Abstract
Some proteolytic enzymes occurring in the leaves of field-grown corn (Zea mays) (B73) were identified and partially characterized. Changes in activities of several proteolytic enzymes and in concentrations of protein and chlorophyll as a function of intraleaf segments (tip to base), leaf position, and leaf senescence during grain development and maturation were followed in crude leaf extracts.
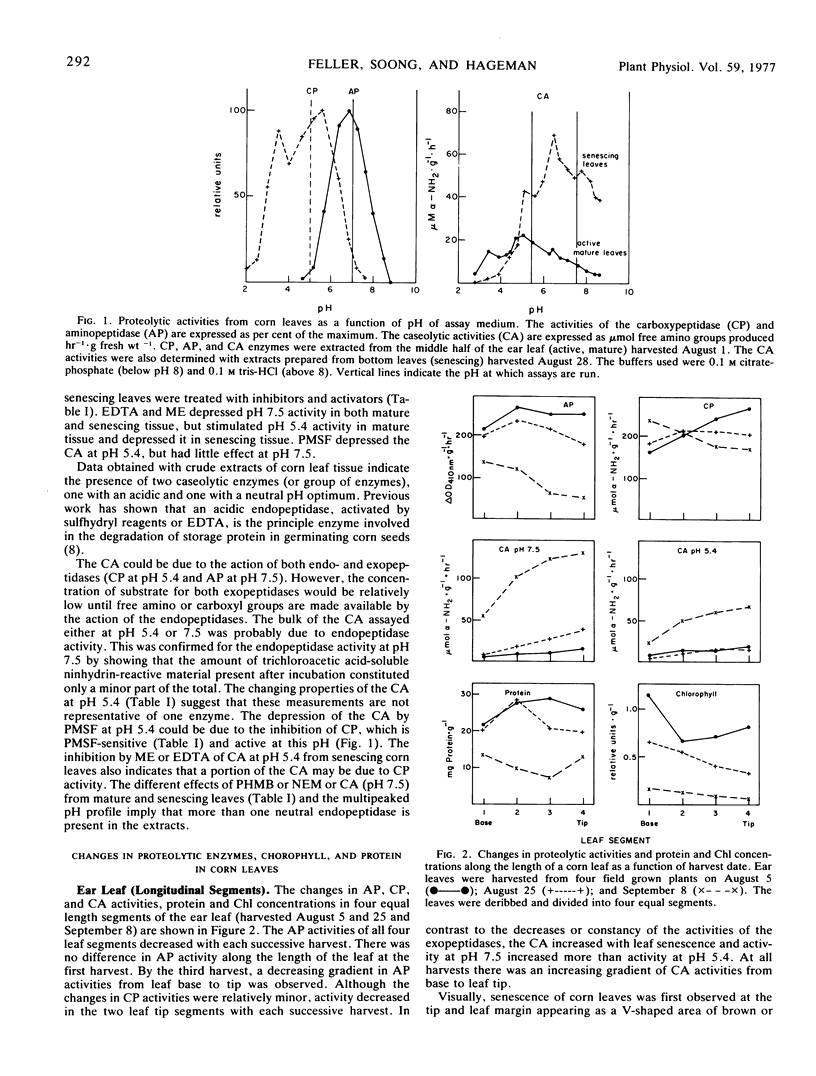
The aminopeptidase (not affected by sulfhydryl or fluoride reagents) was most active at pH 7, while the carboxypeptidase(s) (sensitive to fluoride, but insensitive to sulfhydryl reagents) was most active in the acid range, pH 3 to 6. The presence of two or more endopeptidases is indicated. Endopeptidase (caseolytic) activity at pH 5.4 appeared to be stimulated by sulfhydryl groups or EDTA, while caseolytic activity at pH 7.5 was not.
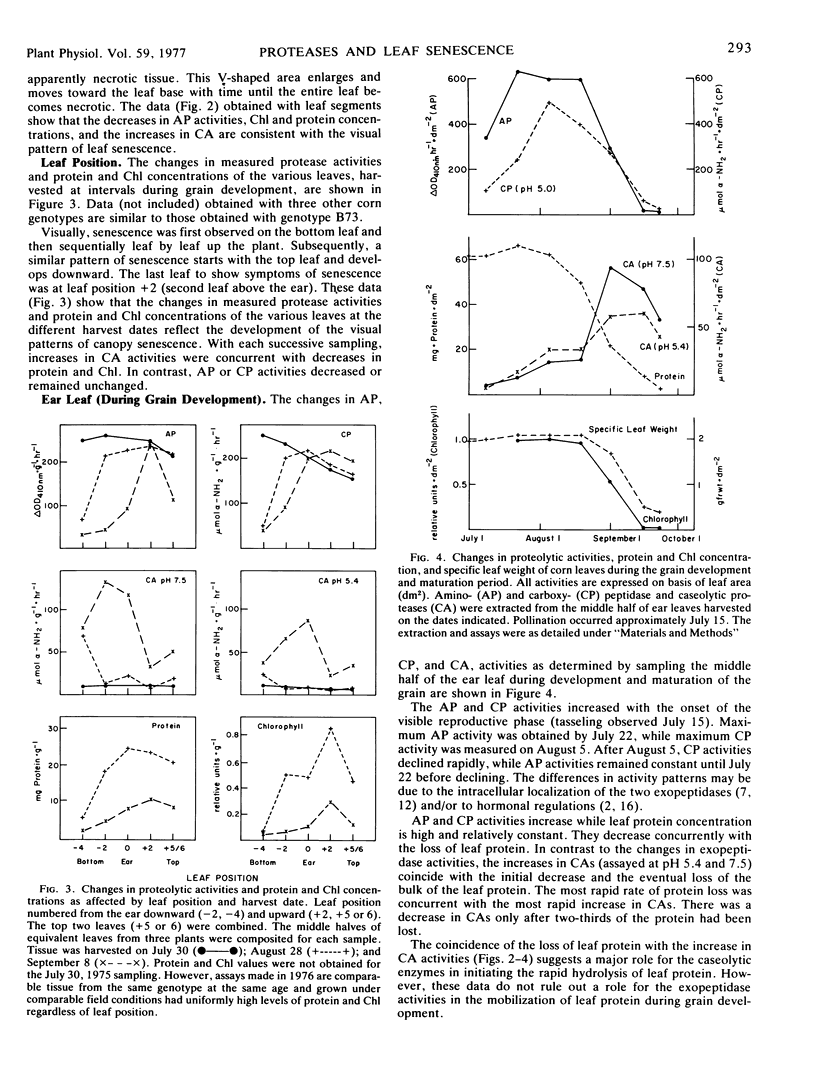
Visually, individual leaf senescence starts at the leaf tip and the necrotic (brown) V-shaped area enlarges progressively toward the leaf base. Canopy senescence occurs in two phases. Foliar symptoms are first observed on the bottom leaf and then in sequential order up the plant. Subsequently, senescence occurs on the top leaf and moves downward. These foliar senescence symptoms are paralleled by decreases in exopeptidase activities, protein, and chlorophyll concentrations and by increases in endopeptidase activities.
During development and maturation of the grain, both aminopeptidase and carboxypeptidase activity of the middle half of the ear leaf increased (2- to 3-fold) during the onset of the visual reproductive phase (tassel and car emergence). However, during grain development and plant senescence, both activities decreased rapidly and concurrently with the loss of protein and chlorophyll from this leaf section. In contrast, caseolytic activity at both pH 5.4 and 7.5 increased gradually during the early reproductive phase and rapidly with leaf senescence. The fastest rate of increase in caseolytic activities was concurrent with the most rapid loss of protein from the leaves. The coincidence of these events suggests a major role for the caseolytic enzymes in initiating the rapid hydrolysis of leaf protein.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. W., Rowan K. S. Activity of peptidase in tobacco-leaf tissue in relation to senescence. Biochem J. 1965 Dec;97(3):741–746. doi: 10.1042/bj0970741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson J. W., Rowan K. S. The effect of 6-furfurylaminopurine on senescence in tobacco-leaf tissue after harvest. Biochem J. 1966 Feb;98(2):401–404. doi: 10.1042/bj0980401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger W. C. Multiple forms of acidic endopeptidase from germinated barley. Plant Physiol. 1973 Jun;51(6):1015–1021. doi: 10.1104/pp.51.6.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrispeels M. J., Boulter D. Control of storage protein metabolism in the cotyledons of germinating mung beans: role of endopeptidase. Plant Physiol. 1975 Jun;55(6):1031–1037. doi: 10.1104/pp.55.6.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris N., Chrispeels M. J. Histochemical and biochemical observations on storage protein metabolism and protein body autolysis in cotyledons of germinating mung beans. Plant Physiol. 1975 Aug;56(2):292–299. doi: 10.1104/pp.56.2.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey B. M., Oaks A. Characteristics of an Acid protease from maize endosperm. Plant Physiol. 1974 Mar;53(3):449–452. doi: 10.1104/pp.53.3.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey B. M., Oaks A. The Hydrolysis of Endosperm Protein in Zea mays. Plant Physiol. 1974 Mar;53(3):453–457. doi: 10.1104/pp.53.3.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichishima E. Purification and characterization of a new type of acid carboxypeptidase from Aspergillus. Biochim Biophys Acta. 1972 Jan 20;258(1):274–288. doi: 10.1016/0005-2744(72)90985-0. [DOI] [PubMed] [Google Scholar]
- Martin C., Thimann K. V. The role of protein synthesis in the senescence of leaves: I. The formation of protease. Plant Physiol. 1972 Jan;49(1):64–71. doi: 10.1104/pp.49.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melville J. C., Scandalios J. G. Maize endopeptidase: genetic control, chemical characterization, and relationship to an endogenous trypsin inhibitor. Biochem Genet. 1972 Aug;7(1):15–31. doi: 10.1007/BF00487006. [DOI] [PubMed] [Google Scholar]
- Peterson L. W., Huffaker R. C. Loss of Ribulose 1,5-Diphosphate Carboxylase and Increase in Proteolytic Activity during Senescence of Detached Primary Barley Leaves. Plant Physiol. 1975 Jun;55(6):1009–1015. doi: 10.1104/pp.55.6.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer P. W., Titus J. S. Biochemical and Enzymatic Changes in Apple Leaf Tissue during Autumnal Senescence. Plant Physiol. 1972 May;49(5):746–750. doi: 10.1104/pp.49.5.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visuri K., Mikola J., Enari T. M. Isolation and partial characterization of a carboxypeptidase from barley. Eur J Biochem. 1969 Jan;7(2):193–199. doi: 10.1111/j.1432-1033.1969.tb19591.x. [DOI] [PubMed] [Google Scholar]
- Yomo H., Srinivasan K. Protein Breakdown and Formation of Protease in Attached and Detached Cotyledons of Phaseolus vulgaris L. Plant Physiol. 1973 Dec;52(6):671–673. doi: 10.1104/pp.52.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]


