Abstract
This research examined human defensive reactivity when exposure to an aversive event could be escaped but not entirely avoided. Prolonged visual cues indicated whether exposure to an upcoming aversive (i.e., disgusting) picture could be terminated after onset (escaped) or not, or that a neutral go signal would appear. Acoustically elicited startle reflexes were measured during each cue interval, as were cardiac and skin conductance activity. Early in the cuing interval, startle reflexes were potentiated during both escape and inescapable exposure trials, compared to the simple motor context. Later in the interval, reflexes remained potentiated for both escapable and inescapable trials, with potentiation further enhanced when aversive exposure could not be escaped compared to when exposure could be escaped. Heart rate deceleration in the cuing interval indicated increased vigilance when preparing any (escape or neutral) action, whereas skin conductance responding indicated enhanced sympathetic action mobilization particularly in an escape context. These data suggest that startle reflexes engaged in an escape context reflect both motor-related response inhibition and aversive potentiation, and they indicate that defensive motivation is engaged whenever aversive exposure is guaranteed, regardless of whether it can be escaped or not.
When an animal confronts danger, its defensive response is adaptively tailored to current context constraints, including whether or not an escape route is accessible. Thus, an animal might freeze (to evade detection) in a context where escape from threat is difficult or impossible, whereas active flight is preferred in contexts where the opportunity to escape is available (Blanchard & Blanchard, 1989). Prior to behavioral enactment, threat contexts that are differentiated by the presence or absence of an escape route also activate at least partially distinct neural networks, which in turn mediate context-specific reflexive, autonomic and hormonal adjustments that support behavior (Timberlake, 1993; Fanselow, 1994). Such neural and physiological activation is then further altered in contexts where threat can be avoided entirely, compared to when danger can be escaped but not completely prevented (e.g., Beninger, Mason, Phillips, & Fibiger, 1980; Weitzikoski, Boschen, Miyoshi, Bortolanza, et al., 2012).
Like nonhuman animals, humans show defensive activation patterns in aversive situations that are context-dependent (Bradley & Lang, in press; Lang & Bradley, 2013). For example, when aversive events can be actively avoided, physiological reactions that typically arise in aversive contexts are replaced by reactions that support rapid active responding to the imperative avoidance signal (Löw, Lang, Smith & Bradley, 2008; Löw, Weymar, & Hamm, 2015). The present research examined whether human defensive activation is similarly tailored to support action when aversive exposure can be escaped but not entirely avoided, using a number of reflex and autonomic responses as complementary indicators of defense system engagement.
A large body of research shows that reflexive blink reactions to startling stimuli are potentiated in various contexts that involve painful or aversive stimulation (see Lang, Bradley, & Cuthbert, 1990 and Lang & Davis, 2006), including during predictive cues that prompt anticipation of an upcoming aversive picture, (Sabatinelli, Bradley & Lang, 2001; Sege, Bradley, & Lang, 2014). Such potentiation is not apparent when an aversive event can be avoided; instead, when a rapid motor response to a visual go signal avoids an aversive outcome such as money loss or shock, reflex reactions to acoustic probes are inhibited in the context of the predictive cue, and the inhibition is equivalent to that found when a visual signal prompts a simple reaction time response (Löw et al., 2008, 2015). These data suggest that the broad defensive reflex priming that potentiates reflexes when aversive exposure is uncontrollable (Lang et al., 1990) is not engaged if aversive exposure can be avoided completely. Instead, startle magnitude in avoidance contexts is modulated by task-related direction of attention towards the imperative action signal (and away from the startle probe), which facilitates rapidly detecting and reacting to that signal (as described in a review by Anthony, 1985).
Inasmuch as supporting action is of similar motive import in escape, compared to avoidance, contexts, motor-related reflex inhibition could similarly predominate when escaping, rather than avoiding, aversive exposure. On the other hand, escape behavior differs from active avoidance in that escape does not completely block aversive exposure. Because startle is potentiated in other contexts where aversive exposure is guaranteed, similar potentiation might occur even if such exposure can be escaped but not entirely prevented. The direction of startle modulation should thus indicate whether escape-related activation primarily supports action or defensive reflex priming, or if modulation instead reflects a net influence of motor and defensive engagement.
Autonomic (heart rate and skin conductance) physiology that might support escape behavior was also investigated in the present study. Cardiac responding in cuing contexts is typically marked by deceleration just prior to the onset of an anticipated stimulus, which, like startle inhibition, indicates preparatory direction of sensory attention towards the awaited event (Graham & Clifton, 1966). Such cardiac deceleration also predominates in an avoidance context, in which deceleration prior to a cued visual avoidance signal is identical to the deceleration found when a cued visual signal prompts a simple reaction time response (Löw et al., 2008, 2015). Equivalent cardiac deceleration in both avoidance and simple motor contexts suggests that such deceleration supports detecting and responding to an upcoming action signal, regardless of the hedonic consequences of action.
Skin conductance responding is enhanced across a variety of motivationally salient tasks, including during cues that predict an aversive picture (Sabatinelli, Bradley, & Lang, 2001) or a visual reaction time signal (e.g., Bortoletto, Lemonis, & Cunnington, 2011). Skin conductance responding is also apparent when a rapid motor response to a cued visual signal avoids an aversive outcome, and this responding is enhanced compared to responding in simple reaction time conditions (Löw et al., 2008, 2015). These findings suggest that, whereas heart rate deceleration is similarly enhanced across motor contexts, skin conductance responding is particularly enhanced in motor contexts that are affectively salient.
The present study examined the physiological responses the support escape behavior in humans by measuring startle, cardiac, and skin conductance responses in an experimental model of escapable and inescapable aversive exposure. In this design, cues reliably indicated whether or not subsequent presentation of an aversive picture could be escaped by a rapid button press. Aversive scenes depicted graphic contamination (e.g., vomit, feces) or death-related content (e.g., mutilated bodies). Such scenes were selected because they prompt a classic rejection response in humans and primates (e.g., Rozin, Haidt, & McCauley, 2000), and prior research has found that participants are highly motivated to reduce exposure to such stimuli as measured by gaze avoidance during free viewing (e.g., Bradley, Houbova, Miccoli, Costa, & Lang, 2011) or following conditioning with a contamination UCS (Armstrong, McClenahan, Kittle, & Olatunji, 2014). On the other hand, as evidenced by its popularity in movies and other media, scenes depicting threat or violence often draw attention, prompting longer voluntary viewing time than neutral scenes (Hamm et al, 1997). Thus, contamination and mutilation scenes were chosen as potentially strong motivators of escape behavior.
To compare responding in escapable and inescapable aversive contexts, one cue indicated that the upcoming aversive picture could be terminated (i.e., escaped) by rapidly pressing a button after picture onset, while a second cue indicated that aversive exposure could not be escaped. In addition, a third cue comprised a simple go task in which a rapid button press at cue offset simply changed the color of the go signal.
Method
Participants
Thirty eight undergraduates (21 female; Mage 18.7 [SD = 1.9] years) from General Psychology courses at University of Florida participated for course credit. Data from 3 participants (2 female) were not included due to very low startle probability (i.e., overt blinks on <10% of [i.e., 10] trials), leaving 35 students with complete data (minimum startle probability was 90% for participants with complete data). The study was approved by the local Institutional Review Board; participants provided written informed consent.
Design and Materials
All stimuli were presented on a commercially-available 19-inch monitor placed 33 inches from the participant. Predictive cues were full-screen (1024 × 768 pixels), vertically-oriented gratings that comprised alternating yellow-and-gray bars, blue-and-gray bars, or yellow-and-blue bars. Cues were presented for 5, 5.25, or 5.5 seconds, and were immediately followed by an aversive picture on “escape” and “no escape” trials, or by a simple visual signal (150 × 150 pixel green circle) on motor control (“go”) trials. On escape trials, a rapid button press within 500 ms after picture onset replaced the aversive scene with a pleasant landscape, whereas slower responses resulted in continued presentation of the aversive scene for 3 s total. On inescapable trials, aversive scenes were always presented for 3 s. Finally, on go trials, a rapid button press to the visual signal resulted in a change in signal color (from green to blue), whereas, for slower responses, the circle remained green for 3 s total.
In addition to cued trials, un-cued trials showed inescapable aversive scenes or nature scenes for 3 s. A variable-length (11 or 14 s) intertrial interval followed every trial.
A total of 100 pictures were selected from the International Affective Picture System (IAPS; Lang, Bradley, & Cuthbert, 2008) and consisted of 50 aversive scenes of disgusting bodily events (e.g., vomit, feces) or mutilated bodies/body parts and 50 pleasant scenes depicting natural landscapes.1,2 Pictures were presented full-screen (1024 X 768 pixels) and in color.
The startle probe was a 50-ms, 98.5-decibel white noise burst with instantaneous rise time, presented binaurally over headphones. Based on research showing that startle modulation changes as an anticipated imperative stimulus approaches in time (e.g., Anthony & Graham, 1985), cue-interval probes were presented either 2.5 s (“early” probes) or 4.5 s (“late” probes) after cue onset. Probes were also presented 2 s after picture onset on each un-cued picture trial. Finally, additional probes were presented during some intertrial intervals, 7.5 s after trial termination.
Following five initial practice trials (each including a startle probe) that were not included in analyses, critical trials included 60 cued trials and 20 un-cued trials. Critical cued trials were divided into 20 escape, 20 no escape, and 20 go trials, and un-cued trials were divided into 10 aversive and 10 landscape trials. For cued trials, one startle probe was presented, either early or late, during each cue, resulting in 10 total probes at each delay for each cue type. One probe was presented during every un-cued trial, resulting in 10 total probes for each picture type. Finally, ten probes were presented sporadically during intertrial intervals.
Trials were presented in counterbalanced order, with no more than two of the same trial type (escape, no escape, go, aversive picture only, or landscape picture only) occuring consecutively. Different presentation orders were generated such that, across orders, each picture was presented in the beginning, middle, and end of the study. The meanings of the yellow-and-gray and blue-and-gray cues were counterbalanced across orders, while the yellow-and-blue compound cue always indicated an escape trial.
Procedure
Following consent and sensor attachment, participants were told that, on some trials, a color would cue whether a button press would escape presentation of an aversive picture, or that aversive exposure was inescapable. Participants were instructed that, when the escape cue was on the screen, a fast button press immediately after aversive picture onset would switch the picture to a pleasant scene, whereas a slow response meant that the aversive picture would remain on the screen. Participants were told that, when a cue signaling no escape was on the screen, aversive picture presentation could not be shortened. For active motor trials, participants were told that a fast button press immediately after go signal onset would change the signal color. Participants were also told that pictures could appear without a preceding cue.
After these instructions, a handheld button was placed in the dominant hand and participants were instructed that responding prior to picture (or go signal) onset would prevent any stimulus change (to discourage early responding). Finally, participants were told to ignore noises heard over headphones.
Following this procedure and sensor removal, participants rated the aversiveness of each cue and picture type on separate 1 (very pleasant) to 9 (very unpleasant) scales (5 = neutral), and they also completed questionnaires measuring individual differences in mutilation fear, depression (Beck Depression Inventory; Beck, Steer, & Brown, 1996), and both current and trait-level anxiety (State-Trait Anxiety Inventory; Spielberger, Gorusch, Lushene, Vagg, & Jacobs, 1983).
Data Collection & Reduction
On each cued trial, reaction time was measured as the latency in ms between stimulus onset and initiation of a buttton press.
Blink reflexes were measured using 2 12mm Ag/Cl sensors placed under the left eyelid over the orbicularis oculi (blink) muscle. The analog signal was band-pass filtered (90–250 Hz), amplified, rectified and integrated (20ms time constant) online and sampled at 1000 Hz (50ms preprobe to 250ms postprobe). Offline, the magnitude of each blink was scored using VPM software (© Cook; Cook, 2001) in which the peak magnitude and the onset latency were determined for blinks occurring in a window from 20 to 150 ms following acoustic probe onset. Trials in which the blink was outside this range or in which the blink could not be discerned from surrounding noise were scored as missing (less than 2% of trials). Blinks were standardized (separately for each participant) on the basis of the mean and standard deviation of blinks elicited during intertrial intervals. Blinks are expressed as T-scores ([z * 10] + 50); in this standardization technique, a T-score of 50 indicates reflexes identical to those elicited during the intertrial interval, and experimental blinks are not in the same distribution as the reference (ITI) distribution, providing independent standardization.
Heart rate was measured using 2 22mm Ag/Cl sensors placed on the inside surface of each forearm. Cardiac signal was input into a Coulbourn© S75-01 bioamplifer with a bandpass filter of 8–40 Hz, and then to a Coulbourn bipolar comparator module that sent a digital trigger every time an R-spike was detected. These interbeat intervals were recorded in ms online and then converted offline to beats-per-minute (BPM) for every half-s bin. The cardiac data were edited for artifacts using VPM software (Cook, 2001), which allows both automated (i.e. based on statistical parameters for each trial) and manual editing of long or short interbeat intervals. To quantify heart rate responding, change scores were calculated for each trial by subtracting average heart rate during a 1-s prestimulus baseline from heart rate values for each half-s trial bin.
For analysis of cued trials, heart rate response during the cuing interval was quantified in accordance with prior research that describes a triphasic response (comprising initial decelerative, subsequent accelerative, and second decelerative components) during anticipation and response preparation (e.g., Headrick & Graham, 1969). In the present study, time windows for each response component were established by visual inspection of the grand mean cardiac waveform, and 3 separate component indices were quantified by deviating the mean heart rate response during the critical window from the peak of the preceding response (Gatchel & Lang, 1973). For un-cued trials, heart rate change-from-baseline was averaged during the final s of picture viewing, when responding was maximal.
Skin conductance was measured by two 22 mm Ag/Ag-Cl electrodes filled with appropriate paste and placed adjacent on the hypothenar eminence of the nondominant palm. A constant current (.5 V) was generated between electrodes by a Coulbourn S71-22 coupler, and the resulting conductance was sampled and digitized at 20 Hz.
Data Analysis
Trials on which a button was inappropriately pressed (including escape and go trials on which the button was pressed prior to cue offset, as well as no escape and un-cued trials on which a button press occurred) were not included in the analyses. Fewer than 1% of such trials, across participants, were removed. Unsuccessful escape and go trials (i.e., reaction time > 500 ms) were also removed from main analysis; this led to removal of 78 (11.4%) escape trials and 96 (13.9%) active motor trials.3
Following trial removal, blink reflexes were analyzed in a 3 (context: escape, no escape, go) X 2 (probe delay: early, late) repeated-measures ANOVA. Heart rate change (early deceleration, acceleration, and late deceleration) and skin conductance were separately analyzed in one-factor (context: escape, no escape, go) ANOVAs.
Postexperimental ratings of the different cues were analyzed using repeated-measures analysis of variance (ANOVA) with context (escape, no escape, go) as a single factor. Un-cued contamination and landscape picture ratings were compared in a single paired-samples t-test.
Results
Self Report
Study participants were, on average, low in mutilation sensitivity (M = 7.5, SD = 5.5, scale range = 0 – 30), depression (M = 5.4, SD = 5.1, scale range = 0 – 63), and both current (M = 36.3, SD = 9.2, scale range = 20 – 80) and trait-level (M = 35.3, SD = 10.8, scale range = 20 – 80) anxiety. After the experiment, participants rated the inescapable context as somewhat more unpleasant (M = 6.6, SEM = 0.3) than the escape context (M = 5.4, SEM = 0.2), t(34) = 3.48, p < .01, d = 1.19, and they rated the escape context as more unpleasant than the neutral motor context (M = 4.9, SEM = 0.2), t(34) = 2.02, p = .05, d = .70; context F(2,33) = 9.95, p < .001, η2 = .38. Participants rated actually viewing aversive scenes pictures as more unpleasant (M = 7.1, SEM = 0.3) than viewing landscape pictures (M = 2.9, SEM = 0.4), t(34) = 6.66, p < .001, d = 2.28.
Reaction Time
Reaction time data indicated greater motivation to respond quickly in an escape than in a neutral motor context. Thus, participants were faster when performing an escape response (M = 356 ms, SEM = 2.3) than they were when performing a simple reaction time response (M = 374 ms, SEM = 2.2), t(34) = 4.76, p < .001, d = 1.62.
Defensive Responding Across Motor and Defensive Contexts
Startle Reflex Modulation
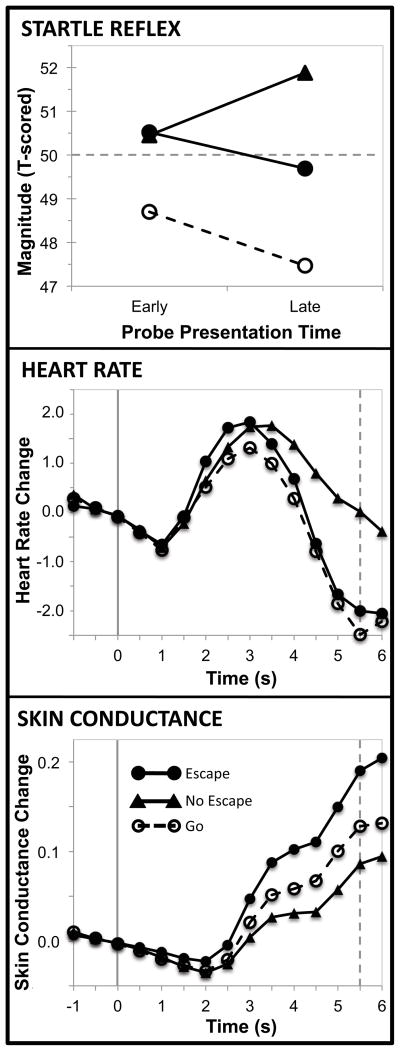
As depicted in Figure 1, patterns of startle reflex modulation differed across escape, no escape, and go contexts, Context X Probe Delay F(2,33) = 4.14, p = .02, η2 = .20. Early in the cuing interval, startle blinks were potentiated, relative to a neutral motor context, whenever aversive picture presentation was guaranteed regardless of whether escape was possible, t(34) = 2.24, p = .03, d = .77, or not, t(34) = 2.75, p = .009, d = .94. Moreover, blink magnitudes early in the cue did not differ whether escape was possible or not, t(34) = 0.02, p = .98, d = .00; context F(2,33) = 3.94, p = .03, η2 = .19. Later in the cue interval, startle potentiation was somewhat, but not completely, reduced when escape was possible – thus, blinks elicited just prior to onset of an escapable picture were enhanced compared to blinks elicited just prior to a go signal, t(34) = 3.40, p = .002, d = 1.17, but attenuated compared to blinks elicited just prior to an inescapable aversive picture, t(34) = 2.50, p = .02, d = .86. Startle remained enhanced in the inescapable aversive context, compared to the simple motor context, late in the cue interval, t(34) = 4.45, p < .001, d = 1.53; context F(2,33) = 10.55, p < .001, η2 = .39.
Figure 1.
Blink startle reflex, heart rate, and skin conductance modulation across escape, no escape, and go conditions. In the startle reflex panel, the horizontal dashed line indicates inter-trial reflex magnitude (ITI = 50). In heart rate and skin conductance panels, the solid vertical line indicates trial onset and the dashed vertical line indicates cue offset.
Analysis of change in reflex magnitude across the cuing interval also suggested reduced, not eliminated, startle potentiation in an escape context. Thus, magnitude of blink reflexes increased as onset of an inescapable aversive picture approached, F(1,34) = 5.34, p = .03, η2 = .14, but not when the scene could be terminated by a fast button press, F(1,34) = 0.80, p = .38, η2 = .02. In addition, magnitude decreased as the time to make a simple reaction time response approached, F(1,34) = 4.10, p = .05, η2 = .11.
Cardiac Response Modulation
Consistent with prior research, a triphasic heart rate response was found across the cue interval comprising an early, brief deceleration maximal 1 s after cue onset, an acceleration that peaked about 3 s after cue onset, and a a second deceleration that continued through cue offset (see Figure 1).
Unlike the startle reflex, cardiac responding was modulated primarily as a function of motor demand rather than aversive exposure. Modulation was specific to the late deceleratory leg of the triphasic response; that is, neither early deceleration, F(2,33) = 0.56, p = .58, η2 = .04, nor subsequent acceleration, F(2,33) = 1.07, p = .36, η2 = .08, differed across escape, no escape, and go cues. Late-interval cardiac deceleration, meanwhile, differed as a function of context, F(2,33) = 10.35, p < .001, η2 = .44, with greater heart rate deceleration prior to making an escape, t(34) = 4.25, p < .001, d = 1.64, or a reaction time response, t(34) = 4.14, p < .001, d = 1.60, than prior to onset of an inescapable picture. Deceleration did not differ when preparing an escape response or a simple motor response, t(34) = .69, p = 49, d = .26.
Skin Conductance Modulation
Skin conductance activity was also modulated across contexts, F(2,33) = 5.18, p = .01, η2 = .25 (see Figure 1). Skin conductance responding was apparent in all three conditions, such that conductance change (from baseline) was greater than 0 on escapable, t(34) = 4.19, p < .001, d = 1.44, inescapable, t(34) = 2.28, p = .03, d = .78, and go, t(34) = 2.21, p = .03, d = .76, trials. Responding was particularly enhanced during escape trials, with electrodermal activity on escape trials significantly larger than on inescapable trials, t(34) = 3.21, p = .003, d = 1.12, and marginally enhanced compared to go trials, t(34) = 1.79, p = .08, d = .63.
Defensive Response During Picture Perception
Table 1 lists reactions when viewing aversive or pleasant pictures on un-cued trials. Consistent with previous data, startle reflexes, skin conductance change, and heart rate deceleration were larger when viewing aversive, compared to nature, scenes (startle: t[34] = 2.92, p = .006, d = 1.00; skin conductance: t[34] = 4.17, p < .001, d = 1.45; cardiac deceleration: t[34] = 3.41, p = .002, d = 1.31).
Table 1.
Mean and standard error of defensive responses when viewing contamination or landscape pictures. Heart rate response is the mean heart rate deceleration during the final second of picture viewing, and skin conductance response is mean response during the final second of picture viewing and first second of the intertrial interval.
| Defensive Response | Contamination Mean (SEM) |
Landscapes Mean (SEM) |
|---|---|---|
| Blink Startle Reflex (T-score) | 50.1 (0.7)a | 48.4 (0.6)b |
| Heart Rate Response (BPM) | −2.4 (0.5)a | −0.3 (0.3)b |
| Skin Conductance Response (μS) | 0.18 (.05)a | −0.05 (.01)b |
Note: Means that significantly differ are denoted by different superscripts.
Discussion
Whereas the ability to completely avoid aversive outcomes eliminates certain defense reactions (i.e., startle potentiation; Löw et al., 2008, 2015), such reactions are not completely abolished when aversive exposure can be escaped but is nonetheless certain. Thus, startle reflexes elicited in the context of escape were potentiated compared to reflexes elicited in a neutral motor context, and skin conductance responding was enhanced in escape compared to neutral motor contexts. Startle reflexes were potentiated early in the anticipatory interval on both escape and inescapable exposure trials, compared to neutral motor contexts, and startle reflexes elicited during inescapable exposure continued to increase as exposure drew near.
The data provide ample evidence that upcoming aversive stimulation was unpleasant in both escape and inescapable contexts, and that participants were motivated to escape aversive exposure when they could. Thus, not only was the context wherein aversive images could be escaped rated as unpleasant post experimentally, but measurable defensive reactivity (e.g., startle potentiation) was apparent when viewing un-cued aversive pictures compared to landscape scenes. In addition, participants were significantly faster when making a motor response that escaped aversive stimulation compared to a simple motor response with no affective outcome. These findings indicate that the use of disgusting contamination and mutilation scenes was effective in prompting measurable motivation to escape.
On the other hand, startle reflexes elicited in an escape context, while potentiated compared to a neutral motor context, were attenuated compared to inescapable aversive trials late in the cue. One hypothesis is that the escapable context was simply less aversive than the inescapable context, leading to less potentiation and postexperimental ratings suggesting that the escapable context was less unpleasant than the inescapable context. On the other hand, this account also predicts a difference in startle potentiation between escapable and inescapable exposure early in the cue interval, whereas startle reflexes were in fact equally potentiated on escape and inescapable exposure trials early in the cue. An alternative hypothesis is that attenuation of startle later in the escape context reflects concurrent inhibition due to the motor task, consistent with previous studies that have demonstrated increasing inhibition of startle in contexts that demand rapid motor responding to visual stimuli (e.g., Anthony, 1985; Low et al., 2008, 2015). This interpretation is also supported by the pattern of startle reflex attenuation occurring to early and late probes in the simple motor control context.
As a whole, the pattern of startle modulation found in the escape, compared to simple motor and inescapable exposure contexts, suggests concurrent modulation of the startle reflex, with potentiation due to imminent aversive exposure and inhibition due to task-related attention. Previous studies have confirmed that the startle reflex can simultaneously be modulated by concurrent processes of attention and emotion (Bradley, Codispoti, & Lang, 2006) and are consistent with an interpretation that both task demand and aversiveness had modulatory effects on the startle reflex in the escape context. Thus, task-related attentional processes attenuate and defensive priming potentiaties the startle response when aversive exposure is escapable.
Whereas startle modulation reflects both defense-related and task-related aspects of upcoming aversive exposure, cardiac and skin conductance responses more specifically reflected task processing that, in this escape context, required orienting (e.g., Graham & Clifton, 1966; Lacey, Kagan, Lacey, & Moss, 1962; Sokolov, 1960) and preparation for action. The orienting response is a multi-component response in which cardiac deceleration and skin conductance index different components, with deceleration associated with vigilance and information intake whereas skin conductance activity supports action mobilization (Bradley, 2009). Cardiac deceleration was significantly enhanced in escapable compared to inescapable contexts and was similarly modulated when preparing to make escape or simple go responses. Skin conductance, on the other hand, was apparent in all experimental contexts but was particularly enhanced in the escape context. These modulatory patterns suggest that sensory orienting (indicated by cardiac deceleration) primarily reflects imperative signal detection regardless of hedonic consequences, whereas action mobilization (indexed by electrodermal reactivity) is most enhanced when active responding is motivationally relevant. Similar patterns of cardiac and electrodermal responding are found in an avoidance, rather than escape, context (Löw et al., 2008, 2015), suggesting that cardiac and skin conductance responses reflect context-dependent aspects of defensive activation whereas startle potentiation represents an “alarm reaction” that is engaged in any situation where aversive exposure is inevitable (Masterson & Crawford, 1982).
Because of the distinct defensive responses that support behavior in different contexts, an interesting avenue for future research is to elucidate the neural mediators of this aversive context specificity. Human neuroscience has already taken steps in this regard; for example, a series of studies (Mobbs, Petrovic, Marchant, Hassabis, et al., 2007; Mobbs, Marchant, Hassabis, Seymour, et al., 2009; Mobbs, Yu, Rowe, Eich, Feldman-Hall, & Dalgleish, 2010) have found that neural activation shifts from forebrain (e.g., amygdala) to brainstem (e.g., periacqueductal gray) regions as aversive exposure become imminent, and that the strength of brainstem activation relates to “panicky” defensive responding in a virtual predator scenario. Future research could examine if analogous neural shifts mediate distinct patterns of peripheral responding that arise in escapable or inescapable (or avoidable) aversive contexts. Additionally, research suggests that individuals differ in their defensive responding to certain aversive contexts in a way that might relate to individual anxiousness or fear (e.g., Lang, Davis, & Öhman, 2000); as such, future research could evaluate anxious or fear-related responding in a variety of different coping contexts.
In conclusion, the startle reflex continued to show potentiation even when an aversive stimulus was escapable, whereas such potentiation is not apparent when aversive stimuli can be completely avoided. Data indicate that defensive mobilization continues to influence reflex responding when exposure can be escaped but not entirely eliminated, with a concurrent influence due to task-related responding. More broadly, findings are consistent with a framework in which defensive activation is considered “tactical” in nature, such that it adapts to specific context demands even as the strategic motivational goal (defense) remains the same (Lang et al., 1997; Bradley, 2000).
Acknowledgments
This research was supported in part by NIMH grants MH098078 and MH094386, awarded to Peter J. Lang.
Footnotes
IAPS numbers: Aversive - 2730, 3000, 3010, 3015, 3016, 3030, 3051, 3053, 3059–64, 3068–9, 3071, 3080, 3100, 3102, 3110, 3120, 3131, 3140, 3150, 3168, 3170, 3213, 3261, 3266, 3400, 9300–2, 9320–2, 9325–6; Pleasant - 5200–1, 5210, 5215, 5220, 5250, 5260, 5270, 5390, 5551, 5593–4, 5600, 5611, 5631, 5660–1, 5665, 5700, 5711, 5720, 5725–6, 5731, 5750, 5760, 5764, 5779–81, 5800, 5811, 5814, 5820, 5825, 5833, 5870, 5891, 5982, 5900, 5990–1, 5994, 6000, 7039, 7492, 7530, 7545, 7580
Based on normative data (Lang et al., 2008), aversive scenes were rated highly unpleasant (scale, 1 = very pleasant, 9 = very unpleasant, 5 = neutral; M = 8.1; SD = 0.4) and arousing (scale, 1 = completely unarousing, 9 = extremely arousing; M = 6.4; SD = 0.6), whereas landscapes were pleasant (M = 3.2; SD = 0.8) and minimally arousing (M = 3.9; SD = 0.9), such that aversive scenes differed from landscape scenes in normative pleasantness (t(99) = 33.8, p < .001) and arousal (t(99) = 14.6, p < .001).
Effects were generally equivalent when successful and unsuccessful trials were analyzed, except that reflex magnitudes late in escape and late in aversive anticipation were not conventionally different (p = .06). Across participants, performance ranged from 0 – 30.0% trials missed on escape trials, and 0 – 36.8% missed on active motor trials.
References
- Anthony BJ. In the blink of an eye: Implications of reflex modification for information processing. In: Ackles PK, Jennings JR, Coles MGH, editors. Advances in Psychophysiology. Vol. 1. Greenwich, CT: JAI Press; 1985. pp. 167–218. [Google Scholar]
- Anthony BJ, Graham FK. Blink reflex modification by selective attention: Evidence for the modulation of ‘automatic’ processing. Biological Psychology. 1985;20:43–59. doi: 10.1016/0301-0511(85)90052-3. [DOI] [PubMed] [Google Scholar]
- Armstrong T, McClenahan L, Kittle J, Olatunji BO. Don’t look now! Oculomotor avoidance as conditioned disgust response. Emotion. 2014;14:95–104. doi: 10.1037/a0034558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- Beninger RJ, Mason ST, Phillips AG, Fibiger HC. The use of extinction to investigate the nature of neuroleptic-induced avoidance deficits. Psychopharmacology. 1980;69:11–18. doi: 10.1007/bf00426515. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Blanchard DC. Attack and defense in rodents as ethoexperimental models for the study of emotion. Progress in Neuro-Psychopharmacology. 1989;13:S3–14. doi: 10.1016/0278-5846(89)90105-x. [DOI] [PubMed] [Google Scholar]
- Bortoletto M, Lemonis MJ, Cunnington R. The role of arousal in the preparation for voluntary movement. Biological Psychology. 2011;87:372–378. doi: 10.1016/j.biopsycho.2011.04.008. [DOI] [PubMed] [Google Scholar]
- Bradley MM. Emotion and motivation. In: Cacioppo JT, Tassinary LG, Berntson GG, editors. Handbook of Psychophysiology. Vol. 2. Cambridge, UK: Cambridge University Press; 2000. pp. 602–642. [DOI] [Google Scholar]
- Bradley MM. Natural selective attention: Orienting and emotion. Psychophysiology. 2009;46:1–11. doi: 10.1111/j.1469-8986.2008.00702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley MM, Lang PJ. Emotion in body & brain: Context-dependent action and reaction. In: Davidson R, Shackman A, Fox A, Lapate R, editors. The Nature of Emotion. Vol. 2. Cambridge, UK: Oxford University Press; in press. [Google Scholar]
- Bradley MM, Codispoti M, Lang PJ. A multi-process account of startle modulation during affective perception. Psychophysiology. 2006;43:486–497. doi: 10.1111/j.1469-8986.2006.00412.x. [DOI] [PubMed] [Google Scholar]
- Cook EW., III . VPM Reference Manual. Birmingham, AL: Author; 2001. [Google Scholar]
- Fanselow MS. Neural organization of the defensive behavior system responsible for fear. Psychonomic Bulletin & Review. 1994;1:429–38. doi: 10.3758/bf03210947. [DOI] [PubMed] [Google Scholar]
- Gatchel RJ, Lang PJ. Accuracy of psychophysical judgments and physiological response amplitude. Journal of Experimental Psychology. 1973;98:175–183. doi: 10.1037/h0034312. [DOI] [PubMed] [Google Scholar]
- Graham FK, Clifton RK. Heart-rate change as a component of the orienting response. Psychological Bulletin. 1966;5:305–320. doi: 10.1037/h0023258. [DOI] [PubMed] [Google Scholar]
- Headrick MW, Graham FK. Multiple-component heart rate responses conditioned under paced respiration. Journal of Experimental Psychology. 1969;79:486–494. doi: 10.1037/h0026975. [DOI] [PubMed] [Google Scholar]
- Lacey JI, Kagan J, Lacey BC, Moss HA. The visceral level: Situational determinants and behavioral correlates of autonomic response patterns. In: Knapp P, editor. Expression of the emotions in man. New York: International Universities Press; 1962. pp. 161–196. [Google Scholar]
- Lang PJ, Bradley MM. Appetive and defensive motivation: goal-directed or goal-determined? Emotion Review. 2013;5:230–234. doi: 10.1177/1754073913477511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Emotion, attention, and the startle reflex. Psychological Review. 1990;97:377–398. doi: 10.1037//0033-295x.97.3.377. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Motivated attention: Affect, activation, and action. In: Lang PJ, Simons RF, Balaban MT, editors. Attention and Orienting: Sensory and Motivational Processes. Mahwah, NJ: Erlbaum; 1997. pp. 41–67. [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Technical Report A-8. Gainesville, FL: University of Florida; 2008. International affective picture system (IAPS): Affective ratings of pictures and instruction manual. [Google Scholar]
- Lang PJ, Davis M. Emotion, motivation, and the brain: Reflex foundations in animal and human research. In: Anders Ende, Junghofer Kissler, Wildgruber, editors. Progress in Brain Research. Vol. 156. Amsterdam, NT: Elsevier; 2006. pp. 3–29. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Davis M, Öhman A. Fear and anxiety: Animal models and human cognitive psychophysiology. Journal of Affective Disorders. 2000;61:137–159. doi: 10.1016/s0165-0327(00)00343-8. [DOI] [PubMed] [Google Scholar]
- Löw A, Lang PJ, Smith JC, Bradley MM. Both predator and prey: Emotional arousal in threat and reward. Psychological Science. 2008;19:865–873. doi: 10.1111/j.1467-9280.2008.02170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löw A, Weymar M, Hamm AO. When threat is near, get out of here: Dynamics of defensive behavior during freezing and active avoidance. Psychological Science. 2015;26:1706–1716. doi: 10.1177/0956797615597332. [DOI] [PubMed] [Google Scholar]
- Masterson FA, Crawford M. The defensive motivation system: A theory of avoidance behavior. The Behavioral and Brain Sciences. 1982;5:661–696. doi: 10.1017/s0140525x00014114. [DOI] [Google Scholar]
- Mobbs D, Petrovic P, Marchant JL, Hassabis D, Weiskopf N, Seymour B, … Frith CD. When fear is near: Threat imminence elicits prefrontal-periacqueductal gray shifts in humans. Science. 2007;317:1079–1083. doi: 10.1126/science.1144298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobbs D, Marchant JL, Hassabis D, Seymour B, Tan G, Gray M, … Frith CD. From threat to fear: The neural organization of defensive fear systems in humans. The Journal of Neuroscience. 2009;29:12236–12243. doi: 10.1523/jneurosci.2378-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobbs D, Yu R, Rowe JB, Eich H, Feldman-Hall O, Dalgleish T. Neural activity associated with monitoring the oscillating threat value of a tarantula. Proceedings of the National Academy of Sciences. 2010;107:20582–20586. doi: 10.1073/pnas.1009076107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatinelli D, Bradley MM, Lang PJ. Affective startle modulation in anticipation and perception. Psychophysiology. 2001;38:719–722. doi: 10.1111/1469-8986.3840719. [DOI] [PubMed] [Google Scholar]
- Sege CT, Bradley MM, Lang PJ. Startle modulation during emotional anticipation and perception. Psychophysiology. 2014;51:977–981. doi: 10.1111/psyp.12244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolov EN. Neuronal models and the orienting reflex. In: Brazier MAB, editor. The Central Nervous System and Behavior. New York: Josiah Macy Jr. Foundation; 1960. pp. 187–276. [Google Scholar]
- Spielberger CD, Gorsuch R, Luschene R, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- Timberlake W. Behavior systems and reinforcement: An integrative approach. Journal of the Experimental Analysis of Behavior. 1993;60:105–128. doi: 10.1901/jeab.1993.60-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitzikoski EC, Boschen SL, Miyoshi E, Bortolanza M, Dos Santos LM, Brandao ML, … Da Cunha C. Roles of D1-like dopamine receptors in the nucleus accumbens and dorsolateral striatum in conditioned avoidance responses. Psychopharmacology. 2012;219:159–169. doi: 10.1007/s00213-011-2384-3. [DOI] [PubMed] [Google Scholar]