Abstract
Atrial fibrillation (AF) is associated with an increased risk for adverse events in patients with heart failure with preserved ejection (HFpEF), but it is currently unknown if gender differences in these outcomes exist. To explore this hypothesis, we examined gender differences in the associations of AF with adverse outcomes in 3,385 (mean age=69±9.6 years; 49% male; 89% white) patients with HFpEF from the Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist Trial (TOPCAT). Baseline AF cases were identified by self-reported history, medical record review, and baseline electrocardiogram data. Outcomes were adjudicated by a clinical end-point committee and included the following: hospitalization, hospitalization for heart failure, stroke, death, and cardiovascular death. Cox regression was used to examine the risk of each outcome associated with AF. Over a median follow-up of 3.4 years, AF was associated with an increased risk for hospitalization (HR=1.49, 95%CI=1.34, 1.66), hospitalization for heart failure (HR=1.49, 95%CI=1.23, 1.81), stroke (HR=2.10, 95%CI=1.43, 2.09), death (HR=1.22, 95%CI=1.02, 1.47), and cardiovascular death (HR=1.31, 95%CI=1.04, 1.65). The association between AF and hospitalization was stronger in women (HR=1.63, 95%CI=1.40, 1.91) than men (HR=1.37, 95%CI=1.18, 1.58; p-interaction=0.032). Although significant interactions were not observed for the other outcomes, we appreciated that the risk estimates were higher for women compared with men. In conclusion, AF increases the risk for adverse cardiovascular outcomes in patients with HFpEF, and the presence of this arrhythmia in women possibly is associated with a greater risk for adverse events than men.
Keywords: heart failure, preserved ejection fraction, atrial fibrillation
INTRODUCTION
Heart failure with preserved ejection fraction (HFpEF) is an emerging public health problem, representing nearly 50% of heart failure cases.1,2 Atrial fibrillation (AF) is commonly found in persons who have HFpEF,3,4 and the link between both conditions likely is explained by shared risk factors which predispose to each condition.5 In patients with HFpEF, AF is associated with an increased risk for adverse events.6–8 Due to the fact that women are more likely to develop HFpEF than men,9 and women who have AF have a higher risk of cardiovascular events compared with their male counterparts,10–14 it is possible that gender differences exist in the outcomes of HFpEF patients who have AF. Therefore, we examined the impact of AF on outcomes in patients with HFpEF in the Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist Trial (TOPCAT),15 and if these outcomes differed by gender.
METHODS
TOPCAT was a multi-center, international randomized, double blind, placebo-control study to examine the efficacy of spironolactone in patients with HFpEF. The design, inclusion criteria, and baseline characteristics of the trial have been published previously.16,17 Briefly, 3,445 patients with symptomatic HFpEF from 270 sites in 6 countries were enrolled between August, 2006 and January, 2012. The primary goal of the trial was to determine if spironolactone was associated with a reduction in the composite outcome of cardiovascular mortality, aborted cardiac arrest, or heart failure hospitalization in patients with HFpEF (e.g., documented ejection fraction ≥45%). For the purpose of this analysis, we excluded TOPCAT participants without complete baseline information or follow-up data.
Patients who participated in TOPCAT underwent a detailed baseline visit to obtain medical histories and a physical examination was performed.17 Baseline AF cases were identified by self-reported history, medical record review, and the baseline electrocardiogram obtained during the initial study visit. AF cases included paroxysmal and chronic cases. Age, gender, race, and smoking were obtained by self-reported history. Smoking was defined as the current use of cigarettes. Medical history for the following diagnoses were obtained by self-report and medical record review: diabetes, coronary heart disease, stroke, New York Heart Association functional classification, and prior heart failure hospitalization. Systolic blood pressure and body mass index were obtained by trained staff and laboratory data included serum creatinine. Medication data also were obtained during the initial study visit and the following were included in this analysis: aspirin, beta blockers, angiotensin-converting enzyme inhibitors/angiotensin II receptor blockers, and statins.
Outcomes in TOPCAT were adjudicated by a clinical end-point committee, and the details of this process and definitions for each outcome examined have been described previously.15,16 The outcomes examined in this analysis included hospitalization, hospitalization for heart failure, stroke, death, and cardiovascular death. Briefly, hospitalization for heart failure was defined as the unexpected presentation to an acute care facility requiring overnight stay with symptoms and physical exam findings consistent with heart failure, and treatment with intravenous vasodilators, inotropes, mechanical fluid removal, or hemodynamic support. Stroke was defined as a focal neurological deficit of sudden onset that was not reversible within 24 hours of onset, or a focal neurological deficit of sudden onset with brain imaging consistent with infarction or hemorrhage. Cardiovascular death was defined as death due to one of the following: myocardial infarction, worsening heart failure, sudden death, stroke, pulmonary embolism, death occurring during a cardiovascular-related procedure, or other cardiovascular death. Death included the composite of cardiovascular and non-cardiovascular death.
Baseline characteristics were compared by the presence of baseline AF. Categorical variables were reported as frequency and percentage, while continuous variables were recorded as mean ± standard deviation. Statistical significance for categorical variables was tested using the chi-square method and for continuous variables to the student’s t-test was used. Kaplan-Meier estimates were used to examine the unadjusted cumulative incidence estimates of each outcome associated with baseline AF. Cox regression was used to examine the risk of each outcome associated with AF. Multivariable models were constructed as follows: Model 1 adjusted for age, race, and gender; Model 2 adjusted for Model 1 covariates with the addition of smoking, systolic blood pressure, diabetes, body mass index, angiotensin-converting enzyme inhibitors/angiotensin II receptor blockers, beta blockers, statin, randomization group, New York Heart Association functional classification, coronary heart disease, and stroke. The risk of each outcome associated with AF was examined in men and women, separately, and effect modification was tested using multiplicative interaction terms. A secondary analysis was performed in patients with prior heart failure hospitalization to determine if the magnitude of the association between AF and each outcome was dependent on prior admission. Additionally, due to differences in the baseline characteristics and event rates observed between patients recruited in Russia and Georgia versus the Americas,18 we examined if our findings varied by region of enrollment (Russia/Georgia vs. the Americas). The proportional hazards assumption was not violated in our analysis. Statistical significance, including interaction terms, was defined as p < 0.05. SAS Version 9.4 (Cary, NC) was used for all analyses.
RESULTS
This analysis included 3,385 (mean age=69±9.6 years; 49% male; 89% white) patients, of whom 1,191 patients (35%) had AF. Patients with AF were more likely to be older, male, white, report prior stroke, and to have a higher New York Heart Association functional classification and serum creatinine values, than those without AF. Patients with AF also were less likely to be current smokers, have diabetes, report prior coronary heart disease, prior hospitalization for heart failure, the use of aspirin and angiotensin-converting enzyme inhibitors/angiotensin II receptor blockers, than patients who did not have AF (Table 1).
Table 1.
Baseline Characteristics (N=3,385)
| Characteristic | AF (n=1,191) |
No AF (n=2,194) |
P-value* |
|---|---|---|---|
| Age (years) | 71 ± 9.3 | 67 ± 9.4 | <0.001 |
| Male | 641 (54%) | 1,002 (46%) | <0.001 |
| White | 1,093 (92%) | 1,916 (87%) | <0.001 |
| Current smoker | 79 (6.6%) | 280 (13%) | <0.001 |
| Diabetes mellitus | 348 (29%) | 743 (34%) | 0.0057 |
| Coronary heart disease | 370 (31%) | 843 (38%) | <0.001 |
| Stroke | 114 (10%) | 147 (6.7%) | 0.0028 |
| Systolic blood pressure, mean ± SD (mm Hg) | 127 ± 14 | 131 ± 14 | <0.001 |
| Body mass index, mean ± SD (kg/m2) | 32 ± 7.0 | 32 ± 7.2 | 0.95 |
| Serum creatinine, mean ± SD (mg/dL) | 1.12 ± 0.30 | 1.07 ± 0.30 | <0.001 |
| New York Heart Association Class III–IV | 471 (40%) | 647 (29%) | <0.001 |
| Prior heart failure hospitalization | 819 (69%) | 1,630 (74%) | <0.001 |
| Aspirin use | 605 (51%) | 1,613 (74%) | <0.001 |
| Beta blockers | 913 (77%) | 1,723 (79%) | 0.21 |
| ACEi/ARB | 977 (82%) | 1,875 (85%) | 0.0089 |
| Statin | 632 (53%) | 1,136 (52%) | 0.47 |
| Spironolacton | 604 (51%) | 1,093 (50%) | 0.62 |
Statistical significance for continuous data was tested using the student’s t-test and categorical data was tested using the chi-square test.
ACEi/ARB= angiotensin-converting enzyme inhibitors/angiotensin II receptor blockers; AF=atrial fibrillation; HDL=high-density lipoprotein; SD=standard deviation.
Over a median follow-up of 3.4 years (25th–75th percentiles=2.0, 4.9 years), a total of 1,524 hospitalizations, 437 hospitalizations for heart failure, 115 strokes, 516 deaths, and 330 cardiovascular deaths occurred. Higher incidence rates for all outcomes were observed in patients with AF than those without AF. The cumulative incidence estimates for hospitalization, hospitalization for heart failure, stroke, death, and cardiovascular death by AF status are depicted in Figures 1, 2, and 3. The cumulative incidence estimates per 100 person-years are shown in Table 2.
Figure 1. Unadjusted Cumulative Incidence of Hospitalization*.
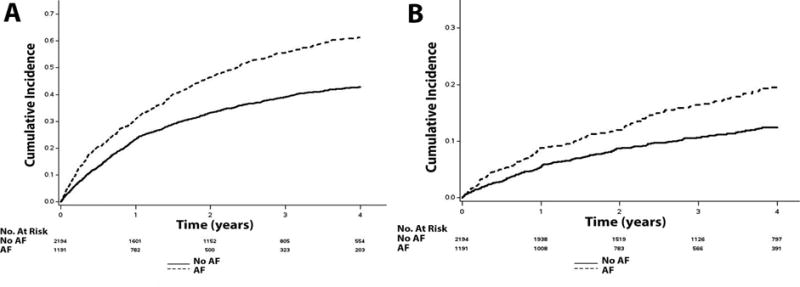
*The cumulative incidence curves for hospitalization (A) and hospitalization for heart failure (B) are shown. The cumulative incidence curves are statistically different for both hospitalization (log-rank p<0.001) and hospitalization for heart failure (log-rank p<0.001).
AF=atrial fibrillation.
Figure 2. Unadjusted Cumulative Incidence of Stroke.
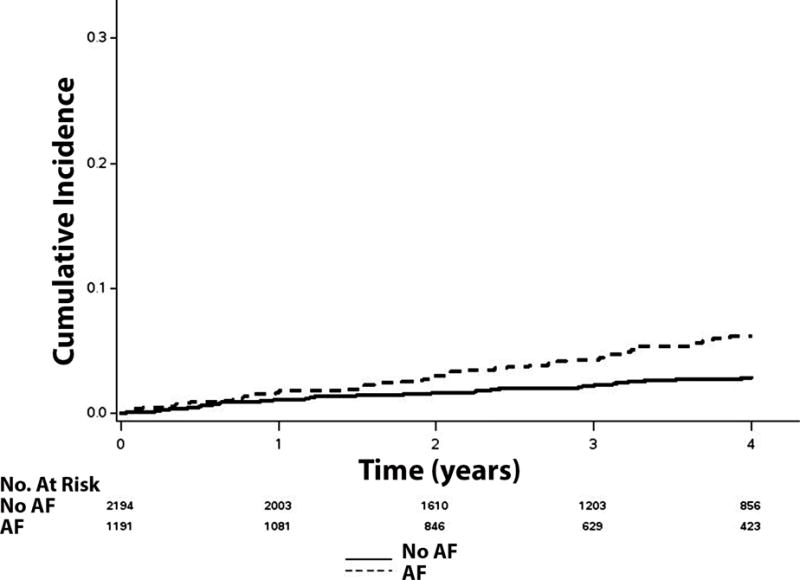
*Cumulative incidence curves are statistically different (log-rank p<0.001).
AF=atrial fibrillation.
Figure 3. Unadjusted Cumulative Incidence of Death.
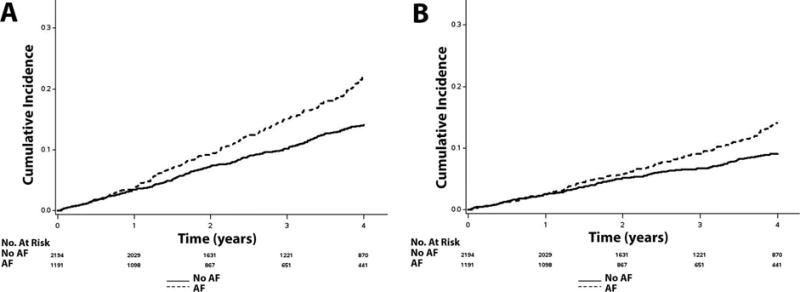
*The cumulative incidence curves for death (A) and cardiovascular death (B) are shown. The cumulative incidence curves are statistically different for both death (log-rank p<0.001) and cardiovascular death (log-rank p<0.001).
AF=atrial fibrillation.
Table 2.
Risk of Hospitalization, Stroke, and Death with Atrial Fibrillation (N=3,385)
| Outcome | Events | Incidence rate per 100 person-years | Model 1* HR (95%CI) | P-value | Model 2† HR (95%CI) | P-value | Interaction P-value |
|---|---|---|---|---|---|---|---|
| Hospitalization | |||||||
| All | 1,524 | 18.9 (18.1, 19.9) | 1.52 (1.37, 1.69) | <0.001 | 1.49 (1.34, 1.66) | <0.001 | |
| Women | 733 | 17.1 (15.9, 18.4) | 1.70 (1.46, 1.98) | <0.001 | 1.63 (1.40, 1.91) | <0.001 | 0.032 |
| Men | 791 | 21.2 (19.7, 22.7) | 1.38 (1.19, 1.58) | <0.001 | 1.37 (1.18, 1.58) | <0.001 | |
|
| |||||||
| Hospitalization for Heart Failure | |||||||
| All | 437 | 4.1 (3.7, 4.5) | 1.55 (1.28, 1.88) | <0.001 | 1.49 (1.23, 1.81) | <0.001 | |
| Women | 207 | 3.7 (3.2, 4.3) | 1.66 (1.25, 2.20) | <0.001 | 1.54 (1.15, 2.06) | 0.0040 | 0.94 |
| Men | 230 | 4.5 (4.0, 5.2) | 1.47 (1.13, 1.92) | 0.0039 | 1.47 (1.12, 1.92) | 0.0048 | |
|
| |||||||
| Stroke | |||||||
| All | 115 | 1.0 (0.85, 1.23) | 1.94 (1.33, 2.83) | <0.001 | 2.10 (1.43, 3.09) | <0.001 | |
| Women | 61 | 1.0 (0.8, 1.3) | 2.31 (1.37, 3.90) | 0.0018 | 2.58 (1.49, 4.48) | <0.001 | 0.54 |
| Men | 54 | 1.0 (0.8, 1.3) | 1.65 (0.96, 2.84) | 0.071 | 1.77 (1.02, 3.08) | 0.042 | |
|
| |||||||
| Death | |||||||
| All | 516 | 4.5 (4.2, 4.9) | 1.27 (1.07, 1.52) | 0.0080 | 1.22 (1.02, 1.47) | 0.031 | |
| Women | 213 | 3.6 (3.1, 4.1) | 1.33 (1.00, 1.76) | 0.047 | 1.31 (0.98, 1.75) | 0.069 | 0.68 |
| Men | 303 | 5.6 (4.9, 6.2) | 1.23 (0.98, 1.55) | 0.078 | 1.17 (0.93, 1.49) | 0.19 | |
|
| |||||||
| Cardiovascular Death | |||||||
| All | 330 | 2.9 (2.6, 3.2) | 1.33 (1.07, 1.66) | 0.012 | 1.31 (1.04, 1.65) | 0.021 | |
| Women | 135 | 2.3 (1.9, 2.7) | 1.51 (1.06, 2.15) | 0.021 | 1.48 (1.03, 2.14) | 0.035 | 0.34 |
| Men | 195 | 3.6 (3.1, 4.1) | 1.22 (0.92, 1.62) | 0.18 | 1.21 (0.90, 1.63) | 0.21 | |
Adjusted for age, race, and gender.
Adjusted for Model 1 covariates plus smoking, systolic blood pressure, diabetes, body mass index, angiotensin-converting enzyme inhibitors/angiotensin II receptor blockers, beta blockers, statin, randomization group, New York Heart Association functional classification, coronary heart disease, and stroke.
CI=confidence interval; HR=hazard ratio.
An increased risk for hospitalization (HR=1.49, 95%CI=1.34, 1.66), hospitalization for heart failure (HR=1.49, 95%CI=1.23, 1.81), stroke (HR=2.10, 95%CI=1.43, 2.09), death (HR=1.22, 95%CI=1.02, 1.47), and cardiovascular death (HR=1.31, 95%CI=1.04, 1.65), was observed for those with AF compared with those without (Table 2). An interaction was observed for hospitalization, with the association between AF and hospitalization being stronger in women (HR=1.63, 95%CI=1.40, 1.91) than men (HR=1.37, 95%CI=1.18, 1.58; p-interaction=0.032) (Table 2). Although significant interactions were not detected for the other outcomes, the risk estimates were greater for women compared with men (Table 2).
When the analysis was limited to participants who reported prior hospitalization for heart failure (N=2,449), the magnitude of the risk for each outcome associated with AF was not substantively different from the main analysis (Table 3). Interactions by gender were not observed for those who reported prior hospitalization for heart failure. When we examined the association between AF and each outcome by region of enrollment (Russia/Georgia vs. the Americas), the associations of AF with hospitalization, hospitalization for heart failure, and stroke, were stronger in the patients from Russia/Georgia compared with the Americas (Supplemental Table 1). Interactions were not detected by gender for each outcome when we stratified the analysis by region.
Table 3.
Risk of Hospitalization, Stroke, and Death with Atrial Fibrillation in Patients with Prior Heart Failure Hospitalization (N=2,449)
| Outcome | Events | Incidence rate per 100 person-years | Model 1* HR (95%CI) | P-value | Model 2† HR (95%CI) | P-value | P-interaction for Gender |
|---|---|---|---|---|---|---|---|
| Hospitalization | 1,082 | 17.8 (16.8, 18.9) | 1.69 (1.49, 1.91) | <0.001 | 1.57 (1.38, 1.78) | <0.001 | 0.22 |
| Hospitalization for Heart Failure | 313 | 3.9 (3.5, 4.4) | 1.86 (1.48, 2.34) | <0.001 | 1.65 (1.31, 2.09) | <0.001 | 0.73 |
| Stroke | 83 | 1.0 (0.8, 1.2) | 2.21 (1.42, 3.44) | <0.001 | 2.31 (1.46, 3.68) | <0.001 | 0.30 |
| Death | 365 | 4.3 (3.9, 4.7) | 1.40 (1.13, 1.72) | 0.0019 | 1.29 (1.04, 1.61) | 0.022 | 0.72 |
| Cardiovascular Death | 244 | 2.9 (2.5, 3.3) | 1.51 (1.17, 1.95) | 0.0018 | 1.42 (1.09, 1.86) | 0.0096 | 0.87 |
Adjusted for age, race, and gender.
Adjusted for Model 1 covariates plus smoking, systolic blood pressure, diabetes, body mass index, angiotensin-converting enzyme inhibitors/angiotensin II receptor blockers, beta blockers, statin, randomization group, New York Heart Association functional classification, coronary heart disease, and stroke.
CI=confidence interval; HR=hazard ratio.
DISCUSSION
In this analysis from TOPCAT, AF was associated with an increased risk for hospitalization, hospitalization for heart failure, stroke, death, and cardiovascular death, in patients with HFpEF. Additionally, we appreciated that the risk estimates associated with each outcome were higher for women with AF than men. Overall, our findings provide evidence that AF is associated with significant morbidity and mortality in HFpEF, and the presence of this arrhythmia in women possibly is associated with a greater risk of adverse events compared with men.
Several large studies have demonstrated an increased risk for adverse events in HFpEF patients who have AF.6–8 For example, data from the Candesartan in Heart failure-Assessment of Reduction in Mortality and morbidity Program have shown that AF in HFpEF is associated with an increased risk for cardiovascular death, hospitalization for worsening heart failure, and all-cause mortality.6 Subsequently, a report using medical claims data from 4 health plans in the Cardiovascular Research Network showed that AF increases the risk of death, hospitalization, hospitalization for heart failure, and ischemic stroke in HFpEF.7 Additionally, a study from the Acute Decompensated Heart Failure National Registry linked with Medicare inpatient claims, reported that AF increases the risk of 30-day mortality after admission for decompensated HFpEF.8 The aforementioned studies have clearly demonstrated that AF portends an increased risk for adverse events in HFpEF, but gender differences in outcomes were not explored.
The findings of the current analysis support that AF in patients with HFpEF is associated with an increased risk for adverse cardiovascular outcomes. In contrast with prior reports, the current study was not limited to claims data, and we were able to examine multiple outcomes that were ascertained by adjudication. Although a significant interaction by gender only was detected between hospitalization and AF, the risk estimates for all outcomes examined were higher in women than men. This suggests that women with AF and HFpEF are a high-risk group for the development of adverse events. To our knowledge this finding has not been previously reported. Since women with AF have a higher risk of adverse events (e.g., stroke, myocardial infarction, death) compared with men,10–14 the higher risk estimates for women in our study were not entirely unexpected. Our data extend the known gender differences in AF outcomes to HFpEF, as we have shown that women with AF and HFpEF have a higher risk of adverse events than their male counterparts. The reasons for these differences are unknown and possibly related to variation in AF or HFpEF management between men and women, and further research is needed to understand these differences.
Due to the reported regional differences in patient characteristics and outcomes in TOPCAT,18 we performed a secondary analysis stratified by region of enrollment. It has been documented that patients in TOPCAT from Russia/Georgia were younger, had less AF and diabetes, and were more likely to have had prior myocardial infarction and prior hospitalization for heart failure.18 Therefore, the adverse outcomes in patients from the Americas possibly were driven by other comorbid conditions (e.g., diabetes) rather than AF. In contrast, it is possible that AF represents a more important marker of illness severity in patients from Georgia/Russia, independent of cardiovascular risk factors. Due to the fact that patients from Russia/Georgia were more likely to have been hospitalized for heart failure prior to randomization, and an increased risk of 30-day mortality exists for patients with AF who are hospitalized for HFpEF,8 the AF profile possibly varies in patients from Russia/Georgia, as they were more likely to have been admitted previously for HFpEF. Although we offer several explanations for the regional differences in outcomes of HFpEF patients with AF, the above ideas are speculative and further research is needed to understand these differences.
The prevalence of heart failure is expected to rise in the coming decades, with annual costs increasing to nearly $53.1 billion.19 HFpEF accounts for 50% of heart failure cases,1 and it is more commonly found among older adults.2 Due to the expected increases in persons older than 65 years of age,20 the prevalence of HFpEF will reach epidemic proportions. Similarly, the burden of AF will continue to increase, as this condition is common among the elderly.21 The findings in this analysis demonstrated that AF is commonly found in HFpEF, and the risk of adverse events dramatically increases in HFpEF patients who have AF. Additionally, careful attention must be given to women with AF and HFpEF, due to the potential increased risk for adverse events compared with men. Therefore, medical providers should be aware of the poor prognosis that AF signifies in HFpEF, and the possible gender differences, and aim to optimize medical therapies and strategies to reduce hospitalization rates and other adverse outcomes in patients who have AF and HFpEF.
The current study should be interpreted in the context of certain limitations. Several baseline characteristics were obtained by self-report and subjected our analysis to recall bias. Similarly, some cases of AF were ascertained at baseline by self-report. Despite rigorous methodology to ascertain all outcomes, it is possible that cases were missed. Additionally, we tried to account for differences between those with and without AF in our multivariable models, but acknowledge the possibility of residual confounding.
Supplementary Material
Acknowledgments
This Manuscript was prepared using Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist Trial (TOPCAT) Research Materials obtained from the National Heart, Lung, and Blood Institute Biologic Specimen and Data Repository Information Coordinating Center and does not necessarily reflect the opinions or views of the TOPCAT Study or the National Heart, Lung, and Blood Institute.
FUNDING
Research reported in this publication was supported by the National Heart, Lung, And Blood Institute of the National Institutes of Health under award number F32-HL134290. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES
None.
References
- 1.Yancy CW, Lopatin M, Stevenson LW, De Marco T, Fonarow GC, Committee ASA, Investigators Clinical presentation, management, and in-hospital outcomes of patients admitted with acute decompensated heart failure with preserved systolic function: a report from the Acute Decompensated Heart Failure National Registry (ADHERE) Database. J Am Coll Cardiol. 2006;47:76–84. doi: 10.1016/j.jacc.2005.09.022. [DOI] [PubMed] [Google Scholar]
- 2.Oktay AA, Rich JD, Shah SJ. The emerging epidemic of heart failure with preserved ejection fraction. Curr Heart Fail Rep. 2013;10:401–410. doi: 10.1007/s11897-013-0155-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bursi F, Weston SA, Redfield MM, Jacobsen SJ, Pakhomov S, Nkomo VT, Meverden RA, Roger VL. Systolic and diastolic heart failure in the community. JAMA. 2006;296:2209–2216. doi: 10.1001/jama.296.18.2209. [DOI] [PubMed] [Google Scholar]
- 4.Zakeri R, Chamberlain AM, Roger VL, Redfield MM. Temporal relationship and prognostic significance of atrial fibrillation in heart failure patients with preserved ejection fraction: a community-based study. Circulation. 2013;128:1085–1093. doi: 10.1161/CIRCULATIONAHA.113.001475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benjamin EJ, Levy D, Vaziri SM, D’Agostino RB, Belanger AJ, Wolf PA. Independent risk factors for atrial fibrillation in a population-based cohort. The Framingham Heart Study. JAMA. 1994;271:840–844. [PubMed] [Google Scholar]
- 6.Olsson LG, Swedberg K, Ducharme A, Granger CB, Michelson EL, McMurray JJ, Puu M, Yusuf S, Pfeffer MA, Investigators C Atrial fibrillation and risk of clinical events in chronic heart failure with and without left ventricular systolic dysfunction: results from the Candesartan in Heart failure-Assessment of Reduction in Mortality and morbidity (CHARM) program. J Am Coll Cardiol. 2006;47:1997–2004. doi: 10.1016/j.jacc.2006.01.060. [DOI] [PubMed] [Google Scholar]
- 7.McManus DD, Hsu G, Sung SH, Saczynski JS, Smith DH, Magid DJ, Gurwitz JH, Goldberg RJ, Go AS, Cardiovascular Research Network PS Atrial fibrillation and outcomes in heart failure with preserved versus reduced left ventricular ejection fraction. J Am Heart Assoc. 2013;2:e005694. doi: 10.1161/JAHA.112.005694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eapen ZJ, Greiner MA, Fonarow GC, Yuan Z, Mills RM, Hernandez AF, Curtis LH. Associations between atrial fibrillation and early outcomes of patients with heart failure and reduced or preserved ejection fraction. Am Heart J. 2014;167:369–375 e362. doi: 10.1016/j.ahj.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 9.Lam CS, Donal E, Kraigher-Krainer E, Vasan RS. Epidemiology and clinical course of heart failure with preserved ejection fraction. Eur J Heart Fail. 2011;13:18–28. doi: 10.1093/eurjhf/hfq121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Soliman EZ, Safford MM, Muntner P, Khodneva Y, Dawood FZ, Zakai NA, Thacker EL, Judd S, Howard VJ, Howard G, Herrington DM, Cushman M. Atrial fibrillation and the risk of myocardial infarction. JAMA Intern Med. 2014;174:107–114. doi: 10.1001/jamainternmed.2013.11912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O’Neal WT, Sangal K, Zhang ZM, Soliman EZ. Atrial fibrillation and incident myocardial infarction in the elderly. Clin Cardiol. 2014;37:750–755. doi: 10.1002/clc.22339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Olesen JB, Torp-Pedersen C, Hansen ML, Lip GY. The value of the CHA2DS2-VASc score for refining stroke risk stratification in patients with atrial fibrillation with a CHADS2 score 0–1: a nationwide cohort study. Thromb Haemost. 2012;107:1172–1179. doi: 10.1160/TH12-03-0175. [DOI] [PubMed] [Google Scholar]
- 13.Benjamin EJ, Wolf PA, D’Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98:946–952. doi: 10.1161/01.cir.98.10.946. [DOI] [PubMed] [Google Scholar]
- 14.Friberg J, Scharling H, Gadsboll N, Truelsen T, Jensen GB, Copenhagen City Heart S Comparison of the impact of atrial fibrillation on the risk of stroke and cardiovascular death in women versus men (The Copenhagen City Heart Study) Am J Cardiol. 2004;94:889–894. doi: 10.1016/j.amjcard.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 15.Pitt B, Pfeffer MA, Assmann SF, Boineau R, Anand IS, Claggett B, Clausell N, Desai AS, Diaz R, Fleg JL, Gordeev I, Harty B, Heitner JF, Kenwood CT, Lewis EF, O’Meara E, Probstfield JL, Shaburishvili T, Shah SJ, Solomon SD, Sweitzer NK, Yang S, McKinlay SM, Investigators T Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014;370:1383–1392. doi: 10.1056/NEJMoa1313731. [DOI] [PubMed] [Google Scholar]
- 16.Desai AS, Lewis EF, Li R, Solomon SD, Assmann SF, Boineau R, Clausell N, Diaz R, Fleg JL, Gordeev I, McKinlay S, O’Meara E, Shaburishvili T, Pitt B, Pfeffer MA. Rationale and design of the treatment of preserved cardiac function heart failure with an aldosterone antagonist trial: a randomized, controlled study of spironolactone in patients with symptomatic heart failure and preserved ejection fraction. Am Heart J. 2011;162:966–972 e910. doi: 10.1016/j.ahj.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 17.Shah SJ, Heitner JF, Sweitzer NK, Anand IS, Kim HY, Harty B, Boineau R, Clausell N, Desai AS, Diaz R, Fleg JL, Gordeev I, Lewis EF, Markov V, O’Meara E, Kobulia B, Shaburishvili T, Solomon SD, Pitt B, Pfeffer MA, Li R. Baseline characteristics of patients in the treatment of preserved cardiac function heart failure with an aldosterone antagonist trial. Circ Heart Fail. 2013;6:184–192. doi: 10.1161/CIRCHEARTFAILURE.112.972794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pfeffer MA, Claggett B, Assmann SF, Boineau R, Anand IS, Clausell N, Desai AS, Diaz R, Fleg JL, Gordeev I, Heitner JF, Lewis EF, O’Meara E, Rouleau JL, Probstfield JL, Shaburishvili T, Shah SJ, Solomon SD, Sweitzer NK, McKinlay SM, Pitt B. Regional variation in patients and outcomes in the Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist (TOPCAT) trial. Circulation. 2015;131:34–42. doi: 10.1161/CIRCULATIONAHA.114.013255. [DOI] [PubMed] [Google Scholar]
- 19.Heidenreich PA, Albert NM, Allen LA, Bluemke DA, Butler J, Fonarow GC, Ikonomidis JS, Khavjou O, Konstam MA, Maddox TM, Nichol G, Pham M, Pina IL, Trogdon JG, American Heart Association Advocacy Coordinating C, Council on Arteriosclerosis T, Vascular B, Council on Cardiovascular R, Intervention, Council on Clinical C, Council on E, Prevention, Stroke C Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013;6:606–619. doi: 10.1161/HHF.0b013e318291329a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Odden MC, Coxson PG, Moran A, Lightwood JM, Goldman L, Bibbins-Domingo K. The impact of the aging population on coronary heart disease in the United States. Am J Med. 2011;124:827–833. doi: 10.1016/j.amjmed.2011.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, Singer DE. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285:2370–2375. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


