. Temporal trends in phthalate exposures: findings from the National Health and Nutrition Examination Survey, 2001–2010. Environ Health Perspect. 2014;122(3):235–41. doi: 10.1289/ehp.1306681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kobrosly RW, Parlett LE, Stahlhut RW, Barrett ES, Swan SH. Socioeconomic factors and phthalate metabolite concentrations among United States women of reproductive age. Environ Res. 2012;115:11–7. doi: 10.1016/j.envres.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 28.Weuve J, Hauser R, Calafat AM, Missmer SA, Wise LA. Association of exposure to phthalates with endometriosis and uterine leiomyomata: findings from NHANES, 1999–2004. Environ Health Perspect. 2010;118(6):825–32. doi: 10.1289/ehp.0901543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.James-Todd TM, Meeker JD, Huang T, Hauser R, Seely EW, Ferguson KK, et al. Racial and ethnic variations in phthalate metabolite concentration changes across full-term pregnancies. J Expo Sci Environ Epidemiol. 2016 doi: 10.1038/jes.2016.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Serrano SE, Karr CJ, Seixas NS, Nguyen RH, Barrett ES, Janssen S, et al. Dietary phthalate exposure in pregnant women and the impact of consumer practices. Int J Environ Res Public Health. 2014;11(6):6193–215. doi: 10.3390/ijerph110606193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wolff MS, Teitelbaum SL, Pinney SM, Windham G, Liao L, Biro F, et al. Investigation of relationships between urinary biomarkers of phytoestrogens, phthalates, and phenols and pubertal stages in girls. Environmental Health Perspectives. 2010;118(7):1039–46. doi: 10.1289/ehp.0901690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wolff MS, Teitelbaum SL, McGovern K, Windham GC, Pinney SM, Galvez M, et al. Phthalate exposure and pubertal development in a longitudinal study of US girls. Human reproduction (Oxford, England) 2014;29(7):1558–66. doi: 10.1093/humrep/deu081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33•.Werner EF, Braun JM, Yolton K, Khoury JC, Lanphear BP. The association between maternal urinary phthalate concentrations and blood pressure in pregnancy: The HOME Study. Environmental health : a global access science source. 2015;14:75. doi: 10.1186/s12940-015-0062-3. This study is among the first to publish on urinary phthalate metabolites and blood pressure in pregnancy, with implications for pregnancy-induced hypertension. With a large proportion of the population being non-white, this study provides an opportunity to further explore racial/ethnic differences in EDC exposures and their contribution to disparities in blood pressure-related outcomes in pregnancy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meeker JD, Sathyanarayana S, Swan SH. Phthalates and other additives in plastics: human exposure and associated health outcomes. Philos Trans R Soc B-Biol Sci. 2009;364(1526):2097–113. doi: 10.1098/rstb.2008.0268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meeker JD, Cantonwine DE, Rivera-Gonzalez LO, Ferguson KK, Mukherjee B, Calafat AM, et al. Distribution, variability, and predictors of urinary concentrations of phenols and parabens among pregnant women in Puerto Rico. Environmental science & technology. 2013;47(7):3439–47. doi: 10.1021/es400510g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Larsson K, Bjorklund KL, Palm B, Wennberg M, Kaj L, Lindh CH, et al. Exposure determinants of phthalates, parabens, bisphenol A and triclosan in Swedish mothers and their children. Environ Int. 2014;73:323–33. doi: 10.1016/j.envint.2014.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Calafat AM, Ye X, Wong LY, Reidy JA, Needham LL. Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003–2004. Environ Health Perspect. 2008;116(1):39–44. doi: 10.1289/ehp.10753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mortensen ME, Calafat AM, Ye X, Wong LY, Wright DJ, Pirkle JL, et al. Urinary concentrations of environmental phenols in pregnant women in a pilot study of the National Children’s Study. Environ Res. 2014;129:32–8. doi: 10.1016/j.envres.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Philippat C, Wolff MS, Calafat AM, Ye X, Bausell R, Meadows M, et al. Prenatal exposure to environmental phenols: concentrations in amniotic fluid and variability in urinary concentrations during pregnancy. Environ Health Perspect. 2013;121(10):1225–31. doi: 10.1289/ehp.1206335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Braun JM, Kalkbrenner AE, Calafat AM, Bernert JT, Ye X, Silva MJ, et al. Variability and predictors of urinary bisphenol A concentrations during pregnancy. Environ Health Perspect. 2011;119(1):131–7. doi: 10.1289/ehp.1002366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McGuinn LA, Ghazarian AA, Joseph Su L, Ellison GL. Urinary bisphenol A and age at menarche among adolescent girls: evidence from NHANES 2003–2010. Environ Res. 2015;136:381–6. doi: 10.1016/j.envres.2014.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Unal ER, Lynn T, Neidich J, Salazar D, Goetzl L, Baatz JE, et al. Racial disparity in maternal and fetal-cord bisphenol A concentrations. Journal of perinatology : official journal of the California Perinatal Association. 2012;32(11):844–50. doi: 10.1038/jp.2012.12. [DOI] [PubMed] [Google Scholar]
- 43.Braun JM, Lanphear BP, Calafat AM, Deria S, Khoury J, Howe CJ, et al. Early-life bisphenol a exposure and child body mass index: a prospective cohort study. Environ Health Perspect. 2014;122(11):1239–45. doi: 10.1289/ehp.1408258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Veiga-Lopez A, Kannan K, Liao C, Ye W, Domino SE, Padmanabhan V. Gender-specific effects on gestational length and birth weight by early pregnancy BPA exposure. 2015:E1394–E403. doi: 10.1210/jc.2015-1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bloom MS, Kim D, Vom Saal FS, Taylor JA, Cheng G, Lamb JD, et al. Bisphenol A exposure reduces the estradiol response to gonadotropin stimulation during in vitro fertilization. Fertility and sterility. 2011;96(3):672–7 e2. doi: 10.1016/j.fertnstert.2011.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Robledo C, Peck JD, Stoner JA, Carabin H, Cowan L, Koch HM, et al. Is bisphenol-A exposure during pregnancy associated with blood glucose levels or diagnosis of gestational diabetes? Journal of Toxicology and Environmental Health - Part A: Current Issues. 2013;76(14):865–73. doi: 10.1080/15287394.2013.824395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Calafat AM, Ye X, Wong LY, Bishop AM, Needham LL. Urinary concentrations of four parabens in the U.S. population: NHANES 2005–2006. Environ Health Perspect. 2010;118(5):679–85. doi: 10.1289/ehp.0901560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Buttke DE, Sircar K, Martin C. Exposures to endocrine-disrupting chemicals and age of menarche in adolescent girls in NHANES (2003–2008) Environ Health Perspect. 2012;120(11):1613–8. doi: 10.1289/ehp.1104748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ranjit N, Siefert K, Padmanabhan V. Bisphenol-A and disparities in birth outcomes: a review and directions for future research. Journal of perinatology : official journal of the California Perinatal Association. 2010;30(1):2–9. doi: 10.1038/jp.2009.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zota AR, Linderholm L, Park JS, Petreas M, Guo T, Privalsky ML, et al. Temporal comparison of PBDEs, OH-PBDEs, PCBs, and OH-PCBs in the serum of second trimester pregnant women recruited from San Francisco General Hospital, California. Environmental science & technology. 2013;47(20):11776–84. doi: 10.1021/es402204y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen A, Chung E, DeFranco EA, Pinney SM, Dietrich KN. Serum PBDEs and age at menarche in adolescent girls: Analysis of the National Health and Nutrition Examination Survey 2003–2004. Environmental Research. 2011;111(6):831–7. doi: 10.1016/j.envres.2011.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Windham GC, Pinney SM, Sjodin A, Lum R, Jones RS, Needham LL, et al. Body burdens of brominated flame retardants and other persistent organo-halogenated compounds and their descriptors in US girls. Environ Res. 2010;110(3):251–7. doi: 10.1016/j.envres.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vuong AM, Webster GM, Romano ME, Braun JM, Zoeller RT, Hoofnagle AN, et al. Maternal Polybrominated Diphenyl Ether (PBDE) Exposure and Thyroid Hormones in Maternal and Cord Sera: The HOME Study, Cincinnati, USA. Environ Health Perspect. 2015;123(10):1079–85. doi: 10.1289/ehp.1408996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu R, Nelson DO, Hurley S, Petreas MX, Park J, Wang Y, et al. Association between Serum Polybrominated Diphenylether Levels and Residential Proximity to Solid Waste Facilities. Environmental science & technology. 2016 doi: 10.1021/acs.est.5b04715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Whitehead TP, Crispo Smith S, Park JS, Petreas MX, Rappaport SM, Metayer C. Concentrations of Persistent Organic Pollutants in California Children’s Whole Blood and Residential Dust. Environmental science & technology. 2015;49(15):9331–40. doi: 10.1021/acs.est.5b02078. [DOI] [PubMed] [Google Scholar]
- 56.Zota AR, Adamkiewicz G, Morello-Frosch RA. Are PBDEs an environmental equity concern? Exposure disparities by socioeconomic status. Environmental science & technology. 2010;44(15):5691–2. doi: 10.1021/es101723d. [DOI] [PubMed] [Google Scholar]
- 57.Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, Hauser R, Prins GS, Soto AM, et al. Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocr Rev. 2009;30(4):293–342. doi: 10.1210/er.2009-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Phthalates and Cumulative Risk Assessment: The Tasks Ahead. Washington (DC): 2008. [PubMed] [Google Scholar]
- 59.Gomez E, Pillon A, Fenet H, Rosain D, Duchesne MJ, Nicolas JC, et al. Estrogenic activity of cosmetic components in reporter cell lines: parabens, UV screens, and musks. J Toxicol Environ Health A. 2005;68(4):239–51. doi: 10.1080/15287390590895054. [DOI] [PubMed] [Google Scholar]
- 60.Okubo T, Yokoyama Y, Kano K, Kano I. ER-dependent estrogenic activity of parabens assessed by proliferation of human breast cancer MCF-7 cells and expression of ERalpha and PR. Food Chem Toxicol. 2001;39(12):1225–32. doi: 10.1016/s0278-6915(01)00073-4. [DOI] [PubMed] [Google Scholar]
- 61.Howdeshell KL, Wilson VS, Furr J, Lambright CR, Rider CV, Blystone CR, et al. A mixture of five phthalate esters inhibits fetal testicular testosterone production in the sprague-dawley rat in a cumulative, dose-additive manner. Toxicol Sci. 2008;105(1):153–65. doi: 10.1093/toxsci/kfn077. [DOI] [PubMed] [Google Scholar]
- 62.Peretz J, Vrooman L, Ricke WA, Hunt PA, Ehrlich S, Hauser R, et al. Bisphenol a and reproductive health: update of experimental and human evidence, 2007–2013. Environ Health Perspect. 2014;122(8):775–86. doi: 10.1289/ehp.1307728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Miller MD, Crofton KM, Rice DC, Zoeller RT. Thyroid-disrupting chemicals: interpreting upstream biomarkers of adverse outcomes. Environ Health Perspect. 2009;117(7):1033–41. doi: 10.1289/ehp.0800247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Meerts IA, van Zanden JJ, Luijks EA, van Leeuwen-Bol I, Marsh G, Jakobsson E, et al. Potent competitive interactions of some brominated flame retardants and related compounds with human transthyretin in vitro. Toxicol Sci. 2000;56(1):95–104. doi: 10.1093/toxsci/56.1.95. [DOI] [PubMed] [Google Scholar]
- 65.Stoker TE, Cooper RL, Lambright CS, Wilson VS, Furr J, Gray LE. In vivo and in vitro anti-androgenic effects of DE-71, a commercial polybrominated diphenyl ether (PBDE) mixture. Toxicol Appl Pharmacol. 2005;207(1):78–88. doi: 10.1016/j.taap.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 66.Hamers T, Kamstra JH, Sonneveld E, Murk AJ, Kester MH, Andersson PL, et al. In vitro profiling of the endocrine-disrupting potency of brominated flame retardants. Toxicol Sci. 2006;92(1):157–73. doi: 10.1093/toxsci/kfj187. [DOI] [PubMed] [Google Scholar]
- 67.Hou L, Wang D, Baccarelli A. Environmental chemicals and microRNAs. Mutat Res. 2011;714(1–2):105–12. doi: 10.1016/j.mrfmmm.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.LaRocca J, Binder AM, McElrath TF, Michels KB. First-Trimester Urine Concentrations of Phthalate Metabolites and Phenols and Placenta miRNA Expression in a Cohort of U.S. Women. Environ Health Perspect. 2016;124(3):380–7. doi: 10.1289/ehp.1408409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ferguson KK, McElrath TF, Chen YH, Mukherjee B, Meeker JD. Urinary phthalate metabolites and biomarkers of oxidative stress in pregnant women: a repeated measures analysis. Environ Health Perspect. 2015;123(3):210–6. doi: 10.1289/ehp.1307996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Euling SY, Herman-Giddens ME, Lee PA, Selevan SG, Juul A, Sorensen TI, et al. Examination of US puberty-timing data from 1940 to 1994 for secular trends: panel findings. Pediatrics. 2008;121(Suppl 3):S172–91. doi: 10.1542/peds.2007-1813D. [DOI] [PubMed] [Google Scholar]
- 71.Lomenick JP, Calafat AM, Melguizo Castro MS, Mier R, Stenger P, Foster MB, et al. Phthalate exposure and precocious puberty in females. The Journal of pediatrics. 2010;156(2):221–5. doi: 10.1016/j.jpeds.2009.09.047. [DOI] [PubMed] [Google Scholar]
- 72.Rais-Bahrami K, Nunez S, Revenis ME, Luban NL, Short BL. Follow-up study of adolescents exposed to di(2-ethylhexyl) phthalate (DEHP) as neonates on extracorporeal membrane oxygenation (ECMO) support. Environ Health Perspect. 2004;112(13):1339–40. doi: 10.1289/ehp.6901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73•.Windham GC, Pinney SM, Voss RW, Sjodin A, Biro FM, Greenspan LC, et al. Brominated Flame Retardants and Other Persistent Organohalogenated Compounds in Relation to Timing of Puberty in a Longitudinal Study of Girls. Environ Health Perspect. 2015;123(10):1046–52. doi: 10.1289/ehp.1408778. This prospective study was important to our review, as it was conducted in a large, multi-racial/ethnic study population, noting racial/ethnic differences in exposure patterns. This diverse study population coupled with assessment of exposures and outcomes that are racially/ethnically patterned enable further assessment of racial/ethnic disparities in this area of research. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wolff MS, Britton JA, Boguski L, Hochman S, Maloney N, Serra N, et al. Environmental exposures and puberty in inner-city girls. Environmental Research. 2008;107(3):393–400. doi: 10.1016/j.envres.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75•.Wolff MS, Teitelbaum SL, McGovern K, Pinney SM, Windham GC, Galvez M, et al. Environmental phenols and pubertal development in girls. Environ Int. 2015;84:174–80. doi: 10.1016/j.envint.2015.08.008. This prospective study was designed to have a racially/ethnically diverse study population that allows for assessment of multiple pubertal outcomes. As one of the more recent studies published from this cohort, it provides important information about racial/ethnic differences in the exposure and outcome, which is critical for future analyses evaluating EDCs’ contribution to racial/ethnic disparities in these outcomes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wolff MS, Teitelbaum SL, Pinney SM, Windham G, Liao L, Biro F, et al. Investigation of relationships between urinary biomarkers of phytoestrogens, phthalates, and phenols and pubertal stages in girls. Environ Health Perspect. 2010;118(7):1039–46. doi: 10.1289/ehp.0901690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jacoby VL, Fujimoto VY, Giudice LC, Kuppermann M, Washington AE. Racial and ethnic disparities in benign gynecologic conditions and associated surgeries. Am J Obstet Gynecol. 2010;202(6):514–21. doi: 10.1016/j.ajog.2010.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Baird DD, Dunson DB, Hill MC, Cousins D, Schectman JM. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188(1):100–7. doi: 10.1067/mob.2003.99. [DOI] [PubMed] [Google Scholar]
- 79.Kjerulff KH, Langenberg P, Seidman JD, Stolley PD, Guzinski GM. Uterine leiomyomas. Racial differences in severity, symptoms and age at diagnosis. J Reprod Med. 1996;41(7):483–90. [PubMed] [Google Scholar]
- 80.Pollack AZ, Buck Louis GM, Chen Z, Sun L, Trabert B, Guo Y, et al. Bisphenol A, benzophenone-type ultraviolet filters, and phthalates in relation to uterine leiomyoma. Environ Res. 2015;137:101–7. doi: 10.1016/j.envres.2014.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Thoma ME, McLain AC, Louis JF, King RB, Trumble AC, Sundaram R, et al. Prevalence of infertility in the United States as estimated by the current duration approach and a traditional constructed approach. Fertility and sterility. 2013;99(5):1324–31 e1. doi: 10.1016/j.fertnstert.2012.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chandra A, Copen CE, Stephen EH. Infertility and impaired fecundity in the United States, 1982–2010: data from the National Survey of Family Growth. Natl Health Stat Report. 2013;(67):1–18. 1 p following 9. [PubMed] [Google Scholar]
- 83.Seifer DB, Frazier LM, Grainger DA. Disparity in assisted reproductive technologies outcomes in black women compared with white women. Fertility and sterility. 2008;90(5):1701–10. doi: 10.1016/j.fertnstert.2007.08.024. [DOI] [PubMed] [Google Scholar]
- 84.McCarthy-Keith DM, Schisterman EF, Robinson RD, O’Leary K, Lucidi RS, Armstrong AY. Will decreasing assisted reproduction technology costs improve utilization and outcomes among minority women? Fertility and sterility. 2010;94(7):2587–9. doi: 10.1016/j.fertnstert.2010.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Alur S, Wang H, Hoeger K, Swan SH, Sathyanarayana S, Redmon BJ, et al. Urinary phthalate metabolite concentrations in relation to history of infertility and use of assisted reproductive technology. Fertility and sterility. 2015;104(5):1227–35. doi: 10.1016/j.fertnstert.2015.07.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Buck Louis GM, Sundaram R, Sweeney AM, Schisterman EF, Maisog J, Kannan K. Urinary bisphenol A, phthalates, and couple fecundity: the Longitudinal Investigation of Fertility and the Environment (LIFE) Study. Fertility and sterility. 2014;101(5):1359–66. doi: 10.1016/j.fertnstert.2014.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hauser R, Gaskins AJ, Souter I, Smith KW, Dodge LE, Ehrlich S, et al. Urinary Phthalate Metabolite Concentrations and Reproductive Outcomes among Women Undergoing Fertilization: Results from the EARTH Study. Environ Health Perspect. 2015 doi: 10.1289/ehp.1509760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jukic AM, Calafat AM, McConnaughey DR, Longnecker MP, Hoppin JA, Weinberg CR, et al. Urinary Concentrations of Phthalate Metabolites and Bisphenol A and Associations with Follicular-Phase Length, Luteal-Phase Length, Fecundability, and Early Pregnancy Loss. Environ Health Perspect. 2015 doi: 10.1289/ehp.1408164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Messerlian C, Souter I, Gaskins AJ, Williams PL, Ford JB, Chiu YH, et al. Urinary phthalate metabolites and ovarian reserve among women seeking infertility care. Human reproduction (Oxford, England) 2015 doi: 10.1093/humrep/dev292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ehrlich S, Williams PL, Missmer SA, Flaws JA, Berry KF, Calafat AM, et al. Urinary bisphenol A concentrations and implantation failure among women undergoing in vitro fertilization. Environ Health Perspect. 2012;120(7):978–83. doi: 10.1289/ehp.1104307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fujimoto VY, Kim D, vom Saal FS, Lamb JD, Taylor JA, Bloom MS. Serum unconjugated bisphenol A concentrations in women may adversely influence oocyte quality during in vitro fertilization. Fertility and sterility. 2011;95(5):1816–9. doi: 10.1016/j.fertnstert.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 92.Lathi RB, Liebert CA, Brookfield KF, Taylor JA, vom Saal FS, Fujimoto VY, et al. Conjugated bisphenol A in maternal serum in relation to miscarriage risk. Fertility and sterility. 2014;102(1):123–8. doi: 10.1016/j.fertnstert.2014.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Minguez-Alarcon L, Gaskins AJ, Chiu YH, Williams PL, Ehrlich S, Chavarro JE, et al. Urinary bisphenol A concentrations and association with in vitro fertilization outcomes among women from a fertility clinic. Human reproduction (Oxford, England) 2015;30(9):2120–8. doi: 10.1093/humrep/dev183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mok-Lin E, Ehrlich S, Williams PL, Petrozza J, Wright DL, Calafat AM, et al. Urinary bisphenol A concentrations and ovarian response among women undergoing IVF. International journal of andrology. 2010;33(2):385–93. doi: 10.1111/j.1365-2605.2009.01014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Souter I, Smith KW, Dimitriadis I, Ehrlich S, Williams PL, Calafat AM, et al. The association of bisphenol-A urinary concentrations with antral follicle counts and other measures of ovarian reserve in women undergoing infertility treatments. Reproductive toxicology (Elmsford, NY) 2013;42:224–31. doi: 10.1016/j.reprotox.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Smith KW, Souter I, Dimitriadis I, Ehrlich S, Williams PL, Calafat AM, et al. Urinary paraben concentrations and ovarian aging among women from a fertility center. Environ Health Perspect. 2013;121(11–12):1299–305. doi: 10.1289/ehp.1205350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Minguez-Alarcon L, Chiu YH, Messerlian C, Williams PL, Sabatini ME, Toth TL, et al. Urinary paraben concentrations and in vitro fertilization outcomes among women from a fertility clinic. Fertility and sterility. 2015 doi: 10.1016/j.fertnstert.2015.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Buck Louis GM, Sundaram R, Schisterman EF, Sweeney AM, Lynch CD, Gore-Langton RE, et al. Persistent environmental pollutants and couple fecundity: the LIFE study. Environ Health Perspect. 2013;121(2):231–6. doi: 10.1289/ehp.1205301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Harley KG, Marks AR, Chevrier J, Bradman A, Sjodin A, Eskenazi B. PBDE concentrations in women’s serum and fecundability. Environ Health Perspect. 2010;118(5):699–704. doi: 10.1289/ehp.0901450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Johnson PI, Altshul L, Cramer DW, Missmer SA, Hauser R, Meeker JD. Serum and follicular fluid concentrations of polybrominated diphenyl ethers and in-vitro fertilization outcome. Environ Int. 2012;45:9–14. doi: 10.1016/j.envint.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Adibi JJ, Hauser R, Williams PL, Whyatt RM, Calafat AM, Nelson H, et al. Maternal urinary metabolites of Di-(2-ethylhexyl) phthalate in relation to the timing of labor in a US multicenter pregnancy cohort study. American Journal of Epidemiology. 2009;169(8):1015–24. doi: 10.1093/aje/kwp001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ferguson KK, McElrath TF, Mukherjee B, Loch-Caruso R, Meeker JD. Associations between Maternal Biomarkers of Phthalate Exposure and Inflammation Using Repeated Measurements across Pregnancy. PloS one. 2015;10(8):e0135601. doi: 10.1371/journal.pone.0135601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103•.Ferguson KK, McElrath TF, Meeker JD. Environmental phthalate exposure and preterm birth. JAMA pediatrics. 2014;168(1):61–7. doi: 10.1001/jamapediatrics.2013.3699. Several studies have been published from this racially/ethnically diverse study population, evaluating an important pregnancy outcome that is racially/ethnically disparate—preterm birth. The study found certain phthalate metabolites to be associated with a higher risk of preterm birth, which could be further explored to determine whether exposure to certain phthalates contributes to racial/ethnic differences in this outcome. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cantonwine DE, Ferguson KK, Mukherjee B, McElrath TF, Meeker JD. Urinary Bisphenol A Levels during Pregnancy and Risk of Preterm Birth. Environ Health Perspect. 2015;123(9):895–901. doi: 10.1289/ehp.1408126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Robledo CA, Peck JD, Stoner J, Calafat AM, Carabin H, Cowan L, et al. Urinary phthalate metabolite concentrations and blood glucose levels during pregnancy. International journal of hygiene and environmental health. 2015;218(3):324–30. doi: 10.1016/j.ijheh.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Smarr MM, Grantz KL, Sundaram R, Maisog JM, Kannan K, Louis GMB. Parental urinary biomarkers of preconception exposure to bisphenol A and phthalates in relation to birth outcomes. Environmental Health: A Global Access Science Source. 2015;14(1) doi: 10.1186/s12940-015-0060-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Whyatt RM, Adibi JJ, Calafat AM, Camann DE, Rauh V, Bhat HK, et al. Prenatal di(2-ethylhexyl)phthalate exposure and length of gestation among an inner-city cohort. Pediatrics. 2009;124(6):e1213–20. doi: 10.1542/peds.2009-0325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wolff MS, Engel SM, Berkowitz GS, Ye X, Silva MJ, Zhu C, et al. Prenatal phenol and phthalate exposures and birth outcomes. Environmental Health Perspectives. 2008;116(8):1092–7. doi: 10.1289/ehp.11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ferguson KK, McElrath TF, Cantonwine DE, Mukherjee B, Meeker JD. Phthalate metabolites and bisphenol-A in association with circulating angiogenic biomarkers across pregnancy. Placenta. 2015;36(6):699–703. doi: 10.1016/j.placenta.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Patel CJ, Yang T, Hu Z, Wen Q, Sung J, El-Sayed YY, et al. Investigation of maternal environmental exposures in association with self-reported preterm birth. Reproductive Toxicology. 2014;45:1–7. doi: 10.1016/j.reprotox.2013.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Peltier MR, Koo HC, Getahun D, Menon R. Does exposure to flame retardants increase the risk for preterm birth? Journal of reproductive immunology. 2015;107:20–5. doi: 10.1016/j.jri.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Smarr MM, Grantz KL, Zhang C, Sundaram R, Maisog JM, Barr DB, et al. Persistent organic pollutants and pregnancy complications. The Science of the total environment. 2016;551–552:285–91. doi: 10.1016/j.scitotenv.2016.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Grindler NM, Allsworth JE, Macones GA, Kannan K, Roehl KA, Cooper AR. Persistent organic pollutants and early menopause in U.S. women. PloS one. 2015;10(1):e0116057. doi: 10.1371/journal.pone.0116057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Committee on Women’s Health Research, Institute of Medicine of the National Academies. Women’s Health Research: Progress, Pitfalls, and Promise. Report Brief. 2010 [Google Scholar]
- 115.Ferguson KK, McElrath TF, Ko YA, Mukherjee B, Meeker JD. Variability in urinary phthalate metabolite levels across pregnancy and sensitive windows of exposure for the risk of preterm birth. Environ Int. 2014;70:118–24. doi: 10.1016/j.envint.2014.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]


