Abstract
Glyoxysome, endoplasmic reticulum, mitochondria, and proplastid fractions were isolated from endosperm of castor beans (Ricinus communis) germinated for 5 days at 30 C. Samples from sucrose density gradients were diluted with 0.15 m KCI and the membranes pelleted. Lipid extracts of these membranes were analyzed for phosphoglyceride, acyl lipid, and sterol content. The endoplasmic reticulum contains 1.24 μmol of phosphoglyceride per mg of protein; the mitochondria, 0.65 μmol/mg; and the glyoxysome membranes, 0.55 μmol/mg. Phosphatidyl choline and phosphatidyl ethanolamine are the most abundant lipids in all membranes studied, accounting for 70% or more of the lipid phosphorus and 50% or more of the fatty acid. Glyoxysome membranes and endoplasmic reticulum also contain phosphatidyl inositol (respectively, 9 and 17% of the lipid phosphorus) and free fatty acids (13% of the total fatty acid in each). Compared with other organelles, mitochondrial membranes have more phosphatidyl ethanolamine relative to phosphatidyl choline and are characterized by the presence of cardiolipin, in which 80% of the fatty acid is linoleate. The relative amounts of linoleate, palmitate, oleate, stearate, and linolenate in each of the phosphotoglycerides are constant regardless of the membrane source. Stimasgasterol and β-sitosterol are present in the membranes (1-9 nmol each/mg protein).
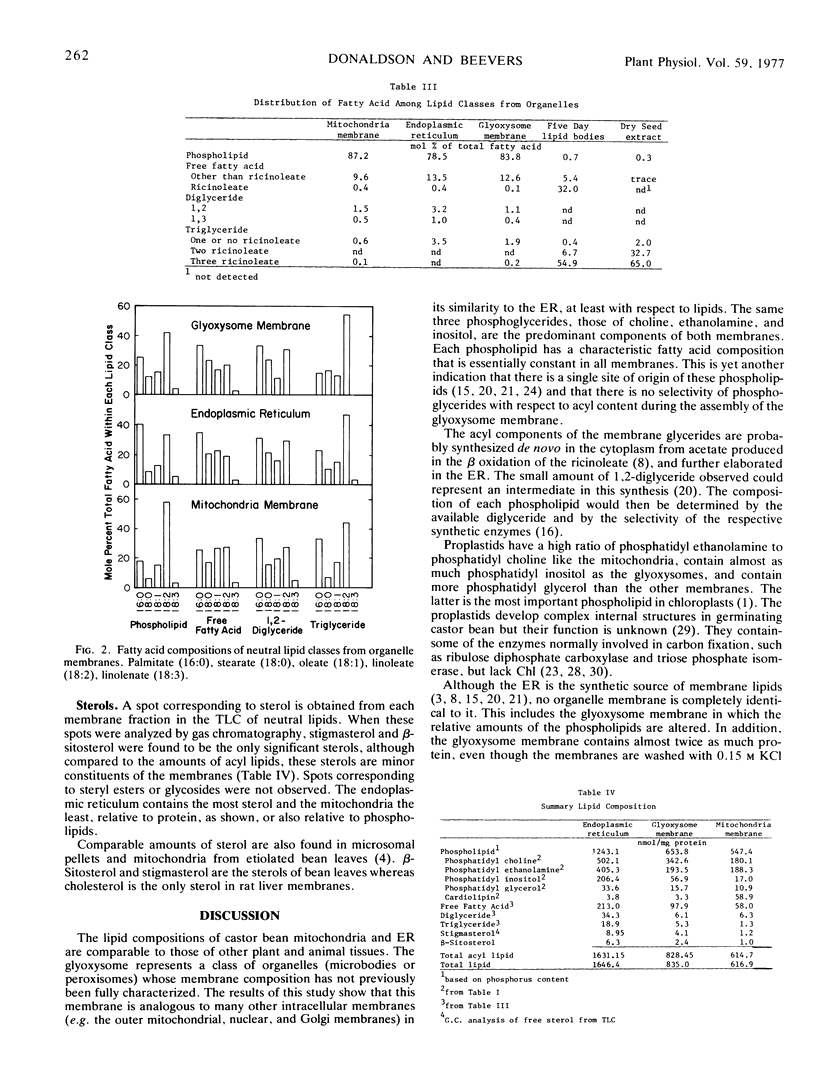
The data provide further evidence that glyoxysome membranes are derived from the endoplasmic reticulum but at the same time indicate some differentiation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brandt R. D., Benveniste P. Isolation and identification of sterols from subcellular fractions of bean leaves (Phaseolus vulgaris). Biochim Biophys Acta. 1972 Sep 1;282(1):85–92. doi: 10.1016/0005-2736(72)90313-6. [DOI] [PubMed] [Google Scholar]
- Colbeau A., Nachbaur J., Vignais P. M. Enzymic characterization and lipid composition of rat liver subcellular membranes. Biochim Biophys Acta. 1971 Dec 3;249(2):462–492. doi: 10.1016/0005-2736(71)90123-4. [DOI] [PubMed] [Google Scholar]
- Devor K. A., Mudd J. B. Structural analysis of phosphatidylcholine of plant tissue. J Lipid Res. 1971 Jul;12(4):396–402. [PubMed] [Google Scholar]
- Donaldson R. P. Membrane lipid metabolism in germinating castor bean endosperm. Plant Physiol. 1976 Apr;57(4):510–515. doi: 10.1104/pp.57.4.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson R. P., Tolbert N. E., Schnarrenberger C. A comparison of microbody membranes with microsomes and mitochondria from plant and animal tissue. Arch Biochem Biophys. 1972 Sep;152(1):199–215. doi: 10.1016/0003-9861(72)90208-1. [DOI] [PubMed] [Google Scholar]
- Gonzalez E., Beevers H. Role of the endoplasmic reticulum in glyoxysome formation in castor bean endosperm. Plant Physiol. 1976 Mar;57(3):406–409. doi: 10.1104/pp.57.3.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins J. A. Studies on the biogenesis of smooth endoplasmic reticulum membranes in hepatocytes of phenobarbital-treated rats. II. The site of phospholipid synthesis in the initial phase of membrane proliferation. J Cell Biol. 1974 Sep;62(3):635–646. doi: 10.1083/jcb.62.3.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A. H., Beevers H. Localization of enzymes within microbodies. J Cell Biol. 1973 Aug;58(2):379–389. doi: 10.1083/jcb.58.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kader J. C. Proteins and the intracellular exchange of lipids. I. Stimulation of phospholipid exchange between mitochondria and microsomal fractions by proteins isolated from potato tuber. Biochim Biophys Acta. 1975 Jan 24;380(1):31–44. [PubMed] [Google Scholar]
- Kagawa T., Lord J. M., Beevers H. The origin and turnover of organelle membranes in castor bean endosperm. Plant Physiol. 1973 Jan;51(1):61–65. doi: 10.1104/pp.51.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanoh H., Ohno K. Substrate-selectivity of rat liver microsomal 1,2-diacylglycerol: CDP-choline(ethanolamine) choline(ethanolamine)phosphotransferase in utilizing endogenous substrates. Biochim Biophys Acta. 1975 Feb 20;380(2):199–207. [PubMed] [Google Scholar]
- Lazarow P. B., de Duve C. The synthesis and turnover of rat liver peroxisomes. V. Intracellular pathway of catalase synthesis. J Cell Biol. 1973 Nov;59(2 Pt 1):507–524. doi: 10.1083/jcb.59.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord J. M., Kagawa T., Beevers H. Intracellular distribution of enzymes of the cytidine diphosphate choline pathway in castor bean endosperm. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2429–2432. doi: 10.1073/pnas.69.9.2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord J. M., Kagawa T., Moore T. S., Beevers H. Endoplasmic reticulum as the site of lecithin formation in castor bean endosperm. J Cell Biol. 1973 Jun;57(3):659–667. doi: 10.1083/jcb.57.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marriott K. M., Northcote D. H. The breakdown of lipid reserves in the endosperm of germinating castor beans. Biochem J. 1975 Apr;148(1):139–144. doi: 10.1042/bj1480139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miflin B. J., Beevers H. Isolation of intact plastids from a range of plant tissues. Plant Physiol. 1974 Jun;53(6):870–874. doi: 10.1104/pp.53.6.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore T. S., Lord J. M., Kagawa T., Beevers H. Enzymes of phospholipid metabolism in the endoplasmic reticulum of castor bean endosperm. Plant Physiol. 1973 Jul;52(1):50–53. doi: 10.1104/pp.52.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muto S., Beevers H. Lipase Activities in Castor Bean Endosperm during Germination. Plant Physiol. 1974 Jul;54(1):23–28. doi: 10.1104/pp.54.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osmond C. B., Akazawa T., Beevers H. Localization and properties of ribulose diphosphate carboxylase from castor bean endosperm. Plant Physiol. 1975 Feb;55(2):226–230. doi: 10.1104/pp.55.2.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philipp E. I., Franke W. W., Keenan T. W., Stadler J., Jarasch E. D. Characterization of nuclear membranes and endoplasmic reticulum isolated from plant tissue. J Cell Biol. 1976 Jan;68(1):11–29. doi: 10.1083/jcb.68.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigil E. L. Cytochemical and developmental changes in microbodies (glyoxysomes) and related organelles of castor bean endosperm. J Cell Biol. 1970 Sep;46(3):435–454. doi: 10.1083/jcb.46.3.435. [DOI] [PMC free article] [PubMed] [Google Scholar]


