Abstract
Introduction
Acute rejection (AR) is lower in pediatric kidney transplant (pKTx) recipients on tacrolimus (Tac) versus cyclosporine (CsA). Data comparing infection outcomes for children treated with these agents are limited.
Methods
We retrospectively studied infection outcomes in 96 pKTx recipients on a rapid discontinuation of prednisone protocol (RDP). Patient survival (PS), death-censored graft survival (DCGS), AR and infection free survival were assessed using Kaplan-Meier/log-rank tests and proportional hazards models.
Results
There were no differences in 1-year PS, DCGS, or AR between Tac and CsA recipients. After adjusting for AR, the hazard of CMV viremia was 4.0 times higher (95%CI: 1.04, 15.5; p=0.044) and that of BK viremia was 3.8 times higher (95%CI: 1.5, 10.2; p=0.007) in Tac recipients. The incidence of EBV viremia was similar between the groups (p=0.56). Posttransplant lymphoproliferative disease was only observed in Tac recipients (3%). There was no difference in the incidence of pneumonia, urinary tract or clostridium difficile infections between Tac and CsA recipients.
Conclusion
Among KTx recipients on RDP, the hazards of CMV and BK viremia within 1-year post-KTx were significantly higher in Tac recipients compared to CsA. Regular assessment for infections and lower Tac trough levels may be warranted in Tac recipients.
Keywords: Cyclosporine, CMV, Kidney transplant, Pediatrics, Steroid avoidance, Tacrolimus
Introduction
The calcineurin inhibitors cyclosporine (CsA) and tacrolimus (Tac) were approved by the United States Food and Drug Administration in 1983 and 1997, respectively, for use in kidney transplantation to prevent acute rejection (1, 2). Clinical trials in kidney transplantation have shown improved kidney allograft survival with Tac compared to CsA when used as a part of maintenance immunosuppression protocols in both adult and pediatric kidney transplant recipients (3, 4).
Increased rates of infectious complications have been observed with the use of more potent immunosuppression (5, 6). Infections are now the leading cause of mortality and hospital readmissions in pediatric kidney transplant recipients (7), accounting for 24 to 56% of deaths (8) and 24% of posttransplant hospitalizations (9). We examined the relationship between calcineurin inhibitor use and infectious complications in pediatric kidney transplant recipients on a rapid discontinuation of prednisone (RDP) protocol at a single center. We did not find prior reports addressing this question in pediatric kidney transplant recipients on steroid minimization protocols. Since Tac may be more potent than CsA in terms of immunosuppression (10), we hypothesized that the incidence of infections would be higher in Tac treated pediatric kidney transplant recipients compared to CsA.
Methods
Patient characteristics
Between January 2006 and November 2014, 96 pediatric kidney transplant recipients, ages 5 to 18 years, were started on RDP immunosuppression protocol following a kidney transplant at the University of Minnesota. We included all 96 pediatric kidney transplant recipients on RDP immunosuppression in our analysis. Prednisone was discontinued on post-operative day 6 in patients on the RDP protocol. When RDP was first introduced (2002), it was restricted to primary kidney transplant recipients who were at least 5 years old, white, not on steroids at the time of transplant, able to take mycophenolate mofetil (MMF), and were Epstein-Barr virus (EBV) seropositive. However, based on the outcomes of the first six recipients, the criteria were expanded in 2003 to include patients who were nonwhite, EBV seronegative and those undergoing retransplantation.
We retrospectively reviewed all donor and recipient data using an Institutional Review Board approved database at the University of Minnesota.
Immunosuppression
Our RDP immunosuppression protocol has been described previously (11). Briefly, thymoglobulin (Thymo) was given to both living and deceased donor recipients at a dose of 1.5 mg/kg intravenously (IV) per day for 5–7 days. Prednisone was given as follows: 10 mg/kg IV intra-operatively, 1 mg/kg IV on day one, 0.5 mg/kg IV days 2 and 3, 0.25 mg/kg IV days 4 and 5, and 0 mg/kg on day 6. MMF was given at a dose of 600 mg/m2/dose twice daily and the first dose was given IV intra-operatively. MMF levels were not routinely measured. CsA was used in 58 recipients transplanted between January 2006 and November 2011 and Tac was used in 38 recipients transplanted after November 2011 to November 2014. For both CsA and Tac recipients, the calcineurin inhibitor was initiated on postoperative day 1–2 and dosed to achieve target levels as shown in Table 1.
Table 1.
Target drug levels
| Tacrolimus monitoring | |
| 0 – 3 months | 10 – 12 mcg/L |
| 3 – 6 months | 8 – 10 mcg/L |
| 6 – 12 months | 6 – 8 mcg/L |
| Cyclosporine monitoring | |
| 0 – 3 months | 175 – 200 mcg/L |
| 3 – 6 months | 150 – 175 mcg/L |
| 6 – 9 months | 125 – 150 mcg/L |
| 9 – 12 months | 100 – 125 mcg/L |
Abbreviations: mcg/L, microgram per liter
Infection prophylaxis and diagnosis
Oral trimethoprim–sulfamethoxazole was given daily to all recipients as prophylaxis against urinary tract infections (UTI) and Pneumocystis jiroveci infections (12). Trimethoprim-sulfamethoxazole was started on day 4 posttransplant and continued indefinitely. Fungal prophylaxis was provided with oral nystatin or clotrimazole for the first six months posttransplant. All recipients received intravenous ganciclovir (5 mg/kg every 12 hours) postoperatively during the period of thymo administration followed by oral valganciclovir (10 to15 mg/kg per day, maximum dose 900 mg/day). The duration of valganciclovir was determined by the pretransplant donor and recipient CMV and EBV serology. Patients with no known EBV or CMV testing ever prior to transplant were considered ‘unknown status’. Patients with at least one positive test pre-transplant were considered ‘positive’ and patients with at least one negative test and no positive test were considered ‘negative’. All recipients who were D+/R− and D+/R+ for CMV and/or EBV received valganciclovir prophylaxis for 12 months. Recipients who were D−/R− for CMV and EBV received valganciclovir for 3 months. If the donor or recipient serologic status for CMV or EBV was unknown, we used the D+/R− protocol and administered valganciclovir for 12 months. If a recipient received Thymo treatment for acute rejection after discontinuation of valganciclovir, prophylaxis was restarted for 6 months.
All viral testing was conducted in the same laboratory and was performed using a quantitative polymerase chain reaction (qPCR) test. Our center started using qPCR for CMV detection in 2006. Hence, the kidney transplant recipients on RDP prior to 2006 were excluded from the study. Recipients were routinely tested for CMV viremia every three months during the first posttransplant year. CMV testing was also performed when clinically indicated such as for febrile episodes, leukopenia/anemia/thrombocytopenia and chronic diarrhea. We treated all recipients with CMV viremia with twice a day dosing of valganciclovir (20 to 30 mg/kg per day divided BID).
We monitored for EBV viremia during the first posttransplant year with monthly qPCR tests. EBV viremia was treated with immunosuppression reduction and therapeutic doses of valganciclovir (20 to 30 mg/kg per day divided BID) until the EBV qPCR became undetectable. All doses of ganciclovir and valganciclovir were adjusted as needed for the degree of renal function. Ganciclovir/Valganciclovir were continued indefinitely if EBV did not clear. Polyoma (BK) virus was monitored with a serum qPCR that was performed every 3 months during the first posttransplant year. If a recipient required antirejection therapy, BK qPCR was checked monthly for 3 months after therapy. Indication for a kidney biopsy in recipients who were positive for BK qPCR was a ≥ 25% increase in the serum creatinine. A positive result for CMV, EBV and BKV was based on a single positive value and sustained viremia was not required for inclusion in the study.
Bacterial infections were diagnosed based on culture results or radiological findings (chest X-ray). PostTx lymphoproliferative disorder (PTLD) or lymphoma were diagnosed on the basis of histopathology and were classified according to the recommendations of the Society of Hematopathology (13).
Rejection
Recipients with a ≥ 25% increase in serum creatinine above baseline underwent a diagnostic kidney biopsy. All biopsies were read by a renal pathologist. Rejection was characterized as acute or chronic, tubulointerstitial and/or vascular by established Banff criteria (14). Acute cellular rejection episodes were treated with steroids only if mild and with Thymo if severe or steroid resistant. Antibody mediated rejection episodes were treated with a combination of plasmapheresis and intravenous immunoglobulin (IVIG) that were given every other day over a 10-day period.
Outcomes and variables
Data collected on pediatric kidney transplant patients included age at transplantation, gender, race, ethnicity, whether this was the primary transplant, pre-transplant dialysis, donor source, donor and recipient CMV and EBV serology pairing and immunosuppression regimens. Patient outcomes included positive test results for infections, first biopsy-proven acute rejection episode, graft failure, and death. Graft failure was defined as a return to chronic dialysis, transplant nephrectomy, re-transplantation, or patient death. CMV, EBV and BK viremia were defined as a positive blood PCR test for these viruses. CMV disease was defined as presence of any of the following signs or symptoms of infection plus a positive blood PCR for CMV: pharyngitis with fever, chronic diarrhea, pneumonia, cytopenia, chorioretinitis, and CMV encephalitis.
Statistical analysis
Patient demographic variables were summarized and compared between Tac and CsA groups using chi-square or Fisher’s Exact tests as appropriate for categorical variables, and Wilcoxon rank sum tests for continuous variables. Kaplan-Meier estimates of 1-year death-censored graft survival, 1-year patient survival, 1-year rejection-free survival, infections and PTLD were computed and compared between CsA and Tac groups using log-rank tests.
A Cox proportional hazards model was used to assess the effect of initial immunosuppression on the risk of infection in the first posttransplant year, adjusting for acute rejection as a time-dependent variable to account for changes in immunosuppression after rejection. Clinically relevant demographic variables (including prior transplant and donor source) and those found to be significant on univariate analysis were also considered as covariates in the model. Backward selection at the significance level of 0.05 was used to select the final model.
Supplementary analyses to consider a secular trend included a Cox model run for each treatment group separately with transplant date as a covariate. This term was run separately for each treatment because of the separate treatment eras, and in this way was used to assess possible changes in care and/or surveillance over time.
All time to an event was calculated from date of transplant. Rejections and infections were censored at date of graft failure or death.
All analysis results were interpreted as statistically significant at the 0.05 level and all analyses were performed using SAS 9.4 (The SAS Institute, Cary, NC).
Results
Our study included 58 kidney transplant recipients who received CsA and 38 who received Tac as part of the RDP maintenance immunosuppression protocol. The demographic and baseline transplant characteristics of the study participants are given in Table 2. There was no significant difference between Tac and CsA recipients in terms of age at transplant, gender, race, prior transplant, pretransplant dialysis, donor characteristics or pretransplant viral serology. The distribution of donor/recipient serology pairing for CMV (p 0.60) and EBV (p 0.67) were also similar between Tac and CsA recipients (Tables 3 and 4).
Table 2.
Recipient and donor characteristics by immunosuppression
| Variable | Tac (n = 38) | CsA (n = 58) | p-value |
|---|---|---|---|
| Age (years) | 0.87 | ||
| 5 – 9 | 10 (26%) | 13 (22%) | |
| 10 – 14 | 14 (37%) | 21 (36%) | |
| 15 – 18 | 14 (37%) | 24 (41%) | |
| Male | 20 (53%) | 34 (59%) | 0.56 |
| White | 29 (78%) | 47 (81%) | 0.75 |
| Dialysis prior to Transplant | 27 (71%) | 34 (59%) | 0.22 |
| Donor age (mean years) | 33 (13) | 34 (11) | 0.76 |
| Deceased donor | 16 (42%) | 18 (31%) | 0.27 |
| Primary Transplant | 35 (92%) | 57 (98%) | 0.30 |
| Recipient CMV negative | 23/38 (61%) | 36/57 (63%) | 0.8 |
| Recipient EBV negative | 15/38 (39%) | 28/55 (51%) | 0.28 |
| Donor CMV negative | 12/37 (23%) | 23/58 (40%) | 0.48 |
| Donor EBV negative | 4/37 (11%) | 4/53 (8%) | 0.71 |
Abbreviations: Tac, tacrolimus; CsA, cyclosporine; CMV, cytomegalovirus; EBV, Epstein-Barr virus
Table 3.
| Donor/recipient | Tac (n = 38) | CsA (n = 58) | p-value* |
|---|---|---|---|
| CMV serostatus | |||
| D+/R+ | 9 (24%) | 15 (26%) | 0.60 |
| D+/R− | 16 (42%) | 19 (33%) | |
| D-/R+ | 5 (13%) | 6 (10%) | |
| D−/R− | 7 (18%) | 17 (29%) | |
| D?/R+ | 1 (3%) | 0 | |
| D?/R− | 0 | 0 | |
| D+/R? | 0 | 1 (2%) | |
| D-/R? | 0 | 0 | |
| D?/R? | 0 | 0 |
P-value comparing proportions of known EBV serology statusAbbreviations: CMV, cytomegalovirus; CsA, cyclosporine; Tac, tacrolimus; D/R, donor/ recipient
Table 4.
| Donor/recipient | Tac (n = 38) | CsA (n = 58) | p-value* |
|---|---|---|---|
| EBV serostatus | |||
| D+/R+ | 20 (53%) | 22 (38%) | 0.67 |
| D+/R− | 13 (34%) | 24 (41%) | |
| D-/R+ | 2 (5%) | 2 (3%) | |
| D−/R− | 2 (5%) | 2 (3%) | |
| D?/R+ | 1 (3%) | 3 (5%) | |
| D?/R− | 0 | 2 (3%) | |
| D+/R? | 0 | 3 (5%) | |
| D-/R? | 0 | 0 | |
| D?/R? | 0 | 0 |
P-value comparing proportions of known EBV serology status
Abbreviations: EBV, Epstein-Barr virus; CsA, cyclosporine; Tac, tacrolimus; D/R, donor/ recipient
Patient and graft survival
There was no difference in the 1-year patient survival between Tac and CsA recipients (100% versus 98%, p 0.42). The CsA recipient who died suffered a sudden death of an unknown cause. The 1-year death-censored graft survival was similar between the two groups (95% versus 97%, p 0.65). One patient lost his/her graft to thrombosis, one to chronic rejection and two patients lost their grafts to recurrent disease. We did not find any difference in the acute rejection free survival between Tac and CsA recipients (75% versus 77%, p 0.89).
Infection outcomes and PTLD
The incidence of CMV viremia was 4 times higher in Tac recipients compared to CsA (19% versus 5%, p 0.03) (Figure 1). The median time to CMV viremia in the Tac group was 4.7 months (Interquartile range (IQR: 3.9 – 6.7) while that in the CsA group was 8.2 months (IQR: 6.8 – 12.0). Of the 10 patients who developed CMV viremia during the first year posttransplant, 5 (50%) were on valganciclovir prophylaxis at the time of the diagnosis. Four of the five patients with CMV viremia were successfully treated with higher/therapeutic doses of valganciclovir (dose was increased from 10–15 mg/kg/day to 20–30 mg/kg/day). One patient had valganciclovir resistant and required foscarnet for eradication of the viremia. After adjusting for acute rejection, the hazard of CMV viremia over the first year posttransplant was 4.01 times higher for Tac recipients compared to CsA (95% CI: 1.04, 15.5; p=0.044). For Tac recipients, their timing within their transplant era (November 2011 to November 2014) did not affect hazard of CMV viremia, controlling for rejection (p=0.76). Likewise, for CsA recipients, their timing within their transplant era (January 2006 to November 2011) did not affect hazard of CMV viremia, controlling for rejection (p=0.94). Three of 7 Tac recipients with CMV viremia had evidence of CMV disease (two patients had diarrhea and one patient had fever and pharyngitis). One (1/3) CsA recipient with CMV viremia had CMV disease characterized by anemia, thrombocytopenia and fatigue.
Figure 1.
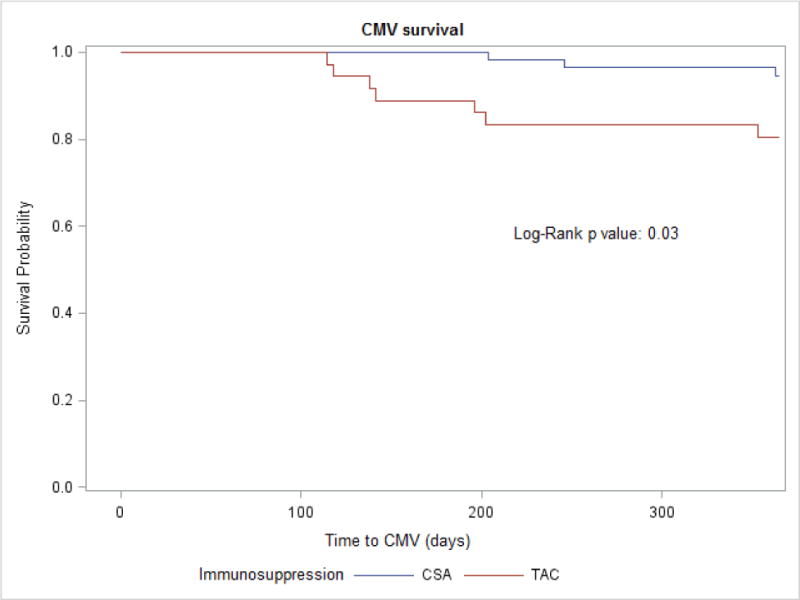
CMV free survival according to maintenance immunosuppression in pediatric kidney transplant recipients
Abbreviations: CMV, cytomegalovirus; CsA, cyclosporine; Tac, tacrolimus
The incidence of BK viremia was also 4 times higher among Tac recipients (34% versus 10%, p 0.005) (Figure 2). The median time to BK viremia in the Tac group was 6 months (IQR: 4.8 – 10.2) while the median time in the CsA group was 3.4 months (IQR 2.4 – 4.7). When adjusted for acute rejection, the hazard of BK viremia over the first year post-transplant was 3.8 times higher for Tac recipients compared to CsA (95% CI 1.5, 10.2; p=0.007). The timing of kidney transplant within Tac (p 0.39) and CsA (p 0.69) eras did not affect the hazard of BK viremia, controlling for rejection. None of the recipients developed BK nephropathy.
Figure 2.
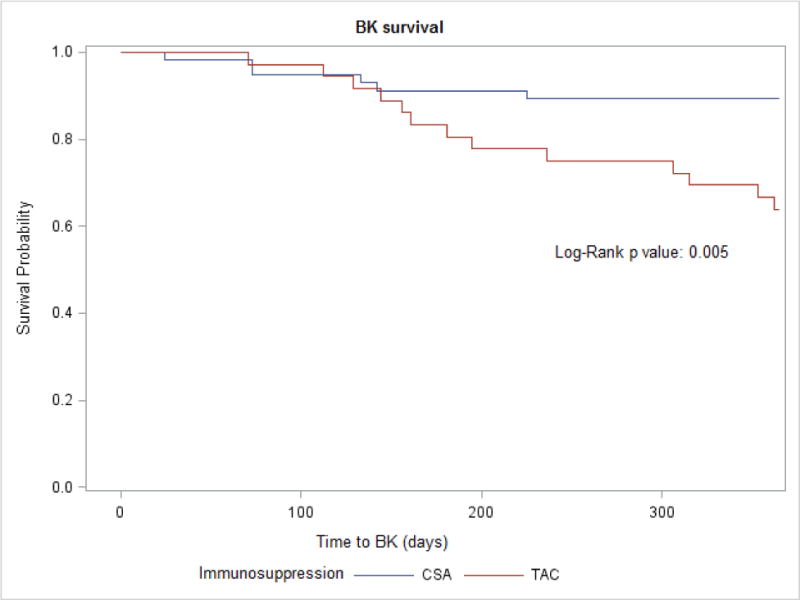
BK free survival according to maintenance immunosuppression in pediatric kidney transplant recipients
Abbreviations: CsA, cyclosporine; Tac, tacrolimus
We did not find any difference in EBV viremia between Tac and CsA groups (21% versus 17%, p 0.56) (Figure 3). Of the 18 patients who developed EBV viremia during the first year posttransplant, 11 (61%) were on valganciclovir prophylaxis at the time of the diagnosis. Similarly, there was no difference in the incidence of UTI (32% versus 31%, p 0.84), pneumonia (11% versus 9%, p 0.73) or clostridium difficile infections (3% versus 7%, p 0.39) between the two groups.
Figure 3.
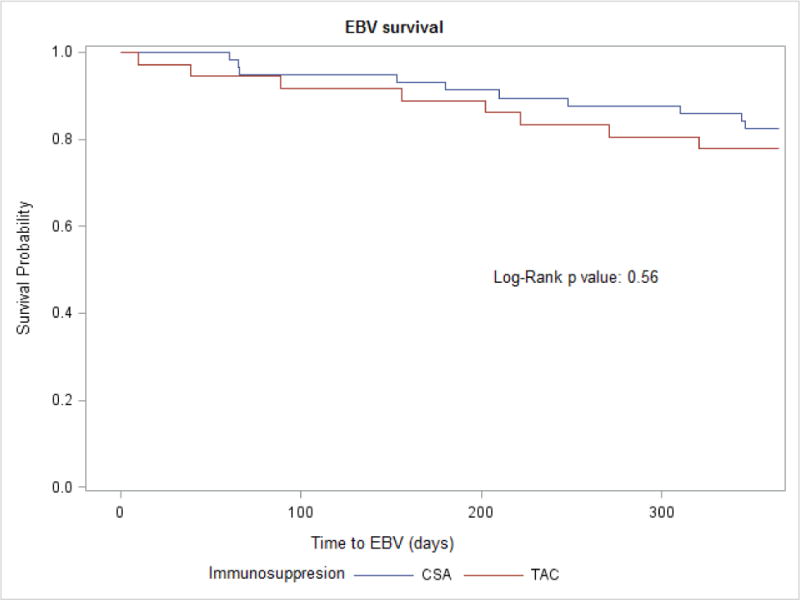
EBV free survival according to maintenance immunosuppression in pediatric kidney transplant recipients
Abbreviations: EBV, Epstein-Barr virus; CsA, cyclosporine; Tac, tacrolimus
PTLD was observed in 1 Tac recipient. We did not document any incidence of PTLD in CsA recipients.
Discussion
This is the first pediatric study to compare the rates of infectious complications after kidney transplantation in Tac and CsA recipients who are on RDP. This is an important comparison due to the widespread use of Tac for maintenance immunosuppression in solid organ transplant recipients. According to the 2012 annual data report of Scientific Registry of Transplant Recipients (SRTR), between 2002 and 2012 the use of CsA declined from 22.4 % to 3.5% while that of Tac increased from 71.1% to 93.8% (15).
Our results show a significantly higher incidence of CMV and BK viremia in Tac recipients compared to CsA. The pretransplant donor and recipient CMV (p 0.60) and EBV (p 0.67) statuses were statistically similar between Tac and CsA recipients, making it unlikely that the associations presented in this paper were confounded by recipients’ pretransplant viral statuses.
CMV is an important pathogen in pediatric and adult kidney transplant recipients with an incidence of 8% to 32% (16, 17). Several risk factors for CMV infection have been identified in kidney transplant recipients of which the most important are D+/R− status and the net state of immunosuppression (16). The latter is determined by the intensity of immunosuppression, co-infection with immunomodulating viruses, as well as rejection episodes requiring an escalation of immunosuppressive therapy. In our study, the two groups followed identical protocols for antiviral chemoprophylaxis. Tac recipients exhibited higher hazards of CMV viremia compared to CsA despite controlling for acute rejection. Nafar et al. in their retrospective study of 427 kidney transplant recipients also found Tac to be an independent risk factor for CMV infection compared to CsA (18).
Half of our patients with CMV viremia developed it on valganciclovir prophylaxis. This may indicate non-adherence to the prophylactic valganciclovir. It is also possible that the viremia was transient and would have resolved spontaneously. However, our protocol called for increasing from prophylactic to therapeutic dosing of valganciclovir. Of the patients who developed EBV viremia, 61% were on valganciclovir prophylaxis at the time of diagnosis. The role of antiviral prophylaxis in the prevention of EBV viremia and PTLD is controversial. In a recent multicenter trial, Hocker et al. found a lower incidence of EBV viremia in high-risk pediatric kidney transplant recipients who received valganciclovir/ganciclovir prophylaxis(19). A recent systemic review from AlDabbagh et al. did not show any benefit of antiviral prophylaxis in prevention of PTLD in high-risk solid organ transplant recipients (20). While most of our patients developed EBV viremia on prophylaxis, only one patient developed PTLD. Whether prophylaxis prevented PTLD in our cohort or not and how many patients would have developed EBV viremia without prophylaxis cannot be determined from our data.
Both asymptomatic CMV viremia and CMV disease have been shown in adult kidney transplant recipients to increase the risk of graft loss and overall mortality beyond 100 days posttransplant (21). In our study, the overall rates of death and graft loss were low and there was no difference in the 1-year graft or patient survival between patients who did or did not develop CMV viremia within the first posttransplant year. This difference in results may have been due to our small sample size.
We found a fourfold higher incidence of BK viremia in Tac recipients within the first posttransplant year. Pediatric data comparing Tac and CsA for BK viremia are lacking, however, our finding is consistent with adult data (6). In a prospective, randomized, multicenter study of 682 adult KTx recipients receiving basiliximab, MMF, steroids and CsA or Tac, Hirsch et al. found a higher incidence of BK viremia in Tac recipients during the first posttransplant year (12.1 vs 4.8, p= 0.004) (22). Similarly, a Chinese prospective study of BK replication and nephropathy in 90 adult kidney transplant recipients receiving prednisone, MMF and either Tac or CsA showed a lower incidence of BK infection in CsA recipients during the first posttransplant year (28.9% vs. 57.7%, p 0.007) (23). Based on recent in vitro evidence it has been proposed that CsA may possess direct antiviral activity against BK (24), possibly accounting for the lower incidence of BK viremia in CsA treated recipients.
In adults, the use of Tac in combination with MMF has been reported to result in higher mycophenolic acid levels compared to co-administration with CsA (25–27). Similar findings were reported in a small pediatric study of liver transplant recipients (28). Equivalent dosing of MMF in Tac and CsA recipients in our study may have resulted in higher net immunosuppression among Tac recipients. Mycophenolic acid levels and area under the curve (MPA-AUC) were not measured in the study population; hence, the exposure of the two groups to MMF could not be determined. Since MPA is associated with higher rates of CMV viremia (29), the higher CMV and BK rates in the Tac recipients may have been a consequence of excessive exposure to MPA. Based on our findings, we recommend that monitoring of MPA levels be considered in kidney transplant recipients on Tac.
We observed only one case of PTLD in our study cohort and that was in a Tac recipient. Dhanidharka et al. reported a higher prevalence of PTLD in Tac treated pediatric kidney transplant recipients compared to CsA (11.5% vs. 1.1% p<0.0001), using the North American Pediatric Renal Transplant Cooperative Study (NAPRTCS) database(30). In Dhanidharka’s study, unlike ours, patients were on a steroid inclusive maintenance immunosuppression protocol and some patients received induction with monoclonal anti CD3 antibody (OKT3).
UTI, clostridium difficile colitis and respiratory infections have been described as common infectious complications in pediatric kidney transplant recipients (31–33). Our study did not demonstrate any significant difference in the incidence of these infections within the first posttransplant year between the two groups.
We did not find any difference in the rejection free survival or graft survival between Tac and CsA recipients. This is in contrast to the well-established superiority of Tac in preventing acute rejections and improving graft survival (10). Webster et al. in their meta-regression of randomized controlled trials found that the benefit of Tac in improving graft survival diminished at higher Tac trough levels (levels > 10 ng/ml) (34). Although there is a paucity of data regarding the ideal Tac trough levels in children, the relatively higher Tac trough levels used at our center may have contributed to the lack of a difference in graft survival in this study. In contrast to Webster, Larkins et al., in their retrospective study of 48 pediatric kidney transplant recipients, found that Tac trough of > 10 ng/ml during the first 3 posttransplant months was associated with a slower rate of decline in GFR with time (35).
Our study has several limitations. Firstly, our CsA and Tac recipients came from two different treatment eras. We tried to account for this difference by including the transplant date as a covariate in our supplementary analysis and did not find any significant secular trend. Secondly, our sample size was small and we may have masked some important associations because of low power. Thirdly, we did not have data on medication adherence. It is possible that CsA recipients were less compliant with their regimen and consequently less immunosuppressed due to undesirable side effects of CsA compared to Tac. However, we did not find higher rates of acute rejection in CsA recipients, making the latter a less likely possibility.
In our single-center study of pediatric kidney transplant recipients on RDP protocol, Tac treated patients had a 4-fold higher incidence of CMV and BK viremia compared to CsA during the first posttransplant year despite an identical antiviral prophylaxis protocol. Based on our results, we recommend frequent viral monitoring with early therapeutic interventions, MPA level monitoring and a modulation of immunosuppression (possible lower Tac trough levels) during the first posttransplant year in Tac recipients on steroid minimization protocols such as RDP.
Acknowledgments
This work was supported by a grant from the National Institutes of Health (DK13083) from the National Institute of Diabetes and Digestive and Kidney Diseases.
Abbreviations
- KTx
kidney transplant
- Tx
transplant
- Tac
tacrolimus
- CsA
Cyclosporine
- RDP
rapid discontinuation of prednisone
- PS
actuarial patient survival
- DCGS
death censored graft survival
- AR
acute rejection
- HR
hazard ratio
- PreTx
pre-transplant
- PostTx
post-transplant
- LD
living donor
- DD
deceased donor
- MMF
mycophenolate mofetil
- ATG
thymoglobulin
- IVIG
intravenous immunoglobulin
- UTI
urinary tract infections
- PCR
polymerase chain reaction
- PTLD
posttransplant lymphoproliferative disorder
- LOS
length of stay
- CMV
cytomegalovirus
- EBV
Epstein-Barr virus
- mcg/L
microgram per liter
Footnotes
Author Contributions:
Sarah J Kizilbash: Participated in data analysis and writing of the paper
Michelle N Rheault: Participated in research design and writing of the paper
Ananta Bangdiwala: Participated in data analysis and writing of the paper
Arthur Matas: Participated in study design and writing up the manuscript.
Srinath Chinnakotla: Participated in study design and writing up the manuscript
Blanche M Chavers: Participated in study design, performance of the research and writing of the manuscript
Disclosures:
The authors of this manuscript have no conflicts of interest to disclose. The results presented in this paper have not been published previously in whole or part, except in abstract format
References
- 1.Bowman LJ, Brennan DC. The role of tacrolimus in renal transplantation. Expert opinion on pharmacotherapy. 2008;9:635–643. doi: 10.1517/14656566.9.4.635. [DOI] [PubMed] [Google Scholar]
- 2.Kolata G. FDA speeds approval of cyclosporin. Science. 1983;221:1273. doi: 10.1126/science.221.4617.1273-a. [DOI] [PubMed] [Google Scholar]
- 3.Pirsch JD, Miller J, Deierhoi MH, Vincenti F, Filo RS. A comparison of tacrolimus (FK506) and cyclosporine for immunosuppression after cadaveric renal transplantation. FK506 Kidney Transplant Study Group. Transplantation. 1997;63:977–983. doi: 10.1097/00007890-199704150-00013. [DOI] [PubMed] [Google Scholar]
- 4.Cavaille-Coll MW, Elashoff MR. Commentary on a comparison of tacrolimus and cyclosporine for immunosuppression after cadaveric renal transplantation. Transplantation. 1998;65:142–145. doi: 10.1097/00007890-199801150-00028. [DOI] [PubMed] [Google Scholar]
- 5.Acott P, Babel N. BK virus replication following kidney transplant: does the choice of immunosuppressive regimen influence outcomes? Annals of transplantation : quarterly of the Polish Transplantation Society. 2012;17:86–99. doi: 10.12659/aot.882640. [DOI] [PubMed] [Google Scholar]
- 6.Suwelack B, Malyar V, Koch M, Sester M, Sommerer C. The influence of immunosuppressive agents on BK virus risk following kidney transplantation, and implications for choice of regimen. Transplantation reviews. 2012;26:201–211. doi: 10.1016/j.trre.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 7.Chavers BM, Solid CA, Gilbertson DT, Collins AJ. Infection-related hospitalization rates in pediatric versus adult patients with end-stage renal disease in the United States. Journal of the American Society of Nephrology : JASN. 2007;18:952–959. doi: 10.1681/ASN.2006040406. [DOI] [PubMed] [Google Scholar]
- 8.Mencarelli F, Marks SD. Non-viral infections in children after renal transplantation. Pediatric nephrology. 2012;27:1465–1476. doi: 10.1007/s00467-011-2099-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dharnidharka VR, Stablein DM, Harmon WE. Post-transplant infections now exceed acute rejection as cause for hospitalization: a report of the NAPRTCS. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2004;4:384–389. doi: 10.1111/j.1600-6143.2004.00350.x. [DOI] [PubMed] [Google Scholar]
- 10.Webster A, Woodroffe RC, Taylor RS, Chapman JR, Craig JC. Tacrolimus versus cyclosporin as primary immunosuppression for kidney transplant recipients. Cochrane Database Syst Rev. 2005:Cd003961. doi: 10.1002/14651858.CD003961.pub2. [DOI] [PubMed] [Google Scholar]
- 11.Chavers BM, Chang YC, Gillingham KJ, Matas A. Pediatric kidney transplantation using a novel protocol of rapid (6-day) discontinuation of prednisone: 2-year results. Transplantation. 2009;88:237–241. doi: 10.1097/TP.0b013e3181ac6833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khositseth S, Matas A, Cook ME, Gillingham KJ, Chavers BM. Thymoglobulin versus ATGAM induction therapy in pediatric kidney transplant recipients: a single-center report. Transplantation. 2005;79:958–963. doi: 10.1097/01.tp.0000158325.12837.a2. [DOI] [PubMed] [Google Scholar]
- 13.Harris NL, Ferry JA, Swerdlow SH. Posttransplant lymphoproliferative disorders: summary of Society for Hematopathology Workshop. Seminars in diagnostic pathology. 1997;14:8–14. [PubMed] [Google Scholar]
- 14.Birk PE, Matas AJ, Gillingham KJ, Mauer SM, Najarian JS, Chavers BM. Risk factors for chronic rejection in pediatric renal transplant recipients–a single-center experience. Pediatric nephrology. 1997;11:395–398. doi: 10.1007/s004670050303. [DOI] [PubMed] [Google Scholar]
- 15.Matas AJ, Smith JM, Skeans MA, et al. OPTN/SRTR 2012 Annual Data Report: kidney. Am J Transplant. 2014;14(Suppl 1):11–44. doi: 10.1111/ajt.12579. [DOI] [PubMed] [Google Scholar]
- 16.Cukuranovic J, Ugrenovic S, Jovanovic I, Visnjic M, Stefanovic V. Viral infection in renal transplant recipients. TheScientificWorldJournal. 2012;2012:820621. doi: 10.1100/2012/820621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith JM, Corey L, Bittner R, et al. Subclinical viremia increases risk for chronic allograft injury in pediatric renal transplantation. Journal of the American Society of Nephrology : JASN. 2010;21:1579–1586. doi: 10.1681/ASN.2009111188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nafar M, Roshan A, Pour-Reza-Gholi F, et al. Prevalence and risk factors of recurrent cytomegalovirus infection in kidney transplant recipients. Iranian journal of kidney diseases. 2014;8:231–235. [PubMed] [Google Scholar]
- 19.Hocker B, Bohm S, Fickenscher H, et al. (Val-)Ganciclovir prophylaxis reduces Epstein-Barr virus primary infection in pediatric renal transplantation. Transplant international : official journal of the European Society for Organ Transplantation. 2012;25:723–731. doi: 10.1111/j.1432-2277.2012.01485.x. [DOI] [PubMed] [Google Scholar]
- 20.AlDabbagh MA, Gitman MR, Kumar D, Humar A, Rotstein C, Husain S. The Role of Antiviral Prophylaxis for the Prevention of Epstein-Barr Virus-Associated Posttransplant Lymphoproliferative Disease in Solid Organ Transplant Recipients: A Systematic Review. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2016 doi: 10.1111/ajt.14020. [DOI] [PubMed] [Google Scholar]
- 21.Sagedal S, Hartmann A, Nordal KP, et al. Impact of early cytomegalovirus infection and disease on long-term recipient and kidney graft survival. Kidney international. 2004;66:329–337. doi: 10.1111/j.1523-1755.2004.00735.x. [DOI] [PubMed] [Google Scholar]
- 22.Hirsch HH, Vincenti F, Friman S, et al. Polyomavirus BK replication in de novo kidney transplant patients receiving tacrolimus or cyclosporine: a prospective, randomized, multicenter study. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2013;13:136–145. doi: 10.1111/j.1600-6143.2012.04320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang G, Chen LZ, Qiu J, et al. Prospective study of polyomavirus BK replication and nephropathy in renal transplant recipients in China: a single-center analysis of incidence, reduction in immunosuppression and clinical course. Clinical transplantation. 2010;24:599–609. doi: 10.1111/j.1399-0012.2009.01141.x. [DOI] [PubMed] [Google Scholar]
- 24.Acott PD, O’Regan PA, Lee SH, Crocker JF. In vitro effect of cyclosporin A on primary and chronic BK polyoma virus infection in Vero E6 cells. Transplant infectious disease : an official journal of the Transplantation Society. 2008;10:385–390. doi: 10.1111/j.1399-3062.2008.00330.x. [DOI] [PubMed] [Google Scholar]
- 25.Zucker K, Rosen A, Tsaroucha A, et al. Unexpected augmentation of mycophenolic acid pharmacokinetics in renal transplant patients receiving tacrolimus and mycophenolate mofetil in combination therapy, and analogous in vitro findings. Transplant immunology. 1997;5:225–232. doi: 10.1016/s0966-3274(97)80042-1. [DOI] [PubMed] [Google Scholar]
- 26.Gregoor PJ, de Sevaux RG, Hene RJ, et al. Effect of cyclosporine on mycophenolic acid trough levels in kidney transplant recipients. Transplantation. 1999;68:1603–1606. doi: 10.1097/00007890-199911270-00028. [DOI] [PubMed] [Google Scholar]
- 27.Meiser BM, Groetzner J, Kaczmarek I, et al. Tacrolimus or cyclosporine: which is the better partner for mycophenolate mofetil in heart transplant recipients? Transplantation. 2004;78:591–598. doi: 10.1097/01.tp.0000129814.52456.25. [DOI] [PubMed] [Google Scholar]
- 28.Brown NW, Aw MM, Mieli-Vergani G, Dhawan A, Tredger JM. Mycophenolic acid and mycophenolic acid glucuronide pharmacokinetics in pediatric liver transplant recipients: effect of cyclosporine and tacrolimus comedication. Therapeutic drug monitoring. 2002;24:598–606. doi: 10.1097/00007691-200210000-00004. [DOI] [PubMed] [Google Scholar]
- 29.Basic-Jukic N, Kes P, Bubic-Filipi LJ, Puretic Z, Brunetta B, Pasini J. Does mycophenolate mofetil increase the incidence of cytomegalovirus disease compared with azathioprine after cadaveric kidney transplantation? Transplantation proceedings. 2005;37:850–851. doi: 10.1016/j.transproceed.2004.12.228. [DOI] [PubMed] [Google Scholar]
- 30.Dharnidharka VR, Sullivan EK, Stablein DM, Tejani AH, Harmon WE. Risk factors for posttransplant lymphoproliferative disorder (PTLD) in pediatric kidney transplantation: a report of the North American Pediatric Renal Transplant Cooperative Study (NAPRTCS) Transplantation. 2001;71:1065–1068. doi: 10.1097/00007890-200104270-00010. [DOI] [PubMed] [Google Scholar]
- 31.Chavers BM, Gillingham KJ, Matas AJ. Complications by age in primary pediatric renal transplant recipients. Pediatric nephrology. 1997;11:399–403. doi: 10.1007/s004670050304. [DOI] [PubMed] [Google Scholar]
- 32.West M, Pirenne J, Chavers B, et al. Clostridium difficile colitis after kidney and kidney-pancreas transplantation. Clinical transplantation. 1999;13:318–323. doi: 10.1034/j.1399-0012.1999.130407.x. [DOI] [PubMed] [Google Scholar]
- 33.Shih CJ, Tarng DC, Yang WC, Yang CY. Immunosuppressant dose reduction and long-term rejection risk in renal transplant recipients with severe bacterial pneumonia. Singapore medical journal. 2014;55:372–377. doi: 10.11622/smedj.2014089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Webster AC, Woodroffe RC, Taylor RS, Chapman JR, Craig JC. Tacrolimus versus ciclosporin as primary immunosuppression for kidney transplant recipients: meta-analysis and meta-regression of randomised trial data. Bmj. 2005;331:810. doi: 10.1136/bmj.38569.471007.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Larkins N, Matsell DG. Tacrolimus therapeutic drug monitoring and pediatric renal transplant graft outcomes. Pediatric transplantation. 2014;18:803–809. doi: 10.1111/petr.12369. [DOI] [PubMed] [Google Scholar]


