Abstract
Objective
Very-low-level viremia (VLLV) is a relatively new concept in the realm of human immunodeficiency virus (HIV) care. Newer generation assays are now able to detect plasma HIV RNA Viral Load (VL) levels as low as 20 copies/mL. The authors characterized patients with VLLV (VL between 20 and 50 copies/mL) in order to identify possible risk factors associated with virologic failure and poor clinical outcomes.
Methods
The authors reviewed 119 consecutive charts of patients with VLLV. Sociodemographic data were extracted and viral load and CD4 counts were trended over a 12 month period (February 2013–February 2014). Regression analysis was used to assess the role of different factors on virologic failure at 1 year.
Results
Of the study participants with evaluable data (n = 100), the median age was 53 years (interquartile range: 43–57.5), 67% were nonwhite, 34% were women, 58% were smokers, 47% were alcoholics, 58% had a history of intravenous drug use, and 40% were coinfected with hepatitis C virus. More than half of the participants had 3 or more comorbidities and their HIV pill burden was high (more than 2 pills daily). After 12 months, 65 participants achieved undetectable viral load levels, whereas 15 experienced virologic failure (2 consecutive viral loads > 50 copies/mL) and the remaining 20 had persistent VLLV. In the virologic failure group, there was a predominance of white males (66%) with a significant number of comorbidities and pill burden. Univariate logistic regression suggested that there was a difference between the failure versus nonfailure groups in terms of race, ethnicity, and alcohol use. Multivariate regression with virological failure as the outcome suggested a trend only in terms of participant’s alcohol use.
Conclusion
Most patients with initial VLLV (70%) achieved virologic suppression at 1 year with no antiretroviral therapy changes. Thus, VLLV does not necessarily predict virologic failure and should not prompt more frequent clinic visits or antiretroviral regimen changes. Further research is needed in order to determine the predictors of virologic failure in this subset of patients and the clinicians’ attitude toward VLLV.
Keywords: HIV, low-level viremia
Introduction
In the setting of antiretroviral therapy (ART), plasma levels of Human Immunodeficiency Virus type-1 (HIV-1) rapidly decay to levels below the limit of detection (LOD) of currently available standard clinical assays. However, reactivation of latently infected memory CD4 can serve as the nidus of continued viral production, requiring patients to remain on ART despite clinically undetectable viral loads.1
With the advent of new viral load assays, very–low-level viremia (VLLV) has become an important clinical question. Some reports show that the switch from Amplicor to TaqMan assay resulted in a nearly 2-fold increase in the number of patients experiencing a plasma HIV-1 RNA level >50 copies/ mL after being suppressed to levels <50 copies/mL consistently during the previous year.2,3
Very-low-level viremia is often defined, in a research context, as an HIV RNA level that is detectable by newer-generation viral load quantification assays but is less than 48 to 50 copies/mL.4
As with HIV viral blips, the exact cause of VLLV is unknown but is likely to be multifactorial. Contributing factors to VLLV may include persistent or intermittent low-level releases of virus from existing viral reservoirs, random laboratory variation or laboratory testing error, ongoing viral replication in various tissues, and decreased ART adherence.2,4
Very-low-level viremia is detected, using more sensitive, newer-generation viral load testing platforms in 20% to 60% of individuals on suppressive ART. Very-low-level viremia has been associated with progressive HIV RNA rebound to levels above 400 copies/mL in several large cohort studies.5,6 Periods of VLLV are associated with immune activation, either directly or through induction of immune activation. Furthermore, VLLV has been associated with immunological and virological consequences in patients receiving ART.7,8 Clinicians cannot often predict the clinical impact of VLLV in patients on ART. Given this uncertainty, clinicians frequently increase patient visits and viral load testing out of concern for subsequent virologic failure. Although low-level viremia does not always lead to treatment failure, it has been associated with reductions in CD4 increase and with T-cell activation.7,9,10
Two recent retrospective studies have supported the hypothesis that virologic rebound is more likely to occur in patients with viral loads >200 copies/mL than in those with low-level viremia between 50 and 200 copies/mL. However, other studies have suggested that viremia, even at very low levels (<200 copies/ mL), can be predictive of progressive viral rebound and can be associated with the evolution of HIV drug resistance.5,6,11
In addition, more recent studies showed that blips are frequent among HIV-infected patients with sustained virologic suppression on ART. HIV-positive patients with blips and at least 3 consecutive detected, but not quantified, HIV-RNA determinations had a higher risk of virologic failure. These findings highlighted the relevance of maintaining HIV-RNA levels below the limits of quantification of current assays, that is, <20 copies/mL.12 Some researchers concluded that undetectable HIV RNA by real-time polymerase chain reaction is significantly associated with a lower 2-year risk of virologic failure along with hepatitis C virus (HCV) antibody negativity, longer viral control, and lower pill burden.13 A study of the Austrian HIV cohort found that for both virologic failure (VF) and low level viremia (LLV), factors associated with adherence play a prominent role. Furthermore, performance characteristics of the diagnostic assay used for VL quantification should also be taken into consideration.14
Thus, further exploration of intermittent or persistent VLLV is needed to understand the impact on patient outcomes. We sought to characterize short-term clinical outcomes and identify potential predictors of such outcomes among HIV-infected patients with VLLV in a large HIV clinic.
Methods
Participants
The study was performed at the Nathan Smith Clinic, the HIV ambulatory clinic of Yale–New Haven Hospital, an urban tertiary care academic teaching hospital and the largest ambulatory HIV clinic in Connecticut. The clinic provides HIV primary care services by infectious disease physicians and trainees specialized in HIV care and serves more than 900 patients with approximately 7500 visits per year.
We conducted a retrospective chart review of HIV-infected patients identified to have VLLV between February 2013 and February 2014. Very-low-level viremia was defined as an HIV RNA viral load more than 20 but less than 50 copies/mL, in patients on ART for at least 6 months.
HIV Viral Load Testing
HIV viral load testing was performed within 1 to 3 days of plasma collection, according to the manufacturer’s instructions. Roche Cobas AmpliPrep/Cobas TaqMan HIV-1 version 2.0 (Roche Molecular Systems, Inc, Pleasanton, California) was used. The assay had a quantification range of 20 to 10 000 000 copies/mL. The LOD was 20 copies/mL (≥95% detection rate), but the assay detects 86%to 90%of samples with viral loads of 10 to 15 copies/mL and 53% of samples with 5 copies/mL.15 Positive results below the lower limit of quantitation of 20 copies/mL were reported as “detected, less than 20 copies/mL.”
Medical Record Review
Review of the electronic medical records (EMR) was performed to abstract sociodemographic and clinical characteristics including age, ethnicity, gender, Body Mass Index (BMI), estimated Glomerular Filtration Rate (eGFR), number and gender of sexual partners, sexual practices, hepatitis infection status, Intravenous Drug Use (IVDU), number of Sexually Transmitted Infections (STIs) and opportunistic infections, number of comorbidities (lung disease, hypertension, anemia, kidney disease, dyslipidemia, diabetes mellitus, cardiovascular disease, cancer, liver, bone, and neurological diseases), number of medications (ART and non-ART), ART history and modifications, current ART regimen, HIV genotype, self-reported ART adherence, number of clinic visits, viral load, and CD4 count laboratory results at the time of the baseline VLLV measurement and throughout the 12-month follow-up period. We examined the following outcomes: change in HIV viral load and CD4 count and virologic failure at 12 months (VL > 50 copies/mL).
The study received ethical approval from the Yale University School of Medicine Human Investigations Committee. Written consent was not obtained from participants for their clinical record use in this study. Patient records and information was anonymized and deidentified prior to analysis.
Statistical Analysis
Statistical analysis was performed using SAS 9.3 (SAS Institute, Cary, North Carolina). Frequencies and percentages are reported for categorical variables; continuous variable data are presented as means (standard deviation) or medians (interquartile range).
Change in viral load (δViralLoad) and CD4 (δCD4) were calculated by subtracting the first available value from the last available measurement. Transformations were performed for HIV viral load (log 10) and CD4 count (square root) to normalize their distribution. Box plots of log 10 viral load, CD4 count and square root (CD4 count) at each of the study’s 3 time points (0, 6, and 12months) were generated. The signed rank test was used to assess whether the median change in CD4 count and VL was significant. A 2-sided α level of .05 was used to assess statistical significance. Logistic regression was used to determine the predictors of virologic failure; variables significant at the P=.1 level in univariate models were entered into the multivariable model.
Results
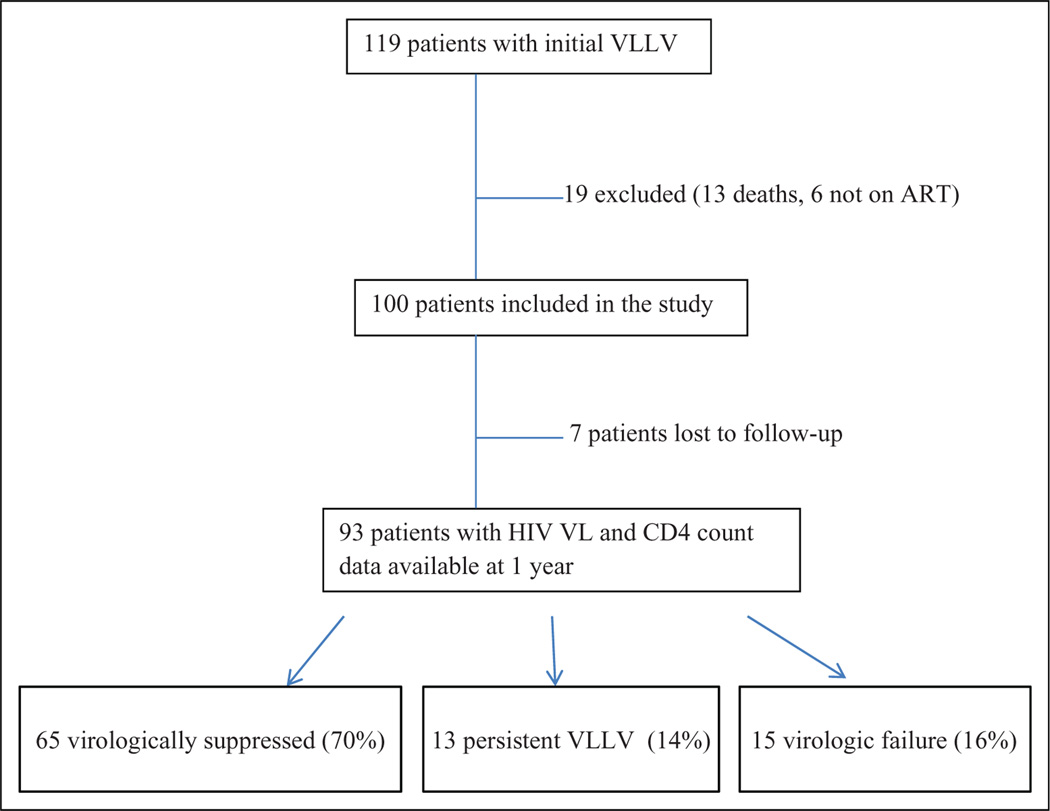
Among 1712 HIV VL measurements performed between February 2013 and February 2014, 119 unique patients were identified as having VLLV (20–50 copies/mL). Nineteen patients were excluded from the analysis: 13 because of death and 6 participants were not on ART (2 elite controllers and 4 declined HIV medications; Figure 1).
Figure 1.
Timetable of outcomes for patient with very-low-level viremia (VLLV) sample over 1 year.
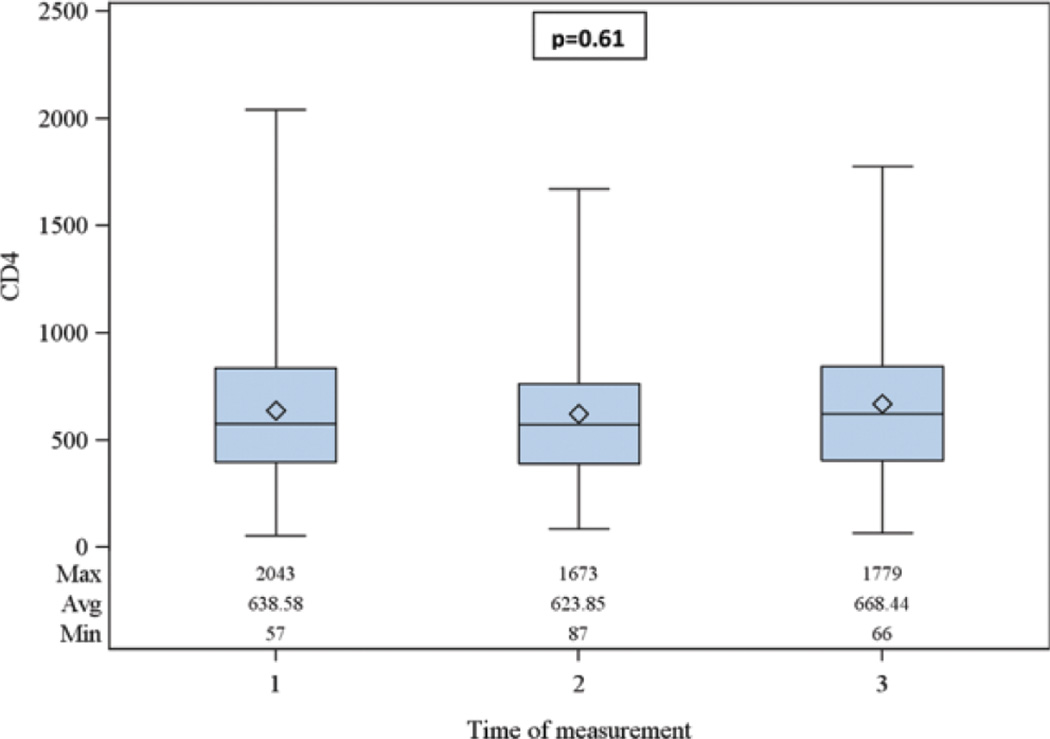
One hundred eligible patients had evaluable data. The median age was 53 years (interquartile range [IQR] 43–57.5); 67% were nonwhite, and 34% were women (Table 1). The median CD4 count at baseline was 639 cells/mm3 (Figure 2). Of those with documentation of substance abuse, 58% had a documentation of a history or active intravenous drug use, 47% alcohol use, and 58% were current or former smokers.
Table 1.
Patient Characteristics and Correlates of Virologic Failure.
| Variable | Frequency | Virologically Suppressed at 1 Year (n = 77) |
Virologic Failure at 1 Year (n = 15) |
P Value |
|---|---|---|---|---|
| Race | ||||
| White | 29 | 21 (72.4%) | 8 (27.6%) | .03 |
| Nonwhite | 67 | 61 (91%) | 6 (9%) | |
| Gender | ||||
| Male | 66 | 53 (80.3%) | 13 (19.7%) | .09 |
| Female | 34 | 32 (94%) | 2 (6%) | |
| IV drug use (n = 52) | ||||
| Yes | 30 | 22 (73.3%) | 8 (26.7%) | .84 |
| No | 22 | 15 (68.2%) | 7 (31.8%) | |
| Smoking (n = 70) | ||||
| Yes | 39 | 28 (72%) | 11 (28%) | .25 |
| No | 31 | 27 (87%) | 4 (13%) | |
| Alcohol use (n = 92) | ||||
| Yes | 43 | 34 (79%) | 9 (21%) | .11 |
| No | 49 | 43 (87.7%) | 6 (12.3%) | |
| HCV coinfection (n = 76) | ||||
| Yes | 30 | 22 (73.3%) | 8 (26.7%) | .31 |
| No | 46 | 39 (84.8%) | 7 (15.2%) | |
| Number of ART pills | ||||
| 1 | 38 | 34 (89.5%) | 4 (10.5%) | .33 |
| 2 or more | 62 | 51 (82.3%) | 11 (17.7%) | |
| Duration of ART (n = 99) | ||||
| More than 1 year |
90 | 76 (84.4%) | 14 (15.6%) | .57 |
| Less than 1 year |
9 | 8 (88.8%) | 1 (11.2%) | |
| Number of comorbidities | ||||
| 2 or less | 74 | 68 (92%) | 6 (8%) | .69 |
| 3 or more | 26 | 17 (65.4%) | 9 (34.6%) | |
| Number of medications (non-ART) | ||||
| 3 or less | 40 | 33 (82.5%) | 7 (17.5%) | .51 |
| 4 or more | 60 | 52 (86.6%) | 8 (13.4%) | |
Abbreviations: ART, antiretroviral therapy; HCV, hepatitis C virus; IV, intravenous.
P < 0.05.
Figure 2.
Distribution of CD4 count over 12 months after baseline very-low-level viremia (VLLV) event. (1 = initial VLLV event, 2 = first follow-up assessment, 3 = second follow-up assessment within 1 year).
The median number of comorbidities was 3 (IQR: 1–5). Fifty percent had psychiatric disease, 40% had arterial hypertension, 30% dyslipidemia, and 20% had diabetes mellitus. Among 76 patients with available serology results, 30 (40%) were HCV antibody positive. Twenty-three patients had a history of AIDS defining illnesses. Sixty-two patients were taking 2 or more ART pills daily and 60% were taking 4 or more non-ART medications daily. Almost ninety percent of patients had their ART initiated more than 12 months prior to the initial VLLV. Thirty-five percent of patients had a nonnucleoside reverse transcriptase inhibitor–based regimen, while 32% were taking a protease inhibitor–based regimen.
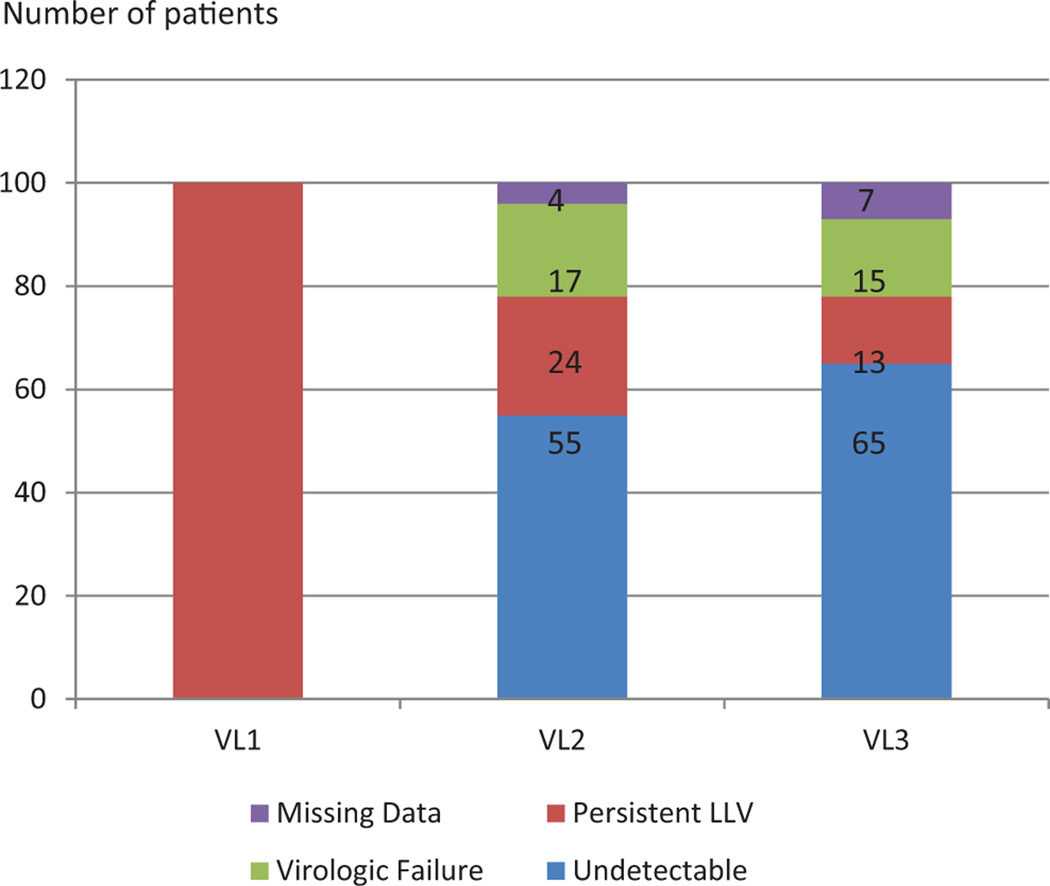
Among the 100 patients with initial VLLV, 55 became undetectable at the second viral load measurement, 23 had persistent LLV and 18 had virologic failure. Data were unavailable for 4 patients who were lost to follow up. The first and second viral load measurements were separated by a median of 14 weeks (IQR = 11–18). The second and third measurements were separated by a median of 14 weeks as well (IQR = 12–19).
At 12 months, viral load and CD4 count data were available for 93 patients. Seven patients were lost to follow up and thus excluded from the statistical analysis. Among the 93 patients, 65 (70%) had virologic suppression including undetectable VL and less than 20 copies/mL, and 13 (14%) had persistent VLLV. There were a total of 15 patients (16%) with virologic failure (VL range: 51–122 000, median = 743; Figures 1 and 3). Of those 15 patients, 11 had documented poor adherence, 13 were men, and 6 had active mental health- or substance-abuse problems. Only 6 of 15 patients had ART resistance genotype testing available for review, and only 3 had new resistance genotype testing performed, but no new mutations were detected.
Figure 3.
Evolution of viral load trends over time (VL1: initial viral load, VL2: viral load at 6 months, VL3: viral load at 12 months; virologic failure: VL > 50 copies/mL, persistent LLV: VL 20–50 copies/mL, undetectable: VL < 20 copies/mL). Abbreviation: LLV, low level viremia.
Seventy-three percent of patients who had virologic failure reported nonadherence to ART, while 27% said they were adherent to their medications. Adherence data were unavailable for the remaining patients. Fifty percent of the patients who were nonadherent had mental health diagnoses, substance abuse, and/or alcoholism as risk factors.
Comparison of patients with virologic failure (n = 15) and those who are suppressed at 1 year (Table 1) showed a significantly higher prevalence of white patients in the failure group (P = .03) and predominance of male gender (P = .09). Among those with virologic failure, 60% had 3 or more comorbidities (P = .69). Notably, 73% of those with virologic failure after initial VLLV were taking more than 1 ART pill daily and 72% were smokers but these were not statistically significant correlates.
The median change in CD4 count over the following 12 months was +10 cells/mm3 (IQR: −70, −99; Figure 3). Very-low-level viremia was not significantly associated with subsequent impaired CD4 count.
Though univariate analysis demonstrated that race, gender, and alcohol use were individually predictive of virologic failure, multivariate regression revealed that only alcohol use trended toward being a significant predictor of virologic failure at 1 year (P = .08).
Regarding deceased patients (13 of 119) who represented a relatively high proportion but were excluded from the analysis, 6 had cancer as a precipitating cause of death (hepatocellular carcinoma in the context of HCV infection, chronic myelogenous leukemia, and Burkitt lymphoma). Four patients had unclear cause of death including GI bleed, seizures, respiratory failure/acute respiratory distress syndrome (ARDS), and septic or other type of shock. Only 2 of 13 patients had initial VLLV, then subsequently developed virologic failure prior to death and had a CD4 count of less than 200 cells/mm3, while the remaining patients had undetectable viral loads after initial VLLV with CD4 counts >200 cells/mm3.
Discussion
Our study demonstrated that the majority of patients with initial VLLV achieved virologic suppression at 1 year with no evidence of development of virologic resistance or decreased CD4 count. Prior published studies have reported inconsistent outcomes regarding the significance of VLLV and the likelihood of virologic failure over time.2,4,5,16,17 In our study, we demonstrated that the majority of patients with VLLV (70%) will achieve a nondetectable viral load over time. A smaller proportion (16%) will proceed to virologic failure with less than 5% due to true virologic failure (>200 copies/mL). This result is similar to other recently reported studies which found that a small number of patients with VLLV (16%) do experience virologic failure at follow-up.2 In our study, we looked indepth into other factors associated with virologic failure. We found that the majority of patients (73%) had documented poor adherence. This finding is similar to previous studies showing that since ART adherence was self-reported, nonadherence could be the most likely cause of low-level viremia and multidrug resistance, even in reportedly adherent patients.18,19
In addition, we demonstrated that white race and male gender were all significantly associated with virologic failure after 1 year. Most of these patients had comorbidities (smoking, IVDU, alcoholism, and HCV coinfection) and high pill burden (ART and non-ART) that may have contributed to poor adherence. Pill burden remains a major concern, with regard to ART as well as medications prescribed for other comorbid conditions. Patients who have to take multiple medications at different times of the day are more likely to miss doses. High prevalence of mental illness could also decrease adherence. In our study, antihypertensive and psychiatric medications were common. In contrast, a recent international trial showed that black race and smoking were negatively associated with adherence, whereas older age, higher education, and area of residence had a positive impact.18
Interestingly, the majority of the patients with VLLV (70%) subsequently achieved HIV VL suppression without changes to the ART regimen, and there was no significant insult to the CD4 count, suggesting little short-term clinical impact of VLLV. Higher immune activation and HIV transmission may be additional undesirable consequences in this population. Previous studies had contradictory outcomes regarding the significance of VLLV and the virologic failure outcome.2,4,5,16,20 The most likely potential mechanisms are low-level viral replication and the intrinsic stability of latently infected cells. Studies have demonstrated that intermittent or persistent VLLV was associated with higher residual viremia, steady or increasing levels of HIV in the latent reservoir, selection of resistant HIV, increased risk of virologic failure, and higher levels of activated T cells.7,21–23 Thus, it is unclear how the VLLV can have an impact on the inflammatory process related to HIV and the risk of added comorbidities including cardiovascular disease, liver disease, and malignancy, although a recent study showed that VLLV was not associated with non-AIDS comorbidities.24,25
In our study, alcohol use trended toward a statistically significant association with virologic failure, though a recent Swiss study concluded that there was no effect of alcohol consumption on either virologic failure or CD4 count in both groups of ART-initiating and ART-naive individuals. However, drinkers were more likely to interrupt ART. Assessing for high alcohol consumption and developing a plan for substance abuse treatment while maintaining ART is critical for optimal care of patients.26
Over the course of 12 months, 51 viral load and 47 CD4 count measurements were performed before the routine 12-week interval, with a mean cost of US$367 per VL and US$383 per CD4 count, which resulted in a combined extra cost of US$36 718. A recent study found even more drastic numbers and cost excess when viral load assays were switched from the branched DNA to viral load assay.27
Several limitations exist in this study. The data were collected in a retrospective manner and so was subject to missing data. In addition, our sample size was limited and derived from 1 clinical site. Lastly, our aim was to investigate the management of VLLV; thus, we did not include the 13 patient deaths in our analysis though it is possible that VLLV contributed to these deaths.
Conclusion
In this study of a large urban HIV clinic, VLLV was not an infrequent occurrence. However, the majority of patients with VLLV subsequently achieve virologic suppression after 1 year of follow-up without changes in ART. Less than 5% of patients with VLLV experienced overt virologic failure (VL > 200 copies/mL) in 1 year of follow-up but none had documented HIV viral resistance. Very-low-level viremia has the potential to increase clinical care costs, given more frequent laboratory testing and clinical visits in addition to raising patient and provider anxiety levels. More studies are needed in order to determine the predictors of virologic failure and how best to monitor patients with VLLV as well as promote adherence.
Acknowledgments
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Footnotes
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Sedaghat AR, Siliciano RF, Wilke CO. Low-level HIV-1 replication and the dynamics of the resting CD4+ T cell reservoir for HIV-1 in the setting of HAART. BMC Infect Dis. 2008;8:2. doi: 10.1186/1471-2334-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antiretroviral Therapy Cohort Collaboration (ART-CC) Vandenhende MA, Ingle S, May M, et al. Impact of low-level viremia on clinical and virological outcomes in treated HIV-1-infected patients. AIDS. 2015;29(3):373–383. doi: 10.1097/QAD.0000000000000544. [DOI] [PubMed] [Google Scholar]
- 3.Lima V, Harrigan R, Montaner JS. Increased reporting of detectable plasma HIV-1 RNA levels at the critical threshold of 50 copies per milliliter with the TaqMan assay in comparison to the Amplicor assay. J Acquir Immune Defic Syndr. 2009;51(1):3–6. doi: 10.1097/QAI.0b013e31819e721b. [DOI] [PubMed] [Google Scholar]
- 4.Ryscavage P, Kelly S, Li JZ, et al. Significance and clinical management of persistent low-level viremia and very-low-level viremia in HIV-1-infected patients. Antimicrob Agents Chemother. 2014;58(7):3585–3598. doi: 10.1128/AAC.00076-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doyle T, Smith C, Vitiello P, et al. Plasma HIV-1 RNA detection below 50 copies/mL and risk of virologic rebound in patients receiving highly active antiretroviral therapy. Clin Infect Dis. 2012;54(5):724–732. doi: 10.1093/cid/cir936. [DOI] [PubMed] [Google Scholar]
- 6.Maggiolo F, Callegaro A, Cologni G, et al. Ultrasensitive assessment of residual low-level HIV viremia in HAART-treated patients and risk of virological failure. J Acquir Immune Defic Syndr. 2012;60(5):473–483. doi: 10.1097/QAI.0b013e3182567a57. [DOI] [PubMed] [Google Scholar]
- 7.Mavigner M, Delobel P, Cazabat M, et al. HIV-1 residual viremia correlates with persistent T-cell activation in poor immunological responders to combination antiretroviral therapy. PLoS One. 2009;4(10):e7658. doi: 10.1371/journal.pone.0007658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ostrowski SR, Katzenstein TL, Pedersen BK, et al. Residual viraemia in HIV-1-infected patients with plasma viral load < or =20 copies/ml is associated with increased blood levels of soluble immune activation markers. Scand J Immunol. 2008;68(6):652–660. doi: 10.1111/j.1365-3083.2008.02184.x. [DOI] [PubMed] [Google Scholar]
- 9.Pascual-Pareja JF, Martínez-Prats L, Luczkowiak J, et al. Detection of HIV-1 at between 20 and 49 copies per milliliter by the Cobas TaqMan HIV-1 v2.0 assay is associated with higher pretherapy viral load and less time on antiretroviral therapy. J Clin Microbiol. 2010;48(5):1911–1912. doi: 10.1128/JCM.02388-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ostrowski SR, Katzenstein TL, Thim PT, et al. Low-level viremia and proviral DNA impede immune reconstitution in HIV-1-infected patients receiving highly active antiretroviral therapy. J Infect Dis. 2005;191(3):348–357. doi: 10.1086/427340. [DOI] [PubMed] [Google Scholar]
- 11.Henrich TJ, Wood BR, Kuritzkes DR. Increased risk of virologic rebound in patients on antiviral therapy with a detectable HIV load <48 copies/mL. PLoS One. 2012;7(11):e50065. doi: 10.1371/journal.pone.0050065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pernas B, Grandal M, Pertega S, et al. Any impact of blips and low-level viraemia episodes among HIV-infected patients with sustained virological suppression on ART? J Antimicrob Chemother. 71(14):1051–1055. doi: 10.1093/jac/dkv433. [DOI] [PubMed] [Google Scholar]
- 13.Calcagno A, Motta I, Ghisetti V, et al. HIV-1 very low level viremia is associated with virological failure in highly active antiretroviral treatment-treated patients. AIDS Res Hum Retroviruses. 2015;31(10):999–1008. doi: 10.1089/AID.2015.0102. [DOI] [PubMed] [Google Scholar]
- 14.Leierer G, Grabmeier-Pfistershammer K, Steuer A, et al. Taylor NAustrian HIV Cohort Study Group. Factors associated with low-level viraemia and virological failure: results from the Austrian HIV Cohort Study. PLoS One. 2015;10(11):e0142923. doi: 10.1371/journal.pone.0142923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pyne MT, Brown KL, Hillyard DR. Evaluation of the Roche Cobas AmpliPrep/Cobas TaqMan HIV-1 test and identification of rare polymorphisms potentially affecting assay performance. J Clin Microbiol. 2010;48(8):2852–2858. doi: 10.1128/JCM.00776-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gianotti N, Galli L, Racca S, et al. Residual viraemia does not influence 1 year virological rebound in HIV-infected patients with HIV RNA persistently below 50 copies/mL. J Antimicrob Chemother. 2012;67(1):213–217. doi: 10.1093/jac/dkr422. [DOI] [PubMed] [Google Scholar]
- 17.Charpentier C, Landman R, Laouenan C, et al. Persistent low-level HIV-1 RNA between 20 and 50 copies/mL in antiretroviral-treated patients: associated factors and virological outcome. J Antimicrob Chemother. 2012;67(9):2231–2235. doi: 10.1093/jac/dks191. [DOI] [PubMed] [Google Scholar]
- 18.O’Connor JL, Gardner EM, Mannheimer SB, et al. INSIGHT SMART Study Group. Factors associated with adherence amongst 5295 people receiving antiretroviral therapy as part of an international trial. J Infect Dis. 2013;208(1):40–49. doi: 10.1093/infdis/jis731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nel A, Kagee A. Common mental health problems and antiretroviral therapy adherence. AIDS Care. 2011;23(11):1360–1365. doi: 10.1080/09540121.2011.565025. [DOI] [PubMed] [Google Scholar]
- 20.Álvarez Estévez M, Chueca Porcuna N, Guillot Suay V, et al. Quantification of viral loads lower than 50 copies per milliliter by use of the Cobas AmpliPrep/Cobas TaqMan HIV-1 test, version 2.0, can predict the likelihood of subsequent virological rebound to >50 copies per milliliter. J Clin Microbiol. 2013;51(5):1555–1557. doi: 10.1128/JCM.00100-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reus S, Portilla J, Sanchez-Paya J, et al. Low-level HIV viremia is associated with microbial translocation and inflammation. J Acquir Immune Defic Syndr. 2013;62(2):129–134. doi: 10.1097/QAI.0b013e3182745ab0. [DOI] [PubMed] [Google Scholar]
- 22.Karlsson AC, Younger SR, Martin JN, et al. Immunologic and virologic evolution during periods of intermittent and persistent low-level viremia. AIDS. 2004;18(7):981–989. doi: 10.1097/00002030-200404300-00005. [DOI] [PubMed] [Google Scholar]
- 23.Chun TW, Nickle DC, Justement JS, et al. HIV-infected individuals receiving effective antiviral therapy for extended periods of time continually replenish their viral reservoir. J Clin Invest. 2005;115(11):3250–3255. doi: 10.1172/JCI26197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Costarelli S, Bernasconi D, Lapadula G, et al. Persistent HIV low-level viremia and cardiovascular risk [Abstract PE13/41]. Paper presented at: 15th European AIDS Conference; October 21–24, 2015; Barcelona, Spain. [Google Scholar]
- 25.Antinori A, Cozzi Lepri A, Ammassari A, et al. Low-level viremia ranging from 50 to 500 copies/mL is associated to an increased risk of AIDS events in the Icona Foundation Cohort. [Abstract PS4/2]. Paper presented at: 15th European AIDS Conference; October 21–24, 2015; Barcelona, Spain. [Google Scholar]
- 26.Conen A, Wang Q, Glass TR, et al. Association of alcohol consumption and HIV surrogate markers in participants of the Swiss HIV cohort study. J Acquir Immune Defic Syndr. 2013;64(5):472–478. doi: 10.1097/QAI.0b013e3182a61ea9. [DOI] [PubMed] [Google Scholar]
- 27.Tipple C, Oomeer S, Dosekun O, et al. Service impact of a change in HIV-1 viral load quantification assay. J Int AIDS Soc. 2014;17(4 suppl 3):19677. doi: 10.7448/IAS.17.4.19677. [DOI] [PMC free article] [PubMed] [Google Scholar]