Abstract
Mycophenolate is often used in the management of systemic lupus erythematosus. It has often been associated with significant fetal embryopathy, including fetal loss and multiple anomalies. The Food and Drug Administration has directed that women should be counseled regarding this prior to initiating treatment with this drug. Isolated total anomalous pulmonary venous return (TAPVR) is a rare association seen with its use in pregnancy.
Keywords: mycophenolate mofetil, mycophenolate sodium, teratogen, embryopathy, congenital anomalies
Introduction
Mycophenolate mofetil (MMF) and Mycophenolate sodium (MFS) are immunosuppressive agents primarily used in preventing rejection after organ transplant.1 2 They are increasingly used in treatment of autoimmune disorders such as systemic lupus erythematosus (SLE).1 When administered during pregnancy, it can cause embryopathy with first-trimester pregnancy loss and congenital malformations, including limb and organ systems anomalies, microtia, and cleft lip and palate.1 3 4 Although these drugs have revolutionized the treatment of organ transplant and rheumatologic disorders, there are serious risks associated with its use. To manage these risks, several initiatives have been taken, and since 2012 the U.S. Food and Drug Administration (FDA) has required all manufacturers to develop a risk evaluation and mitigation strategy (REMS) for all mycophenolate-containing drugs.2 5 6 However, the information is still not widely disseminated, and we report a case of an isolated major congenital cardiac anomaly associated with MMF use during pregnancy.
Case Summary
A preterm male infant was born at 29 weeks' gestation to a 21-year-old Hispanic woman. The maternal history was relevant for SLE, which was diagnosed at the age of 11. One year prior to this delivery, she was noncompliant with her medications and needed admission to hospital, and was treated for deep vein thrombosis, bilateral pleural effusion, and lupus nephritis. During that episode, her antinuclear antibody and anti–double-stranded antibody titers were elevated at 1:160. After an intense initial management, she was maintained on MMF 1,000 mg twice daily, prednisone 10 mg/d, hydroxychloroquine 200 mg/d, low-molecular-weight heparin 65 mg twice daily and aspirin 81 mg once daily. Subsequently she visited an obstetrician for late prenatal care during the fourth month of pregnancy. Her prenatal screening tests were normal and she was continued on her maintenance medications except for a lowering of the dose of MMF to 500 mg twice daily. During her pregnancy, she had chronic hypertension and focal segmental glomerulonephritis. However, she had premature labor and went on to deliver this infant, who did not require significant resuscitation apart from positive airway pressure.
His birth weight was 1295 g (50th percentile), length was 38 cm (50th percentile), and head circumference was 29.5 cm (75th percentile). Gestational age was assessed at 29 weeks and correlated with the maternal obstetric data. There were no signs of dysmorphism or external congenital anomalies. Apart from a cardiac murmur and cardiomegaly on chest radiology, the rest of the physical examination at birth was normal. An echocardiogram revealed mild tricuspid regurgitation, right ventricular hypertrophy, patent ductus arteriosus, atrial septal defect, and total anomalous pulmonary venous return (TAPVR) to a dilated coronary sinus without evidence of pulmonary venous obstruction (Figs. 1, 2). Bubble studies done later did not show evidence of bilateral superior vena cava. The patient had 46 XY karyotype and microdeletion for 22q11.2 was not present.
Fig. 1.
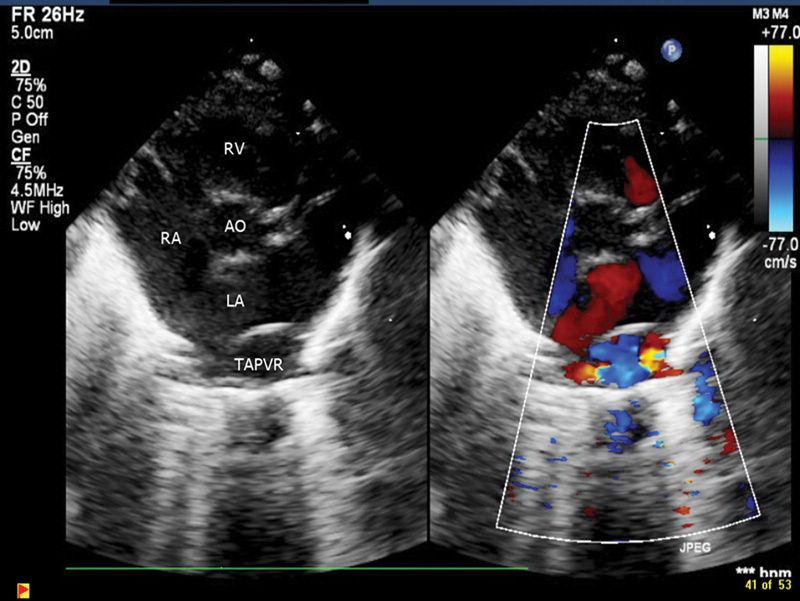
Cardiac ECHO short-axis 2D and color Doppler of TAPVR.
Fig. 2.
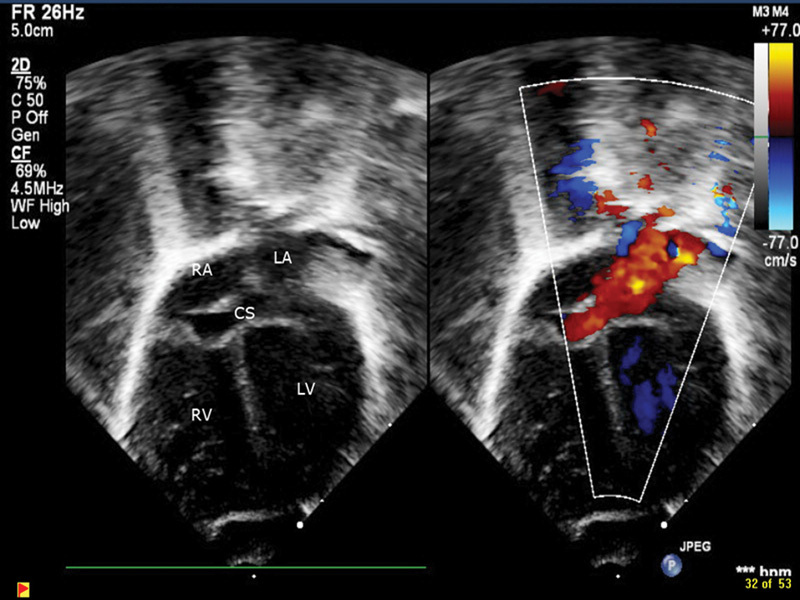
Echocardiograph subcostal four-chamber 2D view along with color Doppler showing TAPVR.
The patient also had bilateral small grade II intraventricular hemorrhages (IVH) that resolved. The patient had an uneventful course in hospital with brief noninvasive respiratory support and maintained optimal growth. He had repair of his TAPVR and ligation of ductus arteriosus at 2 months of age and had an unremarkable postoperative course. At his last follow-up at a corrected age of 18 months, he was scored on the 50th percentile for cognitive, receptive, gross, and fine motor on a Bayley III assessment.
Discussion
MMF is increasingly used as a steroid-sparing treatment in immune-mediated disorders such as Behçet's disease, pemphigus vulgaris, lupus nephritis, immunoglobulin A nephropathy, small vessel vasculitis, psoriasis, and after transplants.7 In the body, it is hydrolyzed to the two-morpholinoethyl ester of mycophenolic acid (MPA), which is the active metabolite. MPA is a reversible inhibitor of inosine monophosphate dehydrogenase and inhibits the pathway of guanosine nucleotide synthesis, thus preventing its incorporation into DNA.1
After the introduction of MMF in the mid 90s, the first case report of a congenital malformation in a child with hypoplastic nails and fifth finger, associated with maternal use, appeared in 2001.8 Subsequently a child with midline anomalies including agenesis of the corpus callosum, microtia with atresia of external auditory canal, and cleft lip and palate was reported.9 As its use became more frequent, more case reports started appearing in the last decade. Sifontis et al reported on 26 women who were on MMF during pregnancy and 4 out of the 15 live born children had congenital anomalies.10
Perez-Aytes et al described the spectrum of the phenotype seen in mycophenolate-associated embryopathy (MMFE). They included cleft lip and palate, microtia with atresia of external auditory canal, micrognathia, hypertelorism, ocular anomalies, corpus callosum agenesis, heart defects, kidney malformations, and diaphragmatic hernia.11 Ang et al postulated in a case with bilateral microtia chorioretinal and iris coloboma that MMFE could be dependent on the dosage and timing of its use.12
Complex congenital heart defects have been described earlier but only in association with other anomalies.13 14 15 In our patient, the cardiac anomaly was the only finding identified apart from prematurity and likely related IVH. This patient had a normal neurodevelopmental assessment at 18 months. The mother was on fairly high doses of MMF, prior to conception and during the entire pregnancy. We are not able to identify whether this cardiac anomaly occurred spontaneously or whether this is related to maternal medication. The complexity and relative infrequency of our finding points toward a possible association with use of MMF. The maternal SLE disease alone increases the baseline risk of prematurity. In this current patient the mother had been on multiple medications, but MMF had the highest teratogenic potential. Most previous reports have been of multiple malformations including, facial, ear, and other anomalies.
There have been attempts to calculate a quantitative risk assessment. The European Network of Teratology is a prospective database of pregnancy outcome after maternal therapy with MFM or MFS. Of 57 prospectively ascertained pregnancies identified, 45% resulted in spontaneous abortion. After first-trimester exposure the incidence of major malformations was 26% in the live born infants.1 The Organization of Teratology Information Specialists over an 8-year period followed prospectively 10 patients. Four resulted in a miscarriage, one had a therapeutic termination due to major congenital malformations, and five resulted in live born infants with no malformations and had normal development.16 The fetal outcome data from the United Kingdom national cohort of transplant recipients showed that seven out of nine MMF exposed pregnancies resulted in poor outcome as still birth, miscarriage, low birth weight, congenital anomaly, or prematurity less than 32 weeks.17 Martin reported on four newborns with esophageal atresia as a feature of MMFE even without the presence of other major craniofacial anomalies.18
In a review of the typical MMFE, 33% had a congenital heart disease, and of those, cono-truncal or aortic arch defects were the most common. They hypothesized that it could be the result of a disturbance of cranial neural crest migration in exposed infants. Several exposed infants have been reported to be minimally affected.15 A retrospective study of kidney transplant recipients showed that birth defects and miscarriages were similar among those who discontinued MPA less than 6 weeks prior to pregnancy and during the first trimester. Ironically, discontinuing MPA during the second trimester or later significantly increased the risk of miscarriages and birth defects.19
The drug's benefits may outweigh its risks in most people. However, it is important that all women of reproductive potential prior to being treated with MFS or MMF have proper education and counseling about pregnancy prevention. They are to be made aware of the increased risk of first-trimester pregnancy loss and congenital malformations. As per this heightened surveillance and risk mitigation strategy, physicians are required to obtain a signed patient-prescriber acknowledgment form from female patients. In addition, women should have a pregnancy test before starting MMF or MFA, subsequently after 8 to 10 days and repeated during routine follow-up office visits. Family planning options should be discussed during treatment and for 6 weeks after stopping mycophenolate-containing medications. The Mycophenolate REMS includes training for health care professionals and the establishment of a pregnancy registry for women who become pregnant during treatment or within 6 weeks following discontinuation.
Footnotes
Conflict of Interest None.
References
- 1.Hoeltzenbein M, Elefant E, Vial T. et al. Teratogenicity of mycophenolate confirmed in a prospective study of the European Network of Teratology Information Services. Am J Med Genet A. 2012;158A(3):588–596. doi: 10.1002/ajmg.a.35223. [DOI] [PubMed] [Google Scholar]
- 2.Cellcept product information http://www.gene.com/medical-professionals/medicines/cellcept. Accessed October 26, 2016
- 3.Coscia L A, Armenti D P, King R W, Sifontis N M, Constantinescu S, Moritz M J. Update on the teratogenicity of maternal mycophenolate mofetil. J Pediatr Genet. 2015;4(2):42–55. doi: 10.1055/s-0035-1556743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perez-Aytes A, Marin-Reina P, Boso V, Ledo A, Carey J C, Vento M. Mycophenolate mofetil embryopathy: a newly recognized teratogenic syndrome. Eur J Med Genet. 2016;7212(16):30318–30324. doi: 10.1016/j.ejmg.2016.09.014. [DOI] [PubMed] [Google Scholar]
- 5.Food and Drug Administration www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm148735.htm. Accessed October 26, 2016
- 6.Risk Evaluation and Mitigation Strategy https://www.mycophenolaterems.com. Accessed October 26, 2016
- 7.Chang C. Unmet needs in the treatment of autoimmunity: from aspirin to stem cells. Autoimmun Rev. 2014;13(04/05):331–346. doi: 10.1016/j.autrev.2014.01.052. [DOI] [PubMed] [Google Scholar]
- 8.Pérgola P E, Kancharla A, Riley D J. Kidney transplantation during the first trimester of pregnancy: immunosuppression with mycophenolate mofetil, tacrolimus, and prednisone. Transplantation. 2001;71(7):994–997. doi: 10.1097/00007890-200104150-00028. [DOI] [PubMed] [Google Scholar]
- 9.Le Ray C Coulomb A Elefant E Frydman R Audibert F Mycophenolate mofetil in pregnancy after renal transplantation: a case of major fetal malformations Obstet Gynecol 2004103(5 Pt 2):1091–1094. [DOI] [PubMed] [Google Scholar]
- 10.Sifontis N M, Coscia L A, Constantinescu S, Lavelanet A F, Moritz M J, Armenti V T. Pregnancy outcomes in solid organ transplant recipients with exposure to mycophenolate mofetil or sirolimus. Transplantation. 2006;82(12):1698–1702. doi: 10.1097/01.tp.0000252683.74584.29. [DOI] [PubMed] [Google Scholar]
- 11.Perez-Aytes A, Ledo A, Boso V. et al. In utero exposure to mycophenolate mofetil: a characteristic phenotype? Am J Med Genet A. 2008;146A(1):1–7. doi: 10.1002/ajmg.a.32117. [DOI] [PubMed] [Google Scholar]
- 12.Ang G S, Simpson S A, Reddy A R. Mycophenolate mofetil embryopathy may be dose and timing dependent. Am J Med Genet A. 2008;146A(15):1963–1966. doi: 10.1002/ajmg.a.32420. [DOI] [PubMed] [Google Scholar]
- 13.Jackson P, Paquette L, Watiker V, Randolph L, Ramanathan R, Seri I. Intrauterine exposure to mycophenolate mofetil and multiple congenital anomalies in a newborn: possible teratogenic effect. Am J Med Genet A. 2009;149A(6):1231–1236. doi: 10.1002/ajmg.a.32715. [DOI] [PubMed] [Google Scholar]
- 14.Parisi M A, Zayed H, Slavotinek A M, Rutledge J C. Congenital diaphragmatic hernia and microtia in a newborn with mycophenolate mofetil (MMF) exposure: phenocopy for Fryns syndrome or broad spectrum of teratogenic effects? Am J Med Genet A. 2009;149A(6):1237–1240. doi: 10.1002/ajmg.a.32684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin A E, Singh K E, Strauss A, Nguyen S, Rawson K, Kimonis V E. An additional patient with mycophenolate mofetil embryopathy: cardiac and facial analyses. Am J Med Genet A. 2011;155A(4):748–756. doi: 10.1002/ajmg.a.33934. [DOI] [PubMed] [Google Scholar]
- 16.Klieger-Grossmann C, Chitayat D, Lavign S. et al. Prenatal exposure to mycophenolate mofetil: an updated estimate. J Obstet Gynaecol Can. 2010;32(8):794–797. doi: 10.1016/s1701-2163(16)34622-9. [DOI] [PubMed] [Google Scholar]
- 17.Mohamed-Ahmed O Nelson-Piercy C Bramham K et al. Pregnancy outcomes in liver and cardiothoracic transplant recipients: a UK national cohort study PLoS One 201492e89151. Doi: 10.1371/journal.pone.0089151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martín M C, Cristiano E, Villanueva M. et al. Esophageal atresia and prenatal exposure to mycophenolate. Reprod Toxicol. 2014;50:117–121. doi: 10.1016/j.reprotox.2014.10.015. [DOI] [PubMed] [Google Scholar]
- 19.King R W, Baca M J, Armenti V T, Kaplan B. Pregnancy outcomes related to mycophenolate exposure in female kidney transplant recipients. . Am J Transplant. 2016 doi: 10.1111/ajt.13928. [DOI] [PubMed] [Google Scholar]


