Abstract
Vitamin A (VA) deficiency in HIV infection has been associated with more progressive HIV disease which may be enhanced by opioid use. In these studies were examined the effects of VA deficiency and morphine on frontal cortex neuronal numbers in the HIV-1 transgenic (Tg) rat. These studies showed that totals numbers of neurons were similar for rats on the VA deficient diet as for rats on the normal diet and these numbers were not affected by treatment with morphine. In contrast, numbers of neurons that expressed the calcium-binding protein parvalbumin, which is a marker interneurons that express the inhibitory neurotransmitter gamma-amino butyric acid (GABAergic neurons) were decreased for wild-type (Wt) rats on the VA deficient diet and for Wt rats treated with morphine. In addition, parvalbumin+ neurons were also decreased for Tg rats on a normal diet but increased to normal levels when these animals were placed on the VA deficient diet and treated with morphine. Analysis of expression of HIV regulatory proteins vif, vpr, and nef in frontal cortex and adjacent subcortical white matter showed that expression of these genes was increased in the Tg rat on the normal diet as compared to a control housekeeping gene. Morphine treatment suppressed expression of the HIV genes; however, expression was increased by VA deficiency and by morphine plus deficiency. These studies therefore suggest that VA deficiency, opioid and HIV infection alone and in combination may either suppress neuronal metabolic activity or induce metabolic stress, resulting in the observed changes in levels of parvalbumin expression. The specific mechanisms that underlie these effects require further study.
Introduction
Neurocognitive impairment is a frequent consequence of HIV infection which has remained common despite the availability of effective antiretroviral therapy (Cysique et al, 2004; Sacktor et al, 2002). HIV-infected opioid users have been shown to have an increased risk of developing neurocognitive and motor impairment not only related to the immunosuppression that occurs in the context of HIV infection but also as a consequence of specific effects of opioids (Bell et al, 1998; Bell, 2004; Perez-Casanova et al, 2007). VA deficiency has been also associated with the development of progressive HIV disease and among individuals with a history of drug use; such deficiency has been associated with lower CD4 counts and a higher mortality. The HIV Tg rat model, which incorporates a non-infectious viral genome that is under similar regulatory control mechanisms in vivo that exist with natural infection (Reid et al, 2001), demonstrates many of the clinical characteristic consequences of HIV infection in humans, including cognitive impairment (Cedeno-Laurent et al, 2009; Reid et al, 2001; Reid et al, 2004; Vigorito et al, 2007).
In brains from HIV infected patients, among the abnormalities that have been observed are cortical thinning with specific loss of neurons in area such as the orbital-frontal cortex and changes in neuronal morphology that correlates with the severity of HIV encephalitis (Masliah et al, 1992; Wiley et al, 1991). In brains from individuals with a history of methamphetamine abuse there has been noted to be a decrease in numbers of neurons that express parvalbumin, a calcium-binding protein that is produced by populations of interneurons that also express the inhibitory neurotransmitter gamma-amino butyric acid (GABA). (Langford et al, 2003). Neurons that express parvalbumin are “fast-spiking” neurons which are thought to promote synchronized electrical activity of primary neuronal cortical output through the formation of networks of chemical synapses and electrical gap junctions (Benes and Berretta, 2001; Gibson et al, 1999). In the cerebral cortex, inhibitory synapses from these interneurons onto the dendrites, proximal axons and soma of primary neurons modulate the electrical activity of these cells (Benes and Berretta, 2001), and loss of such inputs in specific cortical areas may underlie the development of impaired cognitive performance (Lewis and Moghaddam, 2006). In this report we describe studies in which Tg and control rats on a normal or a VA deficient diet were studied for effects of morphine on the numbers of neurons that express parvalbumin. These studies suggest that specific interactions may occur that result in changes in the numbers of these neurons which may potentially impact on cellular function and underlie symptoms that may be associated with HIV related neurocognitive impairment disease in humans.
Results
Analysis of NeuN+ and Parvalbumin+ Neurons
On inspection of the brains from the Tg and Wt rat groups there was no difference in gross appearance or in measures of cortical thickness (data not shown). Staining for NeuN showed an overall pattern in cortex that was similar for the rats in the various groups (figure 1). Quantitative analysis of NeuN+ cells in frontal cortex revealed no within or between group differences in the mean number of stained cells for Tg and Wt rats (figure 2).
Figure 1.
Immunoperoxidase staining (a=10x, b=40x magnification) of NeuN+ neurons in frontal cortex.
Figure 2.
Quantitative analysis of NeuN+ staining in frontal cortex.
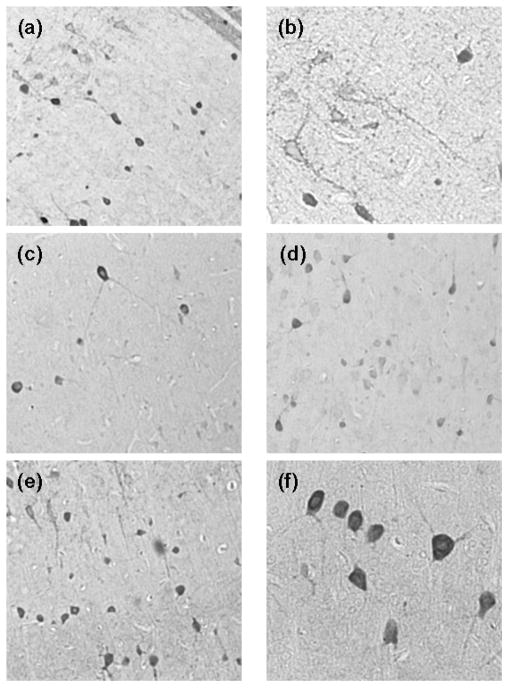
Analysis of the sections stained for parvalbumin revealed cells with rounded or oval-shaped cell bodies, frequently with processes which gave the cell the appearance of small and large basket cells (figure 3). Also seen were outlines of primary cell soma, which result from the formation of synapses formed by processes from basket cells onto primary neuronal cell bodies and on dendrites. Quantitative analysis of the staining showed that the mean number of parvalbumin+ cells was lower for the untreated Tg rats on the normal diet than for the corresponding Wt rat group (figure 4). For the Wt rats, administration of either the VA deficient diet or morphine resulted in an overall decrease in mean numbers of cortical parvalbumin+ cells. However, the mean number of parvalbumin+ cells for the Wt rats with both VA deficiency and morphine exposure showed no difference as compared to numbers for Wt rats on the normal diet. In contrast, for the Tg rats, mean numbers of stained cells increased in the presence of the VA deficient diet and with morphine treatment (figure 4), suggesting an effect by these factors on neuronal parvalbumin expression.
Figure 3.
Immunoperoxidase staining for parvalbumin in frontal cortex from (a, b) an untreated WT rat on a normal diet, (c) an untreated WT rat on a VA deficient diet, (d) an untreated TG rat on a normal diet, and (e, f) a morphine-treated TG rat on a VA deficient diet. Magnification: a, c, d, and e = 10x; b and f = 20x.
Figure 4.
Quantitative analysis of parvalbumin+ staining in frontal cortex.
HIV-1 Regulatory Protein Gene Expression
Tissue blocks form frontal brain cortex and adjacent subcortical white matter from Tg rats were analyzed for expression of the HIV-1 vif, nef, and rev regulatory protein genes. Shown are the results obtained from a rats studied a single experiment. These studies showed an increase in expression vif, nef, and rev gene expression relative to the housekeeping gene for all groups except for the Wt rat treated with morphine (figure 5).
Figure 5.
Analysis of HIV-1 gene expression in frontal cortex of TG rats on the normal diet or the VA deficient diet treated with placebo or morphine.
Discussion
In humans with HIV infection, characteristic neuropathological findings include the presence of marked inflammation with collections of microglial nodules, multinucleated giant cells, lipid-containing macrophages, and inflammatory cells that stain positive for HIV p24 antigen, with such findings being more prominent for individuals with a history of drug use (Anthony and Bell, 2008). These abnormalities are commonly associated with damage to both neuronal and glial elements (Anthony and Bell, 2008; Wiley et al, 1991). In the HIV transgenic rat the viral proteins are present from birth and, therefore, do not elicit an immune response. However, the viral proteins tat, gp120 and nef are capable of exerting direct toxic effects on neurons and glia and such effects can be enhanced by morphine (Bruce-Keller et al, 2008; Giunta et al, 2009; Gurwell et al, 2001; Hauser et al, 2009).
The studies reported here demonstrate that changes in parvalbumin+ neuronal cell numbers can occur in this model in the context of the presence of the HIV transgene, exposure to morphine, and the induction of VA deficiency. Parvalbumin is a calcium-binding protein which is expressed by interneurons that also express gamma-aminobutyric acid (GABA), an inhibitory neurotransmitter which regulates neuronal excitability in the central nervous system (Gerfen et al, 1985). The cortical interneuron cell types include the so-named large and small basket cells, chandelier cells, double bouquet cells and tuft cells. Of this group, only the basket cells and the chandelier cells express parvalbumin (a small percentage of basket cells also express calbindin, which is also a calcium-binding protein). The basket cells project axons that terminate on target cell bodies and receive ascending input from the striatum, making these cells candidates for the establishment of center-surround fields. In contrast, the chandelier cell axons project axonal branches that are oriented at right angles to the cell body and terminate on the initial segments of pyramidal cell axons. With immunocytochemical labeling the stained synapse give the appearance of a “candle.” Both cell types form inhibitory synapses, with the chandelier cell inducing the most prominent inhibitory effect on target cell output.
Parvalbumin expression is regulated by intracellular calcium levels and its synthesis is transcriptionally activated by cyclic AMP. It has also been shown that parvalbumin expression is increased as a result of NMDA receptor activation, which also increases expression of the 67 kDa form of the GABA-synthesizing enzyme, glutamic acid decarboxylase 67 (GAD67) (Cochran et al, 2002; Keilhoff et al, 2004), in association with activation of the Erk1/2 protein kinases and the transcription factor CREB (Kinney et al, 2006). This effect on parvalbumin expression is inhibited at increased levels of calcium (Kinney et al, 2006), and in studies by Potter et al it was shown that parvalbumin can activate cyclic AMP phosphodiesterase in a calcium-dependent manner (Potter et al, 1977), providing feedback mechanisms through which parvalbumin synthesis can be further regulated. Acute morphine exposure has been shown to down-regulate adenyl cyclase activity (Sharma et al, 1975; Traber et al, 1975), thereby producing decreased intracellular cAMP levels, but with repeated or chronic exposure such activity normalizes and will increase above baseline levels in association upon morphine withdrawal (Benalal and Bachrach, 1985; Sharma et al, 1975; Traber et al, 1975). In mice, symptoms that occur with morphine withdrawal can be prevented by cyclic AMP analogues and phosphodiesterase inhibitors, which may act by blocking the decrease in cyclic AMP levels that occur with initial morphine exposure (Mamiya et al, 2001). In our studies the rats were exposed to morphine for 5 days, at which time chronic effects from exposure begin to appear. Therefore, in our studies, the observed changes in the numbers of parvalbumin+ neurons in the Tg and Wt rats may have resulted from concordant changes in levels of intracellular cAMP. In addition to the observed effects from morphine exposure, levels of parvalbumin increased in the Tg rat in the presence of VA deficiency. The mechanisms that are responsible for this effect are not clear at this time since retinoids can either increase or decrease cyclic AMP levels (Hohmann and Greene, 1990). On the other hand, in studies of HIV nephropathy all-trans retinoic acid and 9-cis retinoic acid increased cyclic AMP levels in HIV-infected podocytes (He et al, 2007).
The HIV accessory proteins examined in this report, through a variety of distinct mechanisms, function by either enhancing HIV infectivity (vpr) or replication capacity (nef and vif) (Trono, 1995). Neurons exposed to vpr and nef in vitro have been shown to be toxic to neurons cultured in vitro. In the case of vpr, toxicity results from induction of apoptosis through activation of caspase 8 and by the formation of ion channels in the plasma membrane with the subsequent development membrane depolarization and cell death. Nef can also induce activation of caspases and, purportedly as a result of the proteins structural and functional similarity to scorpion peptides, can aberrantly activate potassium channels. Vif (viral inhibitory factor) enhances the infectivity of viral particles by forming complexes with APOBEC3G (apolipoprotein B mRNA-editing enzyme, catalytic polypeptide-like 3G), which converts cytosine nucleosides present in the viral minus strand reverse transcript to uracil, rendering the viral particle non-infectious (Mangeat et al, 2003; Sheehy et al, 2002). APOBEC3G is ordinarily encapsulated within the viral particle, but this packaging is prevented when the enzyme is complexed with vif. Previous studies have demonstrated that morphine and other opioids promote immune cell replication of HIV (Peterson et al, 1990; Peterson et al, 1993; Peterson et al, 1994). The endogenous opioid endomrophin-1 was shown to increase HIV replication by infected human fetal mixed neuronal/glial cells in culture (Peterson et al, 1999) and this effect was blocked by specific mu opioid receptor antagonism. However, consistent with our findings here in the untreated Tg rat on the normal diet, neither morphine nor the specific mu opioid receptor agonist DAMGO increased HIV expression in the cultures. In fact, the presence of elevated expression of the HIV genes in the Wt rat on a normal diet in our studies suggests that morphine actually decreases expression of these genes. Also implied by the results presented in this report is that VA deficiency, in addition to promoting HIV regulatory gene expression, may reverse the suppressive effect of morphine. Confirmation of these hypotheses and the above observations and whether morphine can have similar effects on the replication of other HIV proteins, such as gp120 and tat will need to be examined in future studies.
It is possible that the observed changes in the numbers of parvalbumin+ cortical neuronal cells for the Tg and Wt rats in the various dietary and treatment groups were due to alterations in levels of expression of the protein that would render the cells more or less detectible. In previous studies by Maharajan et al it was shown that morphine administered to female mice for 1 week prior to the mice being mated, during gestation and for 3 weeks post-partum, during which the offspring were allowed to suckle, resulted in increased numbers of parvalbumin+ neurons in layers II–V of parietal cortex I of the offspring (Maharajan et al, 2000). These results are similar to the observations made in the Tg rats and opposite what was observed in the Wt rat group. However, parvalbumin, like the other calcium-binding proteins calbindin and calretinin, can regulate levels of free intracellular calcium and therefore cellular functions such as synaptic transmission and apoptosis. These calcium-binding function of the proteins can have important neuroprotective effects during periods of excessive neuronal excitation, as can occur in experimental models of epilepsy (Schwaller et al, 2004), brain ischemia (Gerstein et al, 2005), and trauma (Dekkers et al, 2002). In the setting of HIV infection, elevated levels of parvalbumin expression may potentially provide protection from toxicity that can be induced by HIV proteins with such protective effects potentially enhanced by morphine. Conversely, decreased parvalbumin expression may reflect a low metabolic state for the cells, which could potentially occur in association with a number of different scenarios. A better understanding of these potential effects of parvalbumin can be delineated in studies that examine the underlying mechanisms that are involved and in studies that will examine their functional consequences.
Materials and methods
Animals
All experiments were performed using 3–6 month old specific pathogen free Tg and age-matched Wt Fisher 344/NHsd control rats. The details on the construction of the HIV-1 Tg rat have been previously described (Reid et al, 2001). The rats Tg and Wt rats were administered a diet previously used to induce VA deficiency in mice (Carman and Hayes, 1991), except that the rats were fed the Bio-Serv AIN-93M rodent maintenance diet (Bio-Serv; Frenchtown, NJ), which contains 400,000 IU/kg of retinyl palmitate, the major dietary form of VA, or the same diet mix formulated minus retinyl palmitate. Female rats maintained on the normal maintenance diet were mated then randomly divided into two groups at 2 wks gestation. One group of pregnant females was subsequently fed a VA deficient diet and the other was fed the VA-sufficient diet. Weanlings were maintained on the same diets as their dams. For collection of blood, the rats were anesthetized using a combination of 60mg/kg Ketamine and 7.5 mg/kg xylazine and blood was removed by capillary stick from the cavernous sinus. All studies were approved by the University of Maryland Biotechnology Institute of the University of Maryland, Baltimore Animal Care and Use Committee.
Treatments
75 mg morphine tablets and placebo control tablets were obtained through the NIDA Drug Supply Program. Tg and Wt control rats were anesthetized with Ketamine then implanted subcutaneously with initially either a whole morphine or placebo tablet. Subsequently, with the occurrence of unanticipated deaths in Tg rats implanted with the morphine 75 mg tablet, the morphine dose was decreased to 37.5 mg (one half tablet). The rats were then observed for 7 days and then sacrificed using Ketamine inhalation, perfused with 1% paraformaldehyde, and the brains then removed, fixed in 4% paraformaldehyde and embedded with paraffin for subsequent immunocytochemical analysis.
Immunocytochemical Studies
Parvalbumin
The tissue sections were deparaffinized and hydrated through xylene and graded alcohol then rinse for 5 min in tap water. The slides were then incubated with 0.3% H2O2 in methanol for 30min then wash in phosphate buffered saline (PBS) for 5min. Antigen retrieval was performed by incubating in 10mM citrate buffer pH 6.0. The slides were then washed in cold water then the sections incubated with blocking solution (10% horse serum in PBS + 0.1% Triton-X) for 30 min. The slides were then washed and incubated with blocking solution for 1 hour then incubated overnight at 4 degrees Celsius with mouse anti-rat parvalbumin, (SWant, cat#235) diluted 1:50,000 in PBS. The following day the slides were washed three times with PBS then incubated for 1 hour with horse anti-mouse antibody diluted 1:500 with blocking solution (Vectastain ABC kit, cat# PK-4002), washed, incubated with AB reagent and diaminobenzadine and, after processing through graded alcohols and xylene coverslipped.
NeuN
The sections were deparaffinized and incubated through graded alcohols, xylene, methanol, antigen retrieval buffer as described above, and washed as described above. NeuN staining was performed by incubating overnight at 4 degrees Celsius with mouse anti-NeuN (US Biological cat# N2173) antibody diluted 1:500 dilution in PBS. The slides were then washed with PBS for 5 min and then incubated for 1 hr with anti-mouse IgG (Vectastain ABC kit peroxidase Mouse IgG, cat# 4002) diluted 1:200 in blocking serum. The slides were then washed in PBS for 5 min and incubated for 30min. with AB reagent, washed, then incubated with diaminobenzadine as recommended by the manufacturer (Vector Laboratories). The slides were then rinsed, dehydrated and coverslipped.
Quantitative Analysis
Frontal cortex parvalbumin+ cells in four randomly-selected, non-overlapping 10X fields that spanned the cortical region were imaged and manually counted using ImageJ software. Sections were analyzed from three rats per group. For NeuN+ cell quantitation, representative 10X fields were similarly counted and compared.
Polymerase Chain Reaction Assays
RNA extracted from thawed blocks of frontal cortical tissue that had been snap-frozen at the time of necropsy was examined in real-time PCR assays for HIV-1 vpr, nef, and vif gene expression. The primers that were utilized were as follows: vpr: forward – GCAGGAGTGGAAGCCATAAT, reverse – TACTGGCTCCATTTCTTGCTC; nef: forward – GCTAACAATGCTGCTTGTGC, reverse – TGAATTAGCCCTTCCAGTCC; vif: forward – ATTGTGTGGCAAGTAGACAGGATGA, reverse – CTAGTGGGATGTGTACTTCTAAACT, and, as an internal control, the ribosomal protein RPL13A (forward – GCCTACCAGAAAGTTTGC; reverse – CCAAGAGTCCATTGGTCTTG). The thawed tissue was homogenized using Qiagen Qiashredder columns (Qiagen) then subsequently isolated using RNeasy mini-columns (Qiagen) according to the manufacturer’s protocol. Residual contaminating DNA was digested using the RNase-Free DNase set (Qiagen), also as per the manufacturer’s protocol. Synthesis of cDNA was performed using the Reverse Transcription system (Promega; Madison, WI) Reactions were carried out in volumes of 20 μl per sample, to which were added 1 μg of template RNA, dNTPs, oligo-dT primer, RNase inhibitor, reverse transcriptase enzyme, and RNase-free water. The cDNA were synthesized at 37 degrees Celsius for 60 min. Real-time PCR of cDNA were performed using an iCycler (BioRad; Hercules, CA) with detection of double-stranded PCR product using SYBR Green (Sigma; St. Louis, MO). The following iCycler settings were utilized for the primer pairs: 3 min at 95 degrees Celsius followed by amplification at 95 degrees Celsius for 30 sec, then 30 seconds at 58 degrees Celsius for 35 cycles. SYBR Green detection was performed during each amplification cycle at 72 degrees Celsius. Following amplification, a melt curve was performed and analyzed to confirm the absence of primer-dimer formation. Relative gene expression was expressed at the ΔCt.
Statistical Analyses
Neuronal cell count data were analyzed using a between-group ANOVA to evaluate the difference between the untreated and morphine treated TgVA+, TgVA−, WtVA−, and WtVA+ groups followed by a post-hoc between group comparison using the Neuman-Keuls Test.
References
- 1.Anthony IC, Bell JE. The Neuropathology of HIV/AIDS. Int Rev Psychiatry. 2008;20:15–24. doi: 10.1080/09540260701862037. [DOI] [PubMed] [Google Scholar]
- 2.Bell JE. An update on the neuropathology of HIV in the HAART era. Histopathology. 2004;45:549–559. doi: 10.1111/j.1365-2559.2004.02004.x. [DOI] [PubMed] [Google Scholar]
- 3.Bell JE, Brettle RP, Chiswick A, Simmonds P. HIV encephalitis, proviral load and dementia in drug users and homosexuals with AIDS. Effect of neocortical involvement. Brain. 1998;121:2043–2052. doi: 10.1093/brain/121.11.2043. [DOI] [PubMed] [Google Scholar]
- 4.Benalal D, Bachrach U. Opiates and cultured neuroblastoma x glioma cells. Effect on cyclic AMP and polyamine levels and on ornithine decarboxylase and protein kinase activities. Biochem J. 1985;227:389–395. doi: 10.1042/bj2270389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benes FM, Berretta S. GABAergic interneurons: implications for understanding schizophrenia and bipolar disorder. Neuropsychopharmacology. 2001;25:1–27. doi: 10.1016/S0893-133X(01)00225-1. [DOI] [PubMed] [Google Scholar]
- 6.Bruce-Keller AJ, Turchan-Cholewo J, Smart EJ, Geurin T, Chauhan A, Reid R, Xu R, Nath A, Knapp PE, Hauser KF. Morphine causes rapid increases in glial activation and neuronal injury in the striatum of inducible HIV-1 Tat transgenic mice. Glia. 2008;56:1414–1427. doi: 10.1002/glia.20708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carman JA, Hayes CE. Abnormal regulation of IFN-gamma secretion in vitamin A deficiency. J Immunol. 1991;147:1247–1252. [PubMed] [Google Scholar]
- 8.Cedeno-Laurent F, Bryant J, Fishelevich R, Jones OD, Deng A, Eng ML, Gaspari AA, Trujillo JR. Inflammatory papillomatous hyperplasia and epidermal necrosis in a transgenic rat for HIV-1. J Dermatol Sci. 2009;53:112–119. doi: 10.1016/j.jdermsci.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cochran SM, Fujimura M, Morris BJ, Pratt JA. Acute and delayed effects of phencyclidine upon mRNA levels of markers of glutamatergic and GABAergic neurotransmitter function in the rat brain. Synapse. 2002;46:206–214. doi: 10.1002/syn.10126. [DOI] [PubMed] [Google Scholar]
- 10.Cysique LA, Maruff P, Brew BJ. Prevalence and pattern of neuropsychological impairment in human immunodeficiency virus-infected/acquired immunodeficiency syndrome (HIV/AIDS) patients across pre- and post-highly active antiretroviral therapy eras: a combined study of two cohorts. J Neurovirol. 2004;10:350–357. doi: 10.1080/13550280490521078. [DOI] [PubMed] [Google Scholar]
- 11.Dekkers J, Greensmith L, Navarrete R. Changes in the expression of parvalbumin immunoreactivity in the lumbar spinal cord of the rat following neonatal nerve injury. Dev Neurosci. 2002;24:283–293. doi: 10.1159/000066742. [DOI] [PubMed] [Google Scholar]
- 12.Gerfen CR, Baimbridge KG, Miller JJ. The neostriatal mosaic: compartmental distribution of calcium-binding protein and parvalbumin in the basal ganglia of the rat and monkey. Proc Natl Acad Sci U S A. 1985;82:8780–8784. doi: 10.1073/pnas.82.24.8780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gerstein M, Huleihel M, Mane R, Stilman M, Kashtuzki I, Hallak M, Golan H. Remodeling of hippocampal GABAergic system in adult offspring after maternal hypoxia and magnesium sulfate load: immunohistochemical study. Exp Neurol. 2005;196:18–29. doi: 10.1016/j.expneurol.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 14.Gibson JR, Beierlein M, Connors BW. Two networks of electrically coupled inhibitory neurons in neocortex. Nature. 1999;402:75–79. doi: 10.1038/47035. [DOI] [PubMed] [Google Scholar]
- 15.Giunta B, Hou H, Zhu Y, Rrapo E, Tian J, Takashi M, Commins D, Singer E, He J, Fernandez F, Tan J. HIV-1 Tat Contributes to Alzheimer’s Disease-like Pathology in PSAPP Mice. Int J Clin Exp Pathol. 2009;2:433–443. [PMC free article] [PubMed] [Google Scholar]
- 16.Gurwell JA, Nath A, Sun Q, Zhang J, Martin KM, Chen Y, Hauser KF. Synergistic neurotoxicity of opioids and human immunodeficiency virus-1 Tat protein in striatal neurons in vitro. Neuroscience. 2001;102:555–563. doi: 10.1016/s0306-4522(00)00461-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hauser KF, Hahn YK, Adjan VV, Zou S, Buch SK, Nath A, Bruce-Keller AJ, Knapp PE. HIV-1 Tat and morphine have interactive effects on oligodendrocyte survival and morphology. Glia. 2009;57:194–206. doi: 10.1002/glia.20746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He JC, Lu TC, Fleet M, Sunamoto M, Husain M, Fang W, Neves S, Chen Y, Shankland S, Iyengar R, Klotman PE. Retinoic acid inhibits HIV-1-induced podocyte proliferation through the cAMP pathway. J Am Soc Nephrol. 2007;18:93–102. doi: 10.1681/ASN.2006070727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hohmann P, Greene RS. Retinoid induced changes in cAMP-dependent protein kinase activity detected by a new minigel assay. FEBS Lett. 1990;261:81–84. doi: 10.1016/0014-5793(90)80641-u. [DOI] [PubMed] [Google Scholar]
- 20.Keilhoff G, Becker A, Grecksch G, Wolf G, Bernstein HG. Repeated application of ketamine to rats induces changes in the hippocampal expression of parvalbumin, neuronal nitric oxide synthase and cFOS similar to those found in human schizophrenia. Neuroscience. 2004;126:591–598. doi: 10.1016/j.neuroscience.2004.03.039. [DOI] [PubMed] [Google Scholar]
- 21.Kinney JW, Davis CN, Tabarean I, Conti B, Bartfai T, Behrens MM. A specific role for NR2A-containing NMDA receptors in the maintenance of parvalbumin and GAD67 immunoreactivity in cultured interneurons. J Neurosci. 2006;26:1604–1615. doi: 10.1523/JNEUROSCI.4722-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Langford D, Adame A, Grigorian A, Grant I, McCutchan JA, Ellis RJ, Marcotte TD, Masliah E. Patterns of selective neuronal damage in methamphetamine-user AIDS patients. J Acquir Immune Defic Syndr. 2003;34:467–474. doi: 10.1097/00126334-200312150-00004. [DOI] [PubMed] [Google Scholar]
- 23.Lewis DA, Moghaddam B. Cognitive dysfunction in schizophrenia: convergence of gamma-aminobutyric acid and glutamate alterations. Arch Neurol. 2006;63:1372–1376. doi: 10.1001/archneur.63.10.1372. [DOI] [PubMed] [Google Scholar]
- 24.Maharajan P, Prencipe R, Di Francesco P, Paino G, Ravagnan G, Maharajan V. Maternal morphine alters parvalbumin immunoreactivity patterns in neonatal mouse brain. Synapse. 2000;35:265–271. doi: 10.1002/(SICI)1098-2396(20000315)35:4<265::AID-SYN4>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 25.Mamiya T, Noda Y, Ren X, Hamdy M, Furukawa S, Kameyama T, Yamada K, Nabeshima T. Involvement of cyclic AMP systems in morphine physical dependence in mice: prevention of development of morphine dependence by rolipram, a phosphodiesterase 4 inhibitor. Br J Pharmacol. 2001;132:1111–1117. doi: 10.1038/sj.bjp.0703912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mangeat B, Turelli P, Caron G, Friedli M, Perrin L, Trono D. Broad antiretroviral defence by human APOBEC3G through lethal editing of nascent reverse transcripts. Nature. 2003;424:99–103. doi: 10.1038/nature01709. [DOI] [PubMed] [Google Scholar]
- 27.Masliah E, Ge N, Achim CL, Hansen LA, Wiley CA. Selective neuronal vulnerability in HIV encephalitis. J Neuropathol Exp Neurol. 1992;51:585–593. doi: 10.1097/00005072-199211000-00003. [DOI] [PubMed] [Google Scholar]
- 28.Perez-Casanova A, Noel RJ, Jr, Rivera-Amill V, Husain K, Kumar A. Morphine-mediated deterioration of oxidative stress leads to rapid disease progression in SIV/SHIV-infected macaques. AIDS Res Hum Retroviruses. 2007;23:1004–1007. doi: 10.1089/aid.2006.0286. [DOI] [PubMed] [Google Scholar]
- 29.Peterson PK, Gekker G, Hu S, Anderson WR, Kravitz F, Portoghese PS, Balfour HH, Jr, Chao CC. Morphine amplifies HIV-1 expression in chronically infected promonocytes cocultured with human brain cells. J Neuroimmunol. 1994;50:167–175. doi: 10.1016/0165-5728(94)90043-4. [DOI] [PubMed] [Google Scholar]
- 30.Peterson PK, Gekker G, Hu S, Lokensgard J, Portoghese PS, Chao CC. Endomorphin-1 potentiates HIV-1 expression in human brain cell cultures: implication of an atypical mu-opioid receptor. Neuropharmacology. 1999;38:273–278. doi: 10.1016/s0028-3908(98)00167-1. [DOI] [PubMed] [Google Scholar]
- 31.Peterson PK, Gekker G, Schut R, Hu S, Balfour HH, Jr, Chao CC. Enhancement of HIV-1 replication by opiates and cocaine: the cytokine connection. Adv Exp Med Biol. 1993;335:181–188. doi: 10.1007/978-1-4615-2980-4_26. [DOI] [PubMed] [Google Scholar]
- 32.Peterson PK, Sharp BM, Gekker G, Portoghese PS, Sannerud K, Balfour HH., Jr Morphine promotes the growth of HIV-1 in human peripheral blood mononuclear cell cocultures. AIDS. 1990;4:869–873. doi: 10.1097/00002030-199009000-00006. [DOI] [PubMed] [Google Scholar]
- 33.Potter JD, Dedman JR, Means AR. Ca2+-dependent regulation of cyclic-AMP phosphodiesterase by parvalbumin. J Biol Chem. 1977;252:5609–5611. [PubMed] [Google Scholar]
- 34.Reid W, Abdelwahab S, Sadowska M, Huso D, Neal A, Ahearn A, Bryant J, Gallo RC, Lewis GK, Reitz M. HIV-1 transgenic rats develop T cell abnormalities. Virology. 2004;321:111–119. doi: 10.1016/j.virol.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 35.Reid W, Sadowska M, Denaro F, Rao S, Foulke J, Jr, Hayes N, Jones O, Doodnauth D, Davis H, Sill A, O’Driscoll P, Huso D, Fouts T, Lewis G, Hill M, Kamin-Lewis R, Wei C, Ray P, Gallo RC, Reitz M, Bryant J. An HIV-1 transgenic rat that develops HIV-related pathology and immunologic dysfunction. Proc Natl Acad Sci U S A. 2001;98:9271–9276. doi: 10.1073/pnas.161290298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sacktor N, McDermott MP, Marder K, Schifitto G, Selnes OA, McArthur JC, Stern Y, Albert S, Palumbo D, Kieburtz K, De Marcaida JA, Cohen B, Epstein L. HIV-associated cognitive impairment before and after the advent of combination therapy. J Neurovirol. 2002;8:136–142. doi: 10.1080/13550280290049615. [DOI] [PubMed] [Google Scholar]
- 37.Schwaller B, Tetko IV, Tandon P, Silveira DC, Vreugdenhil M, Henzi T, Potier MC, Celio MR, Villa AE. Parvalbumin deficiency affects network properties resulting in increased susceptibility to epileptic seizures. Mol Cell Neurosci. 2004;25:650–663. doi: 10.1016/j.mcn.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 38.Sharma SK, Nirenberg M, Klee WA. Morphine receptors as regulators of adenylate cyclase activity. Proc Natl Acad Sci U S A. 1975;72:590–594. doi: 10.1073/pnas.72.2.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sheehy AM, Gaddis NC, Choi JD, Malim MH. Isolation of a human gene that inhibits HIV-1 infection and is suppressed by the viral Vif protein. Nature. 2002;418:646–650. doi: 10.1038/nature00939. [DOI] [PubMed] [Google Scholar]
- 40.Traber J, Gullis R, Hamprecht B. Influence of opiates on the levels of adenosine 3′:5′-cyclic monophosphate in neuroblastoma X glioma hybrid cells. Life Sci. 1975;16:1863–1868. doi: 10.1016/0024-3205(75)90292-1. [DOI] [PubMed] [Google Scholar]
- 41.Trono D. HIV accessory proteins: leading roles for the supporting cast. Cell. 1995;82:189–192. doi: 10.1016/0092-8674(95)90306-2. [DOI] [PubMed] [Google Scholar]
- 42.Vigorito M, Lashomb AL, Chang SL. Spatial learning and memory in HIV-1 transgenic rats. J Neuroimmune Pharmacol. 2007;2:319–328. doi: 10.1007/s11481-007-9078-y. [DOI] [PubMed] [Google Scholar]
- 43.Wiley CA, Masliah E, Morey M, Lemere C, DeTeresa R, Grafe M, Hansen L, Terry R. Neocortical damage during HIV infection. Ann Neurol. 1991;29:651–657. doi: 10.1002/ana.410290613. [DOI] [PubMed] [Google Scholar]