Abstract
Actin filament turnover underpins several processes in the life cycle of the malaria parasite, Plasmodium falciparum. Polymerization and depolymerization are especially important for gliding motility, a substrate-dependent form of cell movement that underpins the protozoan parasite’s ability to disseminate and invade host cells. To date, given difficulties in extraction of native actins directly from parasites, much of our biochemical understanding of malarial actin has instead relied on recombinant protein extracted and purified from heterologous protein expression systems. Here, using in vitro transcription-translation methodologies and quantitative protein-binding assays, we explored the folding state of heterologously expressed P. falciparum actin 1 (PfACTI) with the aim of assessing the reliability of current recombinant-protein-based data. We demonstrate that PfACTI, when expressed in non-native systems, is capable of binding to and release from bacterial, yeast, and mammalian chaperonin complexes but appears to be incompletely folded. Characterization of the native Plasmodium folding machinery in silico, the chaperonin containing t-complex protein-1 complex, highlights key divergences between the different chaperonin systems that likely underpins this incomplete folded state. These results highlight the importance of characterizing actin’s folded state and raise concerns about the interpretation of actin polymerization kinetics based solely on protein derived from heterologous expression systems.—Olshina, M. A., Baumann, H., Willison, K. R., Baum, J. Plasmodium actin is incompletely folded by heterologous protein-folding machinery and likely requires the native Plasmodium chaperonin complex to enter a mature functional state.
Keywords: malaria, apicomplexa, gliding motility, in vitro translation, rabbit reticulocyte lysate
Apicomplexan parasites are an ancient phylum of obligate intracellular protozoa that includes some of the most significant pathogens affecting global human populations (1). The phylum includes Toxoplasma gondii, Cryptosporidium spp., and parasites from the genus Plasmodium, the causative agent of human malaria disease. Cell movement in these parasites, also referred to as gliding motility, underpins the ability of parasites to infect host tissues and invade host cells and is dependent on the linkage of an intracellular parasite myosin motor with dynamic filaments of actin (2). This actomyosin system provides a rearward propulsive force that, when linked to extracellular substrates, drive parasites forward or into host cells (2).
Plasmodium parasites express two actin isoforms, ACTI and ACTII, that are markedly divergent from their eukaryotic counterparts (3). ACTI is conserved between Plasmodium and other apicomplexan parasites (4) and is the isoform that is directly implicated in gliding motility (5, 6). However, despite the importance of ACTI in cell motility, actin filaments are not readily detected in parasites using various microscopy techniques (7, 8). This is explained by both the reported dominance of globular (G) over filamentous (F) actin in parasite cells (4, 9) and several unusual properties reported for ACTI filaments; namely, their propensity to form very short polymers, measuring only ∼100 nm in length and their highly dynamic, transient, and unstable nature (10, 11). In vivo visualization of native actin-rich structures is only possible following treatment of cells with an actin filament-stabilizing drug such as jasplakinolide (7, 12, 13).
With the exception of those studies using extracted PfACTI (10, 11), most work with ACTI has relied on its expression via heterologous systems (namely yeast and insect cells) to purify enough protein for assaying (10, 11, 14–17). Plasmodium falciparum ACT1 (PfACTI), or that from Toxoplasma gondii (TgACT1), expressed in these conditions only, forms long filaments when assayed in the presence of jasplakinolide or equimolar concentrations of phalloidin, another F-actin binding drug. This supports the observation that native apicomplexan actin forms unstable filaments, which precludes extended elongation (16, 18). Indeed, in support of this, when sites divergent to mammalian actin are mutated to more canonical states in TgACTI, this gives much greater filament stability to actin polymers in Toxoplasma parasites, permitting the formation of long structures that severely disrupt normal cell function (16).
Alongside Toxoplasma work, crystal structures of recombinant Plasmodium PfACTI suggests the instability of ACTI filaments is based on the divergence of several key amino acids, in particular the DNaseI binding-loop (D-loop) and the C terminus, which likely function in maintaining intrastrand contacts (18). In PfACTI, the D-loop is disordered and partially missing in the electron density maps, likely affecting the lateral contacts within the filament and therefore overall filament stability (18). When it is replaced with a canonical D-loop, this restores long filament formation in vitro. The same study revealed that the C terminus of PfACTI is also disordered and unstructured (18) (see Discussion). This latter result is striking given that correct placement of the C terminus is known to be crucial for native G-actin folding (19). Of note, in the PfACTI–D-loop chimera, the C terminus forms a more native order (18). The reappearance of order in this unrelated domain may question whether disorder is actually the native ACTI state or whether the lack of structure at the C terminus is in fact a product of folding in heterologous expression systems.
In eukaryotic cells, actin is folded into its mature, native form by the t-complex protein-1 (TCP-1) ring complex or chaperonin containing TCP-1 (CCT) complex, a 1-MDa protein complex comprised of 8 different subunits arranged into 2 asymmetric rings (20). Actin folding by the CCT complex is an ATP-dependent, multistage process regulated by cofactors from the phosducin-like protein family of proteins. The ability of the CCT complex to fold actin is isoform and species specific, with amino acid divergences leading to incompatibilities between certain actin isoforms and CCTs from different species (21). Given the demonstrated incompatibilities between CCT and heterologous actins, it is possible that the various apicomplexan ACTI expressed proteins may be arrested in intermediate folding states due to incompatibility with the host chaperonin complex.
Here, we sought to address this question using prokaryotic and eukaryotic in vitro transcription and translation systems coupled with native PAGE analysis. We demonstrate that PfACTI and human β-actin expressed in prokaryotic expression systems are not functional and cannot interact with a eukaryote CCT complex in the absence of other cofactors. Furthermore, we show that PfACTI expressed in a mammalian eukaryotic expression system, despite being able to bind and be released by CCT, is incompletely folded by heterologous chaperonin systems. To explore the molecular basis for this incompatibility, we identify the presence of an entity isolated from parasite cell lysate that, in being able to bind actin, is consistent with their being a native PfCCT complex. We back this up by identifying orthologous genes for the CCT complex in the P. falciparum genome. In silico analysis of these identified subunits highlights key divergent residues that likely underpin their incomplete protein folding by non-native CCT. These observations suggest that functional PfACTI likely requires homologous CCT to complete its native monomer fold.
MATERIALS AND METHODS
In vitro translation and analysis
In vitro translation
Human β-actin cDNA, in the pET-11d vector (22), and pET-28b-PfACTI were translated in vitro using the Expressway Cell-Free Escherichia coli Expression System (Life Technologies, Carlsbad, CA, USA) or the TNT Coupled Reticulocyte Lysate Systems (Promega, Madison, WI, USA) according to the manufacturers’ instructions. Both systems were coupled with the inclusion of [35S]methionine (>1000 Ci/mmol; PerkinElmer, Waltham, MA, USA). Aliquots were taken at appropriate time points and stored on ice until the conclusion of the time course. For the titration experiments, [35S]PfACTI, expressed in either the prokaryotic or eukaryotic lysate for 40 min, was added to P. falciparum cell extract at a volume ratio 1:10 (in vitro translation lysate: P. falciparum lysate) and incubated for another 15 min. The P. falciparum cell extract was prepared from saponin-lysed parasite pellets, lysed via bead-beating after resuspension in extract preparation buffer [45 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, 250 mM sucrose, 100 mM KOAc, 2.5 mM Mg(OAc)2, and 2 mM DTT] prior to a 10-min centrifugation (12,000 g; 4°C) to remove cell debris. Samples were adjusted with 4× Native PAGE Sample Buffer (Life Technologies) and separated on 3–12% Native PAGE Bis-Tris Protein gels (Life Technologies) according to the manufacturer’s instructions, incorporating NativeMark Unstained Protein Standard (Life Technologies). Native PAGE running buffers (Life Technologies) were supplemented with 1 mM ATP.
Autoradiography
Native PAGE gels were incubated in 100% v/v DMSO for 20 min at room temperature on an orbital shaker. The gel was incubated in fresh 100% (v/v) DMSO for 20 min, followed by incubation in 22% (w/v) 2,5-diphenyloxazole in DMSO overnight. The gels were washed multiple times in fresh, deionized water until all precipitated residue had been removed. The gels were dried onto Whatman filter paper (GE Healthcare, Little Chalfont, United Kingdom) using a Bio-Rad Gel Dryer 583. 35S-Labeled proteins were visualized by autoradiography using BioMax MR film (Bio-Rad, Hercules, CA, USA) or a Fuji FLA-5000 PhosphorImager (Fujifilm, Tokyo, Japan). Band intensities were quantified using AIDA software (FinalWire, Budapest, Hungary), and images were analyzed and processed using ImageJ (U.S. National Institues of Health, Bethesda, MD, USA).
Protein expression and/or purification
Yeast CCT
Calmodulin-binding peptide tagged Saccharomyces cerevisiae CCT was expressed and purified as previously described (23). Briefly, cells were lysed, and crude extract was collected and incubated with calmodulin resin (Stratagene, Cedar Creek, Texas, USA). The protein was eluted and subjected to 2 sucrose gradients, followed by concentration of the CCT-containing fractions. The concentrated sample was applied to a 20 ml Superose 6 gel-filtration column (10/300 GL), and the CCT was peak concentrated, adjusted to 50% glycerol, and flash frozen in liquid N2.
PfADF1/HsCof
Full-length P. falciparum actin depolymerizing factor 1 (PfADF1) and Homo sapiens cofilin (HsCof) were expressed and purified as N-terminal glutathione S-transferase fusion proteins using the pGEX4T vector (GE Healthcare) as previously described (24). Briefly, GST-PfADF1 and GST-HsCof were expressed in BL21 (DE3) E. coli cells on addition of 1 mM isopropyl β-d-1-thiogalactopyranoside for 4 h at 37°C, and the cells were harvested, resuspended in lysis buffer (20 mM Tris, pH 8.0, 0.3 M NaCl, 0.3% Triton X-100, and 5 mM 2-mercaptoethanol) supplemented with complete EDTA-free protease inhibitors and 1 mg/ml lysozyme, and lysed by sonication. The proteins were bound to glutathione agarose (Sigma-Aldrich, St. Louis, MO, USA), washed with 100 ml wash buffer 1 (20 mM Tris, pH 8.0, 1 M NaCl, 2% Triton X-100, and 5 mM 2-mercaptoethanol) and 100 ml wash buffer 2 (20 mM Tris, pH 8.0, 1 M NaCl, and 5 mM 2-mercaptoethanol), and eluted with elution buffer (20 mM Tris, pH 8.0, 150 mM NaCl, 20 mM glutathione, and 5 mM 2-mercaptoethanol). The eluted proteins were dialyzed against PBS, and the GST tag was removed by thrombin protease. The cleaved proteins were subjected to size exclusion chromatography on a Superdex 200 10/300 gel filtration column (GE Healthcare) pre-equilibrated in storage buffer (20 mM 2-(N-morpholino)ethanesulfonic acid, pH 7.0, and 10 mM NaCl). PfADF1 eluted at ∼18 ml. HsCof eluted at ∼17 ml. The purity of the proteins was assessed by Coomassie Brilliant Blue-stained SDS-PAGE, with positive fractions concentrated and the proteins stored at 4°C.
PfProfilin
Full-length P. falciparum profilin (PfPfn) was expressed and purified as an N-terminal 6xHis fusion protein using the pProEX-HTb (Invitrogen, Carlsbad, CA, USA) as previously described (25). In brief, His-PfPfn was expressed in BL21 (DE3) E. coli cells on addition of 1 mM IPTG for 3 h at 37°C. The cells were harvested, resuspended in lysis buffer (20 mM Tris, pH 8.0, 150 mM NaCl, 0.3% Triton X-100, 20 mM imidazole, pH 8.0, and 5 mM 2-mercaptoethanol) supplemented with complete EDTA-free protease inhibitors, 1 mg/ml lysozyme, and 1 mg/ml DNAseI. The suspension was incubated on a rolling platform at 4°C for 30 min, followed by centrifugation at 30,000 g for 30 min. The supernatant was collected and incubated for 2 h at 4°C with 4 ml Profinity IMAC resin (Bio-Rad). The resin was washed sequentially with wash buffer 1 (20 mM Tris, pH 8.0, 150 mM NaCl, 2% Triton X-100, 20 mM imidazole, pH 8.0, and 5 mM 2-mercaptoethanol) and wash buffer 2 (20 mM Tris, pH 8.0, 150 mM NaCl, 20 mM imidazole, pH 8.0, and 5 mM 2-mercaptoethanol) and eluted with elution buffer (20 mM Tris, pH 8.0, 150 mM NaCl, 250 mM imidazole, pH 8.0, and 5 mM β-mercaptoethanol). The protein was dialyzed against Tris-buffered saline (150 mM Tris, pH 8.0, and 150 mM NaCl), and the N-terminal 6×His tag was removed by cleavage with tobacco etch virus protease. The cleaved protein was subjected to size exclusion chromatography on a Superdex 200 10/300 gel filtration column (GE Healthcare) pre-equilibrated in Tris-buffered saline. PfPfn eluted at ∼15.5 ml, and the protein purity was assessed by Coomassie-stained SDS-PAGE. PfPfn was concentrated to 1 mg/ml and stored at 4°C.
Sequestration assays
Rabbit skeletal muscle actin (2 μM) in Ca-buffer G (2 mM Tris, pH 8.0, 0.2 mM ATP, 0.5 mM DTT, and 0.1 mM CaCl2) was induced to polymerize in the presence of PfPfn/PfADF1 (0–16 μM) by the addition of 10× KMEI (0.5 M KCl, 0.1 M imidazole, pH 7.0, 0.01 EGTA, pH 8.0, and 0.01 M MgCl2) and incubated for 2 h at room temperature. The samples were centrifuged at 60,000 rpm in a Beckman preparative ultracentrifuge for 1 h at room temperature. The supernatant was carefully removed and adjusted with 5× reduced sample buffer. The pellet was rinsed with MgBG (2 mM Tris, pH 8.0, 0.2 mM ATP, 0.5 mM DTT, and 0.1 mM MgCl2) and centrifuged at 60,000 rpm in a Beckman preparative ultracentrifuge for 1 h at room temperature. The supernatant was carefully removed and discarded, and the pellet was resuspended in 2× reduced sample buffer to a volume equivalent to the first supernatant after addition of reduced sample buffer. The supernatant and pellet samples were boiled for 5 min, equal volumes were separated by SDS-PAGE, the gels were stained with Coomassie Brilliant Blue (Bio-Rad), and the bands were analyzed by densitometry (Supplemental Data).
Computational analyses
BLASTp searches and sequence alignments
The sequences of CCT1 from H. sapiens and S. cerevisiae were used as BLASTp queries to search the P. falciparum genome to identity potential homologes of CCT (www.plasmodb.org). Selected P. falciparum sequences were used for reciprocal BLASTp queries of the H. sapiens (taxid: 9606) and S. cerevisiae (taxid: 4932) databases (blast.ncbi.nlm.nih.gov) to confirm their identities. Multiple sequence alignments were generated by ClustalW2 (www.ebi.ac.uk/Tools/msa/clustalw2) and collated using ESPript 3.0 (www.espript.ibcp.fr/ESPript/ESPript).
In silico modeling
Modeling was performed by submitting protein sequences to the I-TASSER server (www.zhanglab.ccmb.med.umich.edu/I-TASSER) (26). Generated models were analyzed and prepared using PyMOL (DeLano Scientific LLC, Palo Alto, CA, USA; www.pymol.org).
RESULTS
PfACTI can be expressed in prokaryotic and eukaryotic in vitro translation systems
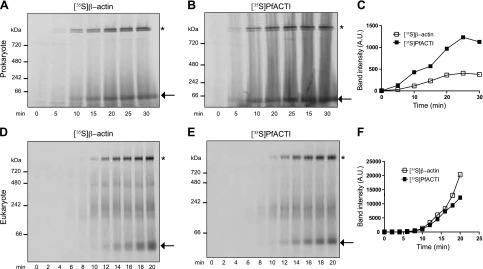
Well-established methods for analyzing the folding state of actin monomers incorporating in vitro transcription and translation coupled with 35S labeling, native PAGE analysis and autoradiography have been used extensively to characterize the folding kinetics of canonical actins, such as human β-actin (β-actin) and yeast ACT1 (23). To determine whether PfACTI can be expressed using the same systems, a time course of protein expression was performed using prokaryotic (E. coli) and eukaryotic (rabbit reticulocyte) cell lysate expression systems. It has been well established that actin expressed in E. coli is not functional; therefore, this system was included alongside the eukaryotic expression system as a negative control. Time courses for human β-actin expression were run in parallel. Both [35S]β-actin (Fig. 1A, D) and [35S]PfACTI (Fig. 1B, E) were translated efficiently in both systems, with an increase in signal in a discrete band seen below the 66-kDa marker. Quantification of band intensity by densitometry demonstrates the increase in protein expression over time for both actins (Fig. 1C, F). Although there does appear to be a higher level of expression of [35S]PfACTI compared with [35S]β-actin in the prokaryotic expression system (Fig. 1C), this could be a result of slight variation in DNA template concentration, which is amplified during RNA transcription and translation.
Figure 1.
In vitro translation of actins in E. coli cell extract and rabbit reticulocyte cell extract. A, B) Time course of expression of [35S]β-actin (A) and [35S]PfACTI (B), translated in E.coli in vitro transcription/translation extract, monitored by native 3–12% PAGE analysis. Arrow, [35S]β-actin/PfACTI; asterisk, [35S]β-actin/PfACTI bound to endogenous GroEL present in the E. coli cell extract. C) Densitometry of band intensities over the time course of expression, for both [35S]β-actin (open squares) and [35S]PfACTI (filled squares). D, E) Time course of expression of [35S]β-actin (D) and [35S]PfACTI, translated in rabbit reticulocyte in vitro transcription/translation extract, monitored by native 3–12% PAGE analysis (E). Arrow, monomeric [35S]β-actin and [35S]PfACTI; asterisk, [35S]β-actin and [35S]PfACTI bound to endogenous RbCCT. F) Densitometry of the monomeric actin band intensities over the time course of expression, for both [35S]β-actin (open squares) and [35S]PfACTI (filled squares).
In addition to the band representing monomeric actin in each experiment, there are higher-molecular-weight bands appearing toward the top of the gels above the 720-kDa marker, as indicated by the asterisks (Fig. 1A, B, D, E). It has been previously reported that nascent, unfolded actin can form a tight complex with GroEL, the primary chaperonin present in bacterial cells, although GroEL cannot process actin into its native state (27). This higher banding in the prokaryotic expression system therefore denotes the complex formed by endogenous E. coli GroEL and the translated [35S]β-actin or [35S]PfACTI (Fig. 1A, B). In the eukaryotic system, however, the discrete, higher-molecular-weight band appearing toward the top of the gels represents the complex formed between incompletely folded [35S]β-actin or [35S]PfACTI and endogenous rabbit CCT (RbCCT), which is present in the rabbit reticulocyte cell extract (Fig. 1D, E). This banding pattern for CCT bound to actin has been validated in multiple studies in both yeast and rabbit expression systems (23). This indicates that [35S]PfACTI can bind to RbCCT, although whether it is being processed into its native, folded state is not clear.
PfACTI expressed in prokaryotic cells can bind to the CCT complex in the eukaryotic cell lysate
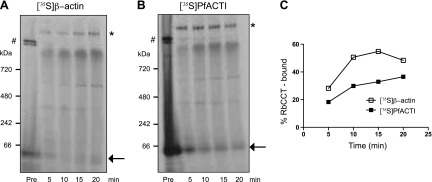
To determine whether the actins expressed in the prokaryotic system are capable of binding to RbCCT in the eukaryotic cell extract, [35S]β-actin and [35S]PfACTI were expressed for 30 min in the prokaryotic in vitro translation system followed by titration into the eukaryotic cell extract. Both [35S]β-actin and [35S]PfACTI bind to RbCCT, as denoted by the change in banding pattern before and after titration (Fig. 2A, B). As the time course progresses, the RbCCT-actin band increases in intensity for both [35S]β-actin and [35S]PfACTI, whereas the GroEL-actin complex band disappears, indicating total competition of the actins off GroEL and onto RbCCT. Quantification of the relative percentage of [35S]β-actin or [35S]PfACTI bound to RbCCT at each time point demonstrates this increase in RbCCT binding (Fig. 2C).
Figure 2.
Competition of E. coli extract in vitro-translated actins onto endogenous rabbit CCT. A, B) [35S]β-Actin (A) and [35S]PfACTI (B), translated in E. coli cell extract for 30 min (Pre), added to rabbit reticulocyte extract and monitored by native 3–12% PAGE analysis for 20 min. Arrow, [35S]β-actin/PfACTI; asterisk, [35S]β-actin/PfACTI bound to endogenous CCT present in the rabbit reticulocyte cell extract; #, [35S]β-actin/PfACTI bound to GroEL. C) Quantification by densitometry of the percentage of actin bound to RbCCT over time for both [35S]β-actin (open squares) and [35S]PfACTI (filled squares).
PfACTI expressed in eukaryotic cell lysate can bind to S. cerevisiae CCT
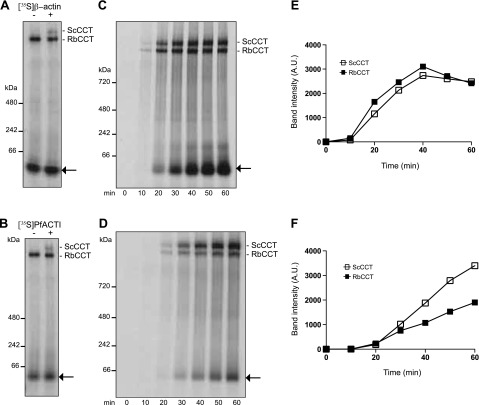
To explore the affinity of PfACTI for an alternative eukaryotic CCT complex, we next determined whether it could interact with that from S. cerevisiae. Purified S. cerevisiae CCT (ScCCT) (23) was added to the rabbit reticulocyte in vitro translation system following [35S]β-actin and [35S]PfACTI expression, thus discriminating between actin binding to endogenous RbCCT over the added ScCCT (Fig. 3A, B). Comparison of the banding pattern at the top of the gels indicates that the band corresponding to ScCCT bound to either [35S]β-actin (Fig. 3A) or [35S]PfACTI (Fig. 3B) runs slightly slower than endogenous RbCCT-actin. To compare the binding efficiency for RbCCT and ScCCT for each actin over the course of translation, ScCCT was added at the initiation of translation, with samples taken every 10 min for 60 min (Fig. 3C, D). [35S]β-Actin binds to both endogenous RbCCT and added ScCCT with approximately the same efficiency (Fig. 3C) with further analysis by densitometry confirming this similarity (Fig. 3E). In contrast, [35S]PfACTI shows a distinct preference for binding to ScCCT (Fig. 3D). Analysis by densitometry clearly demonstrates this effect, with a large proportion of [35S]PfACTI partitioning to ScCCT (Fig. 3F). It is also important to note that [35S]β-actin peaks in binding to both CCTs at 40 min and then begins to show release from the CCTs (Fig. 3E), whereas [35S]PfACTI continues to accumulate on the CCTs and does not show increased release (Fig. 3F). This explains why there appears to be a higher yield of unbound [35S]β-actin compared with [35S]PfACTI (indicated by the arrows, Fig. 3C, D), as native [35S]β-actin is released from CCT, whereas [35S]PfACTI is not. These data hint toward complications in the completion of the folding cycle by CCT for PfACTI, which should release actin once it is natively folded (28).
Figure 3.
Rabbit reticulocyte actins can bind to ScCCT. Native PAGE analysis of [35S]β-actin and [35S]PfACTI translated in rabbit reticulocyte extract in the presence of purified ScCCT (400 nM). A, B) [35S]β-Actin (A) or [35S]PfACTI (B) was expressed for 40 min (left lane), followed by addition of ScCCT (right lane). [35S]β-Actin/[35S]PfACTI bound to ScCCT runs higher that [35S]β-actin/[35S]PfACTI bound to endogenous RbCCT as indicated by ScCCT/RbCCT labels. C, D) [35S]β-Actin (C) and [35S]PfACTI (D) were translated in the presence of purified ScCCT (400 nM) for 60 min with samples taken every 10 min. Arrow indicates translated [35S]β-actin/[35S]PfACTI. E, F) Quantification of band intensities over the time course of expression for [35S]β-actin (E) and [35S]PfACTI (F) bound to ScCCT (open squares) and RbCCT (filled squares).
PfACTI expressed in eukaryotic cell lysate is unable to bind to G-actin binding proteins and is therefore not natively folded
Although PfACTI is able to bind to different eukaryotic CCTs, ScCCT and RbCCT, it is unclear whether even a small proportion is being processed by CCT and folded into its native state. A method of determining the formation of natively folded actin in the context of in vitro translation is the ability of added G-actin binding proteins to interact with monomeric actin (23). If β-actin is natively folded, it will be able to form a complex with the added G-actin binding protein DNAseI, forming a complex that will be visible as a higher-molecular-weight band after native PAGE analysis (29). However, sequence analysis and experimental evidence have suggested that the DNAseI binding site on PfACTI is highly divergent, leading to a 200-fold reduction in DNAseI binding affinity (15). Therefore, although DNAseI is an appropriate choice to assess the native fold of β-actin, 2 additional G-actin–binding proteins, PfADF1 (24, 30) and PfPfn (25), were used for analysis of PfACTI. These proteins have been previously validated as functional G-actin interacting proteins when expressed recombinantly in E. coli (25, 30) and were subjected to rabbit skeletal muscle actin sequestration assays prior to use to ensure G-actin binding activity (Supplemental Fig. S1). To mirror the use of alternative G-actin binding proteins, HsCof (24) was used alongside DNAseI for assessment of β-actin folding.
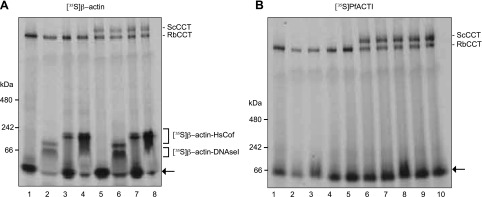
Prior to the addition of the G-actin binding proteins, [35S]β-actin and [35S]PfACTI were synthesized for 40 min in the eukaryotic in vitro translation system with and without ScCCT, which may be able to bind and fold PfACTI if RbCCT cannot. One microgram DNAseI or 1 μg/10 μg of HsCof was added to the [35S]β-actin translation reactions (Fig. 4A), and 1 μg/10 μg PfADF1 and 1 μg/10 μg PfPfn were added to the [35S]PfACTI translation reactions (Fig. 4B). [35S]β-Actin formed complexes with both DNAseI and HsCof both in the presence and absence of ScCCT (Fig. 4A). Although there is still a residual amount of monomeric actin remaining that is not in a complex, a concentration-dependent shift is evident when comparing the amount of [35S]β-actin shifting to the complex with 1 or 10 μg added HsCof. This indicates that [35S]β-actin is natively folded in the eukaryotic in vitro translation system by endogenous RbCCT. In contrast, [35S]PfACTI was not able to form complexes with either PfADF1 or PfPfn, regardless of the amount of G-actin binding protein added, or the presence or absence of added ScCCT (Fig. 4B). This inability to bind to G-actin binding proteins would appear to indicate that [35S]PfACTI is not natively folded when expressed in the heterologous eukaryotic system, and, furthermore, ScCCT is not capable of mediating its folding into a native state. Combined, these data demonstrate that the production of PfACTI in heterologous eukaryotic expression systems is unlikely to yield fully folded native protein. This suggests that evolutionary specificity may have evolved between parasite actin and its native folding machinery as it has with other actin isoforms in other species (21).
Figure 4.
Assessment of native folding of actins by complex formation with G-actin binding proteins. Native 3–12% PAGE analysis of actins translated in rabbit reticulocyte extract in the absence or presence of purified ScCCT (400 nM) for 40 min followed by addition of G-actin binding proteins for 15 min. A) Lanes 1 and 5, [35S]β-actin prior to G-actin binding protein addition synthesized in the presence or absence of ScCCT, respectively; lanes 2 and 6, +1 μg DNAseI; lanes 3 and 7, +1 μg HsCof; lanes 4 and 8, +10 μg HsCof. Complexes formed between [35S]β-actin and DNAseI/HsC of marked by square brackets. B) Lanes 1 and 6, [35S]PfACTI prior to G-actin binding protein addition synthesized in the presence or absence of ScCCT, respectively; lanes 2 and 7, +1 μg PfADF1; lanes 3 and 8, +10 μg PfADF1; lanes 4 and 9, +1 μg PfPfn; lanes 5 and 10, +10 μg PfPfn. Arrows, [35S]β-actin/[35S]PfACTI uncomplexed.
Addition of in vitro-translated PfACTI into P. falciparum lysate
The inability of PfACTI to fold into its native state in the context of a heterologous expression system indicates a potential requirement for homologous protein folding machinery found within the Plasmodium parasite cell. If [35S]PfACTI is in a partially unfolded state after expression in an in vitro translation system, it may be able to bind to the homologous P. falciparum CCT homolog (PfCCT) within P. falciparum cell extract and be folded into its native form.
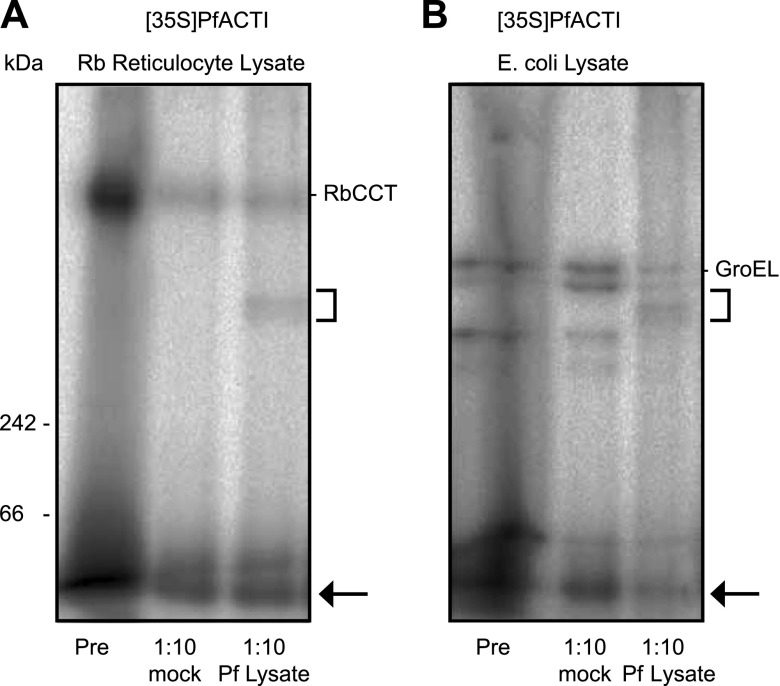
To investigate this possibility, [35S]PfACTI, expressed in either the eukaryotic or the prokaryotic in vitro translation system, was titrated into P. falciparum cell extract or the extract preparation buffer as a negative control (Fig. 5 and Supplemental Fig. S2). For the reticulocyte expression system lysate, the band corresponding to [35S]PfACTI bound to RbCCT persists after addition to the P. falciparum cell extract, however, with a diminished intensity. Concurrently, an additional band, which would be consistent with binding of [35S]PfACTI to its native PfCCT present in the lysate, became apparent (Fig. 5A). Critically, an identical banding pattern also appeared when the prokaryotic in vitro translation lysate was incubated with Plasmodium cell extract (Fig. 5B). Of particular note, the bands [35S]PfACTI bound to GroEL in the prokaryotic cell extract almost entirely disappeared when titrated into the P. falciparum cell extracts, supporting the notion that GroEL-bound [35S]PfACTI preferentially disengaged to rebind to the native Plasmodium CCT complex. These findings demonstrate the presence of a cytosolic P. falciparum complex, which can bind to unfolded actin and likely indicates the presence of the PfCCT complex. This would be predicted if, as a eukaryotic cell with a complex cytoskeleton including both actin and tubulin, Plasmodium has a conventional homolog of the CCT complex to mediate the folding of cytoskeletal components.
Figure 5.
Addition of [35S]PfACTI to P. falciparum cell extract. Native PAGE analysis of [35S]PfACTI translated in rabbit reticulocyte (A) or E. coli lysate (B) for 40 min (Pre), added to extract preparation buffer (mock) or P. falciparum cell extract (Pf lysate) at a 1:10 ratio of [35S]PfACTI:P. falciparum cell extract for 15 min. Square bracket indicates possible complex formation in P. falciparum lysate. Arrow, monomeric [35S]PfACTI.
In silico identification of P. falciparum CCT
Toward identifying if a CCT complex is present in the Plasmodium genome, BLASTp searches were performed on public databases (31) using the protein sequences of CCT1 from H. sapiens and S. cerevisiae. The reference genome for P. falciparum (strain 3D7) yielded 10 CCT subunit hits, of which 2 nonspecific hits could readily be discounted [the 60-kDa chaperonin (CPN60) and heat shock protein 60 (HSP60), both of which have previously been characterized as mitochondrial chaperones (32, 33)]. The remaining 8 genes are all described as putative T-complex protein subunits or members of the TCP-1 chaperonin family. To classify each of the 8 putative genes as subunits of the CCT complex, a reciprocal BLASTp search was performed on the databases of H. sapiens (taxid: 9606) and S. cerevisiae (taxid: 4932) by submitting the protein sequence for each putative subunit and selecting the top hit. Both the H. sapiens and S. cerevisiae databases were in agreement regarding the subunit categorization, and all 8 CCT subunits were accounted for. As outlined in Table 1, the percentage identity between the putative P. falciparum CCT subunits and those from H. sapiens and S. cerevisiae were low, ranging from 34% to 60%, indicating a high level of sequence divergence in PfCCT.
TABLE 1.
Results of CCT subunit protein BLAST search using UniProtKB/SwissProt database
| P. falciparum gene ID | Description | H. sapiens gene ID | % Identity | S. cerevisiae gene ID | % Identity | Description |
|---|---|---|---|---|---|---|
| PF3D7_0214000 | T-complex protein 1, putative | P50990 | 34 | P47079 | 34 | TCP-1 subunit θ |
| PF3D7_0306800 | T-complex protein β subunit, putative | P78371 | 57 | P39076 | 55 | TCP-1 subunit β |
| PF3D7_0308200 | TCP-1/cpn60 chaperonin family, putative | Q99832 | 57 | P42943 | 54 | TCP-1 subunit η |
| PF3D7_0320300 | T-complex protein 1 ε subunit, putative | P48643 | 55 | P40413 | 51 | TCP-1 subunit ε |
| PF3D7_0608700 | chaperone, putative | P40227 | 49 | P39079 | 43 | TCP-1 subunit ζ |
| PF3D7_1132200 | TCP-1/cpn60 chaperonin family, putative | P17987 | 59 | P12612 | 55 | TCP-1 subunit α |
| PF3D7_1229500 | T-complex protein 1 γ subunit, putative | P49368 | 54 | P39077 | 51 | TCP-1 subunit γ |
| PF3D7_1357800 | TCP-1/cpn60 chaperonin family, putative | P50991 | 60 | P39078 | 51 | TCP-1 subunit δ |
Conservation of signature residues and the nucleotide-binding site in PfCCT
Highly conserved residues, termed “signature residues” have been identified in each CCT subunit across many species (34). These signature residues are located throughout each of the subunits, with those in the apical domains involved in substrate binding, whereas those in the intermediate and equatorial domains are involved in ATP hydrolysis and stability of the complex. To determine whether these residues are conserved in the P. falciparum CCT subunits, multiple sequence alignments were generated for each CCT subunit, using protein sequences from H. sapiens, Mus musculus, Drosophila melanogaster, Caenorhabditis elegans, S. cerevisiae, and P. falciparum. Of most interest, in all species analyzed, with the exception of P. falciparum, the signature residues are completely conserved (data not shown). In P. falciparum, approximately 21% of the signature residues are divergent, with ∼42% of these divergent residues located in the apical domain (Table 2). Given that the apical domain is responsible for substrate binding, this high level of sequence divergence in P. falciparum is likely an indication of species-specific interactions with substrates, further lending support to the potential necessity of native CCT for folding PfACTI into a mature state. It is also notable that the CCT-binding sites of mammalian β-actin (35) are divergent in PfACT1, which further strengthens the suggestion of coevolution and divergence of the PfCCT-actin system.
TABLE 2.
Divergent signature residues in the PfCCT complex
| CCT subunit domain | CCT1 | CCT2 | CCT3 | CCT4 | CCT5 | CCT6 | CCT7 | CCT8 |
|---|---|---|---|---|---|---|---|---|
| Equatorial | Q28Aa | D131S | T42S, A77S | P114G | E126D | |||
| Intermediate | G186D | G175Y | ||||||
| Apical | L206I, A208V | D209E, A259Q | G255A, P292C | E317D | L340I | M199I, H201R, D223N, V284L, A336P | A292K | |
| Intermediate | S385T, P410G | D402H | ||||||
| Equatorial | T512S | S506T | Q502I | E476K | Y479I |
Residue numbering is given as per ScCCT (34).
Within each subunit of CCT is a conserved cluster of aspartic acid residues, located in the equatorial domain, that are involved in nucleotide binding and ATP hydrolysis. As described for ScCCT6, D58, D84, and D397 coordinate the catalytic water molecule, whereas D89, located within the sequence GDGTT, is the catalytic residue for ATP hydrolysis (34). This sequence is a conserved ATP hydrolysis motif also found in the archaeal thermosome (a homolog of CCT in Archaea) and GroEL (36). To determine whether this nucleotide binding site is conserved in PfCCT, a multiple sequence alignment of all PfCCT subunits was generated, and the aspartic acid residues potentially involved in ATP hydrolysis were identified across the subunits, demonstrating complete conservation of the catalytic site (Supplemental Fig. S3). The motif GDGTT is conserved in subunits PfCCT1-5 and 7. In subunit PfCCT6, the motif is instead GDGSS; however, the latter serines represent somewhat conservative substitutions. PfCCT8 is the most divergent, with the motif consisting of GDFTN. These differences hint toward possible heterogeneity in the ATP hydrolysis potential of the PfCCT subunits as has been demonstrated in ScCCT (36), indicating that PfCCT may act with a similar sequential mechanism for ATP hydrolysis and consequent conformational change during substrate folding.
DISCUSSION
ACTI is increasingly implicated in diverse processes in the apicomplexan parasite cell, from endocytic trafficking (37, 38) to nuclear positioning of var genes associated with parasite virulence (39, 40), gametocyte development (6, 41), cell-cell communication (42), and, of perhaps the most intensive interest, the molecular basis for parasite gliding motility and host cell invasion (43). Gliding motility is known to rely on dynamic actin filaments that are thought to act as a “molecular clutch,” engaging the myosin motor and together generating a rearward force sufficient to drive the parasite forward (2).
Detailed investigation of apicomplexan actin in recent years has demonstrated that it is remarkably different from conventional eukaryotic actins. Indeed, unlike canonical actins, which conform to a nucleation-elongation mechanism of polymerization, it has even been proposed that apicomplexan actins polymerize in a unique fashion, using isodesmic polymerization that involves identical association and dissociation rates throughout polymerization with no lag phase (17). Combined with observations that these actins also form very short, highly dynamic, and unstable polymers (10, 11, 14–16, 18), this has placed apicomplexan actin (ACTI specifically) as one of the most divergent known, both in its sequence and kinetic properties. Some of these properties are thought to be specific evolutionary adaptations of the parasite that allow for the rapid polymer turnover required for invasion and gliding motility (16) and the skewing of the whole actin regulatory system toward disassembly (43).
Of key concern, however, is the paucity of biochemical studies that have focused on native actin from these parasites (10, 11). Most biochemical and kinetic measurements described in the literature have instead relied on purification of ACTI (both Toxoplasma and Plasmodium) using heterologous yeast or insect cell expression systems (14–16, 18). Here, we set out to test whether heterologous expression of PfACT, the primary actin required for motility in Plasmodium parasites, is correctly folded in prokaryotic and eukaryotic coupled transcription/translation systems. This demonstrated that PfACTI produced in both expression systems appears to be incompletely folded into a native, and therefore, fully functional state. This is most keenly demonstrated by the inability of monomeric PfACTI to bind to two of its native G-actin binding proteins (Fig. 4). Although the in vitro expression systems used are not identical to those in the published literature, these data certainly raise a critical question surrounding the characterization of PfACTI expressed in either yeast or insect cells.
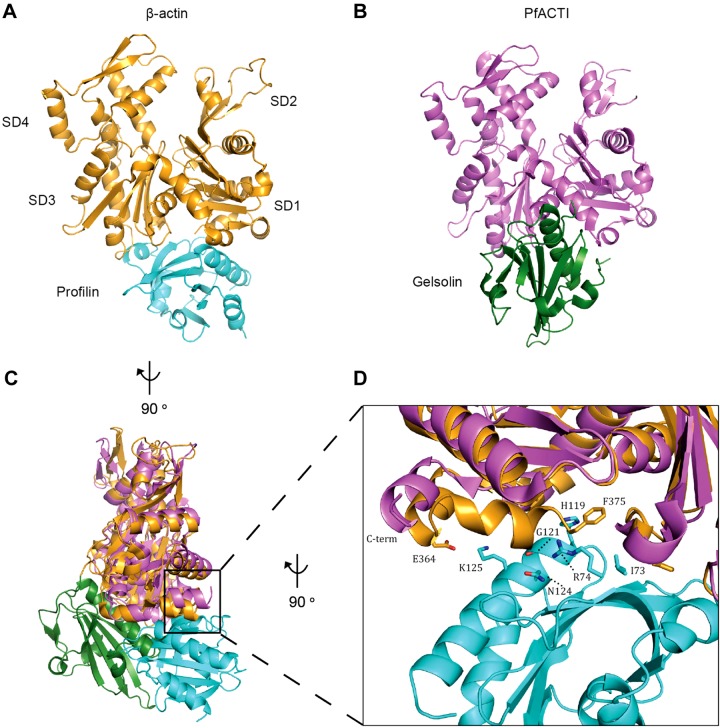
On this point, and of particular interest, is a recent study in which the crystal structure of monomeric PfACTI was solved in complex with the mammalian G-actin binding protein gelsolin (18). Apicomplexan parasites, Plasmodium included, do not contain a homolog of gelsolin (43). Close inspection of the PfACTI:gelsolin structure reveals that the last 8 residues, SIVHRKCF, of PfACTI are not visible, indicating disorder at the C terminus (Fig. 6). Of note, the terminal phenylalanine, F375, in human β-actin is critical for binding to profilin (44) (it interacts with I73, R74, H119, G121, and N124 of profilin via its phenyl ring and C terminus; Fig. 6D). If the C-terminal region is consistently disordered in heterologously, and potentially incompletely folded PfACTI, this might therefore caution interpretation that recombinant PfPfn shows only weak affinity for PfACTI in vitro (45).
Figure 6.
Comparison of structures of β-actin:profilin and PfACTI/gelsolin complexes. A, B) The crystal structures of β-actin bound to profilin (A), where β-actin is in orange and profilin is in cyan [2BTF.pdb (44)] and PfACTI bound to gelsolin (B), where PfACTI is in pink and gelsolin is in green [4CBU.pdb (18)]. SDs 1-4 are labeled in A. C) Overlay of A and B rotated 90° to the left along the vertical axis, demonstrating the different binding modes of profilin and gelsolin. D) Close up view of the interaction between the C terminus of β-actin and profilin, viewed from a 90° rotation to the left along the vertical axis from C, overlayed with PfACTI. F375 of β-actin forms contacts with I73, R74, H119, G121, and N124 of profilin, whereas E364 of β-actin interacts with K125 of profilin. Residue side chains are represented as sticks. Note the absence of the C-terminal helix in the structure of PfACTI.
The contribution of the C terminus to actin function has been extensively examined across many other eukaryotic systems. For example, exposure of the thiol groups in cysteine residues including C374 (the penultimate actin residue), using ATP analogs, results in a loss of actin polymerizability (46). Similarly, direct modification of C374 to glutathionyl, a mixed disulfide, leads to the formation of filaments that are easily disrupted by mechanical stress, indicating that these filaments are more unstable (47). This same study demonstrated that the stability of the filaments formed by this modified actin could be rescued by the addition of equimolar phalloidin (47). The same effect on filament stability can also be achieved by limited proteolysis of the last 2 residues of actin, C374 and F375, which could also be rescued by the addition of equimolar phalloidin (48). In each study, C-terminal truncations have also been shown to increase the critical concentration, polymerization rate, and rate of ATP hydrolysis of the filaments (47–49). C-terminal truncations have also been shown to lead to conformational changes in the d-loop of SD2, disrupting the interactions between neighboring monomers in the filament, affecting filament stability, and causing observed structural changes to the actin filament (49–53). Finally, in vivo, truncation of the last 2 residues, C374 and F375, of yeast actin (the last 3 are lethal) leads to a complete loss of actin filamentous structures (54) with a similar effect seen in mammalian cells containing actin having the same mutated C374, which led to a disruption and disorganization of observable filamentous structures with an accompanied increase in diffuse actin staining (55). It is important to note that, from both experimental data (19, 28, 56, 57) and computational modeling (57), the final stage of actin monomer folding via CCT is thought to involve the correct positioning of the C terminus in SD1 (19, 28, 56, 57), which is also the final stage that precedes release of native actin from CCT (19, 28). Therefore, although the rest of the protein may be correctly folded, without correct packing of the C terminus in SD1, the protein is not completely folded into its native, and therefore, fully functional, state.
Given this wealth of experimental evidence about the critical role that the C terminus of actin plays in the formation of stable filaments in vitro and in vivo, the fact that many of the unusual kinetic properties or phenotypic characteristics described above for mutant actins have been ascribed to native apicomplexan actins, based on studies with heterologous expression ACTI, a degree of caution in the interpretation of biochemical assays may now be warranted. Assays with native actin or actin refolded with the native PfCCT complex would now appear to be an imperative.
Supplementary Material
Acknowledgments
The authors thank Jacqui Gulbis for ongoing support and advice throughout the project’s duration. Supported by Human Frontier Science Program Young Investigator Program Grant RGY0071/2011 (to J.B.). M.A.O. is supported through Australia National Health and Medical Research Council Dora Lush Scholarship APP1018002 and was supported in the United Kingdom with a travel award from the Australian Society for Biochemistry and Molecular Biology. J.B. is supported by the Wellcome Trust, through New Investigator Award 100993/Z/13/Z. The authors declare no conflicts of interest.
Glossary
- ADF
actin depolymerizing factor
- CCT
chaperonin-containing TCP-1
- Cof
cofilin
- d-loop
DNaseI binding loop
- Hs
Homo sapiens
- PfACTI
Plasmodium falciparum actin I
- PfCCT
P. falciparum CCT homolog
- Pfn
profilin
- RbCCT
rabbit CCT
- ScCCT
S. cerevisiae CCT
- TgACT1
Toxoplasma gondii actin 1
- TCP-1
t-complex protein-1
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1.Sibley L. D. (2004) Intracellular parasite invasion strategies. Science 304, 248–253 [DOI] [PubMed] [Google Scholar]
- 2.Baum J., Gilberger T.-W., Frischknecht F., Meissner M. (2008) Host-cell invasion by malaria parasites: insights from Plasmodium and Toxoplasma. Trends Parasitol. 24, 557–563 [DOI] [PubMed] [Google Scholar]
- 3.Wesseling J. G., Smits M. A., Schoenmakers J. G. (1988) Extremely diverged actin proteins in Plasmodium falciparum. Mol. Biochem. Parasitol. 30, 143–153 [DOI] [PubMed] [Google Scholar]
- 4.Dobrowolski J. M., Niesman I. R., Sibley L. D. (1997) Actin in the parasite Toxoplasma gondii is encoded by a single copy gene, ACT1 and exists primarily in a globular form. Cell Motil. Cytoskeleton 37, 253–262 [DOI] [PubMed] [Google Scholar]
- 5.Egarter S., Andenmatten N., Jackson A. J., Whitelaw J. A., Pall G., Black J. A., Ferguson D. J., Tardieux I., Mogilner A., Meissner M. (2014) The toxoplasma Acto-MyoA motor complex is important but not essential for gliding motility and host cell invasion. PLoS One 9, e91819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deligianni E., Morgan R. N., Bertuccini L., Kooij T. W. A., Laforge A., Nahar C., Poulakakis N., Schüler H., Louis C., Matuschewski K., Siden-Kiamos I. (2011) Critical role for a stage-specific actin in male exflagellation of the malaria parasite. Cell. Microbiol. 13, 1714–1730 [DOI] [PubMed] [Google Scholar]
- 7.Angrisano F., Riglar D. T., Sturm A., Volz J. C., Delves M. J., Zuccala E. S., Turnbull L., Dekiwadia C., Olshina M. A., Marapana D. S., Wong W., Mollard V., Bradin C. H., Tonkin C. J., Gunning P. W., Ralph S. A., Whitchurch C. B., Sinden R. E., Cowman A. F., McFadden G. I., Baum J. (2012) Spatial localisation of actin filaments across developmental stages of the malaria parasite. PLoS One 7, e32188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kudryashev M., Lepper S., Baumeister W., Cyrklaff M., Frischknecht F. (2010) Geometric constrains for detecting short actin filaments by cryogenic electron tomography. PMC Biophys. 3, 6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Field S. J., Pinder J. C., Clough B., Dluzewski A. R., Wilson R. J., Gratzer W. B. (1993) Actin in the merozoite of the malaria parasite, Plasmodium falciparum. Cell Motil. Cytoskeleton 25, 43–48 [DOI] [PubMed] [Google Scholar]
- 10.Schmitz S., Grainger M., Howell S., Calder L. J., Gaeb M., Pinder J. C., Holder A. A., Veigel C. (2005) Malaria parasite actin filaments are very short. J. Mol. Biol. 349, 113–125 [DOI] [PubMed] [Google Scholar]
- 11.Schmitz S., Schaap I. A., Kleinjung J., Harder S., Grainger M., Calder L., Rosenthal P. B., Holder A. A., Veigel C. (2010) Malaria parasite actin polymerization and filament structure. J. Biol. Chem. 285, 36577–36585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mizuno Y., Makioka A., Kawazu S., Kano S., Kawai S., Akaki M., Aikawa M., Ohtomo H. (2002) Effect of jasplakinolide on the growth, invasion, and actin cytoskeleton of Plasmodium falciparum. Parasitol. Res. 88, 844–848 [DOI] [PubMed] [Google Scholar]
- 13.Shaw M. K., Tilney L. G. (1999) Induction of an acrosomal process in Toxoplasma gondii: visualization of actin filaments in a protozoan parasite. Proc. Natl. Acad. Sci. USA 96, 9095–9099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sahoo N., Beatty W., Heuser J., Sept D., Sibley L. D. (2006) Unusual kinetic and structural properties control rapid assembly and turnover of actin in the parasite Toxoplasma gondii. Mol. Biol. Cell 17, 895–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schüler H., Mueller A.-K., Matuschewski K. (2005) Unusual properties of Plasmodium falciparum actin: new insights into microfilament dynamics of apicomplexan parasites. FEBS Lett. 579, 655–660 [DOI] [PubMed] [Google Scholar]
- 16.Skillman K. M., Diraviyam K., Khan A., Tang K., Sept D., Sibley L. D. (2011) Evolutionarily divergent, unstable filamentous actin is essential for gliding motility in apicomplexan parasites. PLoS Pathog. 7, e1002280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Skillman K. M., Ma C. I., Fremont D. H., Diraviyam K., Cooper J. A., Sept D., Sibley L. D. (2013) The unusual dynamics of parasite actin result from isodesmic polymerization. Nat. Commun. 4, 2285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vahokoski J., Bhargav S. P., Desfosses A., Andreadaki M., Kumpula E. P., Martinez S. M., Ignatev A., Lepper S., Frischknecht F., Sidén-Kiamos I., Sachse C., Kursula I. (2014) Structural differences explain diverse functions of Plasmodium actins. PLoS Pathog. 10, e1004091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stuart S. F., Leatherbarrow R. J., Willison K. R. (2011) A two-step mechanism for the folding of actin by the yeast cytosolic chaperonin. J. Biol. Chem. 286, 178–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lundin V. F., Leroux M. R., Stirling P. C. (2010) Quality control of cytoskeletal proteins and human disease. Trends Biochem. Sci. 35, 288–297 [DOI] [PubMed] [Google Scholar]
- 21.Altschuler G. M., Dekker C., McCormack E. A., Morris E. P., Klug D. R., Willison K. R. (2009) A single amino acid residue is responsible for species-specific incompatibility between CCT and alpha-actin. FEBS Lett. 583, 782–786 [DOI] [PubMed] [Google Scholar]
- 22.Llorca O., Martín-Benito J., Grantham J., Ritco-Vonsovici M., Willison K. R., Carrascosa J. L., Valpuesta J. M. (2001) The ‘sequential allosteric ring’ mechanism in the eukaryotic chaperonin-assisted folding of actin and tubulin. EMBO J. 20, 4065–4075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pappenberger G., McCormack E. A., Willison K. R. (2006) Quantitative actin folding reactions using yeast CCT purified via an internal tag in the CCT3/gamma subunit. J. Mol. Biol. 360, 484–496 [DOI] [PubMed] [Google Scholar]
- 24.Wong W., Skau C. T., Marapana D. S., Hanssen E., Taylor N. L., Riglar D. T., Zuccala E. S., Angrisano F., Lewis H., Catimel B., Clarke O. B., Kershaw N. J., Perugini M. A., Kovar D. R., Gulbis J. M., Baum J. (2011) Minimal requirements for actin filament disassembly revealed by structural analysis of malaria parasite actin-depolymerizing factor 1. Proc. Natl. Acad. Sci. USA 108, 9869–9874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baum J., Tonkin C. J., Paul A. S., Rug M., Smith B. J., Gould S. B., Richard D., Pollard T. D., Cowman A. F. (2008) A malaria parasite formin regulates actin polymerization and localizes to the parasite-erythrocyte moving junction during invasion. Cell Host Microbe 3, 188–198 [DOI] [PubMed] [Google Scholar]
- 26.Yang J., Yan R., Roy A., Xu D., Poisson J., Zhang Y. (2015) The I-TASSER Suite: protein structure and function prediction. Nat. Methods 12, 7–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tian G., Vainberg I. E., Tap W. D., Lewis S. A., Cowan N. J. (1995) Specificity in chaperonin-mediated protein folding. Nature 375, 250–253 [DOI] [PubMed] [Google Scholar]
- 28.Neirynck K., Waterschoot D., Vandekerckhove J., Ampe C., Rommelaere H. (2006) Actin interacts with CCT via discrete binding sites: a binding transition-release model for CCT-mediated actin folding. J. Mol. Biol. 355, 124–138 [DOI] [PubMed] [Google Scholar]
- 29.Grantham J., Llorca O., Valpuesta J. M., Willison K. R. (2000) Partial occlusion of both cavities of the eukaryotic chaperonin with antibody has no effect upon the rates of beta-actin or alpha-tubulin folding. J. Biol. Chem. 275, 4587–4591 [DOI] [PubMed] [Google Scholar]
- 30.Schüler H., Mueller A.-K., Matuschewski K. (2005) A Plasmodium actin-depolymerizing factor that binds exclusively to actin monomers. Mol. Biol. Cell 16, 4013–4023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aurrecoechea C., Brestelli J., Brunk B. P., Dommer J., Fischer S., Gajria B., Gao X., Gingle A., Grant G., Harb O. S., Heiges M., Innamorato F., Iodice J., Kissinger J. C., Kraemer E., Li W., Miller J. A., Nayak V., Pennington C., Pinney D. F., Roos D. S., Ross C., Stoeckert C. J. Jr., Treatman C., Wang H. (2009) PlasmoDB: a functional genomic database for malaria parasites. Nucleic Acids Res. 37, D539–D543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Das A., Syin C., Fujioka H., Zheng H., Goldman N., Aikawa M., Kumar N. (1997) Molecular characterization and ultrastructural localization of Plasmodium falciparum Hsp 60. Mol. Biochem. Parasitol. 88, 95–104 [DOI] [PubMed] [Google Scholar]
- 33.Holloway S. P., Min W., Inselburg J. W. (1994) Isolation and characterization of a chaperonin-60 gene of the human malaria parasite Plasmodium falciparum. Mol. Biochem. Parasitol. 64, 25–32 [DOI] [PubMed] [Google Scholar]
- 34.Dekker C., Roe S. M., McCormack E. A., Beuron F., Pearl L. H., Willison K. R. (2011) The crystal structure of yeast CCT reveals intrinsic asymmetry of eukaryotic cytosolic chaperonins. EMBO J. 30, 3078–3090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hynes G. M., Willison K. R. (2000) Individual subunits of the eukaryotic cytosolic chaperonin mediate interactions with binding sites located on subdomains of beta-actin. J. Biol. Chem. 275, 18985–18994 [DOI] [PubMed] [Google Scholar]
- 36.Amit M., Weisberg S. J., Nadler-Holly M., McCormack E. A., Feldmesser E., Kaganovich D., Willison K. R., Horovitz A. (2010) Equivalent mutations in the eight subunits of the chaperonin CCT produce dramatically different cellular and gene expression phenotypes. J. Mol. Biol. 401, 532–543 [DOI] [PubMed] [Google Scholar]
- 37.Lazarus M. D., Schneider T. G., Taraschi T. F. (2008) A new model for hemoglobin ingestion and transport by the human malaria parasite Plasmodium falciparum. J. Cell Sci. 121, 1937–1949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smythe W. A., Joiner K. A., Hoppe H. C. (2008) Actin is required for endocytic trafficking in the malaria parasite Plasmodium falciparum. Cell. Microbiol. 10, 452–464 [DOI] [PubMed] [Google Scholar]
- 39.Volz J. C., Bártfai R., Petter M., Langer C., Josling G. A., Tsuboi T., Schwach F., Baum J., Rayner J. C., Stunnenberg H. G., Duffy M. F., Cowman A. F. (2012) PfSET10, a Plasmodium falciparum methyltransferase, maintains the active var gene in a poised state during parasite division. Cell Host Microbe 11, 7–18 [DOI] [PubMed] [Google Scholar]
- 40.Zhang Q., Huang Y., Zhang Y., Fang X., Claes A., Duchateau M., Namane A., Lopez-Rubio J. J., Pan W., Scherf A. (2011) A critical role of perinuclear filamentous actin in spatial repositioning and mutually exclusive expression of virulence genes in malaria parasites. Cell Host Microbe 10, 451–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hliscs M., Millet C., Dixon M. W., Siden-Kiamos I., McMillan P., Tilley L. (2015) Organization and function of an actin cytoskeleton in Plasmodium falciparum gametocytes. Cell. Microbiol. 17, 207–225 [DOI] [PubMed] [Google Scholar]
- 42.Regev-Rudzki N., Wilson D. W., Carvalho T. G., Sisquella X., Coleman B. M., Rug M., Bursac D., Angrisano F., Gee M., Hill A. F., Baum J., Cowman A. F. (2013) Cell-cell communication between malaria-infected red blood cells via exosome-like vesicles. Cell 153, 1120–1133 [DOI] [PubMed] [Google Scholar]
- 43.Baum J., Papenfuss A. T., Baum B., Speed T. P., Cowman A. F. (2006) Regulation of apicomplexan actin-based motility. Nat. Rev. Microbiol. 4, 621–628 [DOI] [PubMed] [Google Scholar]
- 44.Schutt C. E., Myslik J. C., Rozycki M. D., Goonesekere N. C., Lindberg U. (1993) The structure of crystalline profilin-beta-actin. Nature 365, 810–816 [DOI] [PubMed] [Google Scholar]
- 45.Kursula I., Kursula P., Ganter M., Panjikar S., Matuschewski K., Schüler H. (2008) Structural basis for parasite-specific functions of the divergent profilin of Plasmodium falciparum. Structure 16, 1638–1648 [DOI] [PubMed] [Google Scholar]
- 46.Stournaras C., Merkler I., Faulstich H. (1988) Thiol group reactivity and polymerization of actin in the presence of ATP analogs. Biochem. Biophys. Res. Commun. 155, 962–970 [DOI] [PubMed] [Google Scholar]
- 47.Stournaras C., Drewes G., Blackholm H., Merkler I., Faulstich H. (1990) Glutathionyl(cysteine-374) actin forms filaments of low mechanical stability. Biochim. Biophys. Acta 1037, 86–91 [DOI] [PubMed] [Google Scholar]
- 48.Drewes G., Faulstich H. (1993) Cooperative effects on filament stability in actin modified at the C-terminus by substitution or truncation. Eur. J. Biochem. 212, 247–253 [DOI] [PubMed] [Google Scholar]
- 49.Strzelecka-Gołaszewska H., Mossakowska M., Woźniak A., Moraczewska J., Nakayama H. (1995) Long-range conformational effects of proteolytic removal of the last three residues of actin. Biochem. J. 307, 527–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Orlova A., Egelman E. H. (1995) Structural dynamics of F-actin: I. Changes in the C terminus. J. Mol. Biol. 245, 582–597 [DOI] [PubMed] [Google Scholar]
- 51.O’Donoghue S. I., Miki M., dos Remedios C. G. (1992) Removing the two C-terminal residues of actin affects the filament structure. Arch. Biochem. Biophys. 293, 110–116 [DOI] [PubMed] [Google Scholar]
- 52.Crosbie R. H., Miller C., Cheung P., Goodnight T., Muhlrad A., Reisler E. (1994) Structural connectivity in actin: effect of C-terminal modifications on the properties of actin. Biophys. J. 67, 1957–1964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xia D., Peng I. (1995) Deletion of amino acids from the carboxy-terminal end of actin. Cell Motil. Cytoskeleton 32, 163–172 [DOI] [PubMed] [Google Scholar]
- 54.Johannes F. J., Gallwitz D. (1991) Site-directed mutagenesis of the yeast actin gene: a test for actin function in vivo. EMBO J. 10, 3951–3958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tsapara A., Kardassis D., Moustakas A., Gravanis A., Stournaras C. (1999) Expression and characterization of Cys374 mutated human beta-actin in two different mammalian cell lines: impaired microfilament organization and stability. FEBS Lett. 455, 117–122 [DOI] [PubMed] [Google Scholar]
- 56.Rommelaere H., De Neve M., Melki R., Vandekerckhove J., Ampe C. (1999) The cytosolic class II chaperonin CCT recognizes delineated hydrophobic sequences in its target proteins. Biochemistry 38, 3246–3257 [DOI] [PubMed] [Google Scholar]
- 57.Lee J. Y., Duan L., Iverson T. M., Dima R. I. (2012) Exploring the role of topological frustration in actin refolding with molecular simulations. J. Phys. Chem. B 116, 1677–1686 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.