Abstract
We report two cases of Kaposi sarcoma associated herpesvirus (KSHV) - and Epstein Barr Virus (EBV)-associated germinotropic lymphoproliferative disorder (GLPD). Both cases arose in patients from regions endemic for KSHV, Cape Verde and the Democratic Republic of the Congo, presenting as localized lymphadenopathy. The affected lymph nodes showed colonization of the follicles by clusters of large atypical plasmablasts, but also showed regressive changes with vascular proliferation and interfollicular plasmacytosis, both reminiscent of HHV8-positive multicentric Castleman disease (MCD). The atypical plasmablasts showed dual positivity for HHV-8 and EBV, being positive for LANA and viral IL-6, as well as EBER by in-situ hybridization. They showed a Latency I phenotype, being negative for LMP1, EBNA2 and BZLF-1. The plasmablasts were negative for immunoglobulin light chains, and in one case with successful DNA amplification had a polyclonal immunoglobulin rearrangement pattern. GLPD is a rare disorder, with only 6 cases reported in the literature. We demonstrate for the first time the expression of HHV-8 viral IL-6 and provide evidence for Latency I phenotype for EBV. Additionally, one case progressed to an EBV-positive diffuse large B-cell lymphoma, but interestingly was negative for KSHV/HHV-8, likely indicative of tumor derived from an independent clone.
Keywords: Kaposi Sarcoma Herpes Virus, HHV-8, Epstein Barr virus, Castleman disease, germinotropic
Introduction
Kaposi sarcoma associated herpesvirus (KSHV), also known as human herpesvirus 8 (HHV8) is a lymphotropic virus associated with distinct lymphoproliferative disorders including primary effusion lymphoma (PEL), multicentric Castleman disease (MCD) and MCD-associated plasmablastic lymphoma [1].
KSHV/HHV8 has also been associated with a germinotropic lymphoproliferative disorder (GLPD), which has distinctive clinical features, presenting with localized lymphadenopathy in an asymptomatic and immunocompetent patient, and having a good prognosis. The atypical cells, which show coinfection with EBV and HHV-8, have a propensity to grow within germinal centers [2].
Previously, six cases of this rare entity have been described in the literature; Du et al [2] described 3 cases, followed by three case reports with one in a 60-year-old male [3], the other in a 49-year-old African American male [4], and the last being successful management in a 75-year-old male [5] (Table 1). We report two additional cases of KSHV-and EBV- associated lymphoproliferative disorder characterized by KSHV and EBV co-infected plasmablasts preferentially involving the germinal centers. In addition we defined the viral phenotype of this lesion, examining the EBV Latency phenotype and the expression of HHV-8 related markers.
Table 1.
Clinical and Biological Features of GLPD
| Current Case 1 | Current Case 2 | Lit Case 1 | Lit Case 2 | Lit Case 3 | Lit Case 4 | Lit Case 5 | Lit Case 6 | |
|---|---|---|---|---|---|---|---|---|
| Reported by | Du et al., 2002 | Du et al., 2002 | Du et al., 2002 | D’antonio Antonio et al., 2007 | Oh et al., 2012 | Taris et al., 2014 | ||
|
Age (years)/sex |
84/Female | 58/ Male | 41/Male | 61/Male | 63/Female | 60/Male | 75/Male | 49/Female |
|
Clinical presentation |
Multifocal bulky lymphadenopathy involving left common femoral (largest node, 5 × 3.7 cm), common, external, and internal iliac chain lymph nodes and right axillary lymph nodes |
10-year-history of localized right axillary mass, extending to chest wall, slowly growing (26 ×16 × 11 cm). Mild splenomegaly, no B symptoms |
Axillary (6 × 6 × 6 cm) and cervical lymph node enlargement for 6 years; staging revealed right perirenal lymphadenopathy; bone marrow trephine and aspirate were normal |
Submandibular and inguinal lymph node enlargement for 4 years; staging showed slightly enlarged spleen and right “paratracheal” lymphadenopathy |
Presented with left leg swelling and paraesthesia; enlarged paraaortic lymph node (5 × 4 × 3.5 cm) |
Cervical lymphadenopathy (2.8 ×1.2 cm); no other lymphadenopathy; post-operative radiological studies and bilateral bone marrow biopsies were normal |
Incidental (3 × 2.5 × 1.5 cm) mass in the neck; enlarged, solid, enhancing lymph node with an increased FDG uptake in left submandibular area; 1.5 cm partially cystic lymph node in left cervical level II area; bilateral bone marrow trephine and aspirate were normal |
Right jugulo-cervical isolated nodal mass, slowly evolving for 4 months; no systemic symptoms; 9 nodes including two nodes (greater than 3.5 cm × 3 cm) |
|
Viral serology |
KSHV+ EBV+ HCV− HIV− |
KSHV+ EBV+ HIV+ | KSHV+ EBV+ HCV+ HIV− |
KSHV+ EBV+ HIV− | Not available | KSHV+ EBV+ HIV− |
KSHV+ EBV+ HIV− | KSHV+ EBV+ HIV− HBV− HCV− HTLV1− HTLV2− |
| Treatment | 6 cycles of CHOP | Resection | 6 cycles of CHOP | Surgical excision and radiotherapy |
Not available | 4 cycles of CHOP | Surgical excision and radiotherapy |
|
| Outcome | Complete remission after 6 months |
Subsequent EBV+ DLBCL (HHV8−) Patient expired, exact date unknown. |
Complete remission for 7 years |
Complete remission for 15 years |
NA | No clinical evidence of relapse after 2 years |
Remained disease- free for 19 months |
Complete remission for more than 1 year |
|
B-cell antigens |
CD20− PAX5− CD79a− CD138− BCL6− CD10−, MUM1+, EMA+ focal, CD30− |
CD20− PAX5− BCL6− MUM1+, CD10− CD30− |
CD20− CD79a− CD138− CD38−BCL6− CD10− CD27− CD30− |
CD20− CD79a− CD138− CD38+ BCL6− CD10− EMA− CD27− CD30+ |
CD20− CD79a− CD138− CD38+ BCL6− CD10− EMA− CD27− BCL2− CD30− |
CD20− CD79a− CD138− BCL6− CD10− CD22− BCL2− CD19− CD45− CD30− |
CD20− CD79a− CD138+ CD10− EMA+ CD30− |
CD20− PAX5− CD79a− CD138− CD38+ weak, EMA+ MUM1+ BOB1+ OCT2+ BCL2+ CD30− |
|
T/NK cell & other antigens |
CD56− ALK1− CD3+ partial, CD5− CD43+ partial, CD7− CD4− CD8− |
CD3+ partial, CD5− CD4− CD8− CD2− |
ND | ND | ND | ND | CD3− CD5− CD43+ CD15− CD21− CD23− CD45− CD56− CD68− S100− |
ALK1− CD3− CD15− CD21− CD23− CD45− CD56− CD68− S100− |
|
Viral Antigens |
vIL-6+ LANA+ EBER+ LMP1− EBNA2− BZLF1− |
vIL-6+ LANA+ EBER+ LMP1− EBNA2− BZLF1− |
largely vIL-6+ LANA+ EBER+ |
largely vIL-6+ LANA+ EBER+ |
largely vIL-6+ LANA+ EBER+ |
LANA+ EBER+ | HHV8+ EBER+ | HHV8+ EBER+ |
|
Ig light chain |
None | None | lambda | lambda | kappa | kappa | kappa | lambda |
|
Ig heavy chain |
Not done | Not done | IgM+ D+ | IgA+ | IgM− IgD− IgG− IgA− |
IgM+ | Not done | Not done |
|
IG Clonality by PCR |
Polyclonal for both B and T cell gene rearrangements |
No amplification | Weak dominant band in a polyclonal background |
PCR failed | Polyclonal | Polyclonal | Not done | Polyclonal |
Case Reports
Case 1
An 84-year-old Cape Verdean female presented with extensive lymphadenopathy. An initial FNA diagnosis from the right inguinal lymph node was “atypical lymphocytes suspicious for lymphoma”. Her serology was negative for HIV and hepatitis. The pre-treatment PET scan showed multifocal bulky lymphadenopathy involving the left common femoral chain, common, external, and internal iliac chain lymph nodes and right axillary lymph nodes. The largest and most PET avid lymph node was the left common femoral lymph node (5 × 3.7 cm; SUV max of 10.7).
The patient was due to have 6 cycles of R-CHOP; however, after the first two cycles, she developed neutropenic fever with sepsis and further chemotherapy was not pursued. Approximately 6 months after her last chemotherapy, a CT scan (chest/abdomen/pelvic) showed resolution of the previously described right axillary, inguinal and iliac chain lymphadenopathy suggestive of favorable response to chemotherapy. New lymphadenopathy or focal lesions suggestive of recurrent disease was not detected, and she remains largely asymptomatic at 1.5 year follow-up.
Case 2
A 58-year-old black male from the Democratic Republic of the Congo presented to physicians in Johannesburg, South Africa with a 10-year history of a localized right axillary mass, with extension to the chest wall, which had been growing slowly. No other sites of lymphadenopathy were noted on physical examination. He had mild splenomegaly and no hepatomegaly, and was otherwise asymptomatic. The complete blood cell count was normal, but patient was later determined to be HIV-positive. The resected specimen had the appearance of matted lymph nodes, measuring 26 × 16 × 11 cm, and weighed 870 grams.
Over the course of the following year, the patient developed generalized lymphadenopathy. A biopsy of an inguinal lymph node was performed, which showed different histological findings. The patient was begun on CHOP chemotherapy. He subsequently died of his disease; details of his subsequent course were not available as the patient had returned to his homeland in the Congo.
Microscopic and immunohistochemistry findings
Sections of the initial lymph node biopsies in both cases showed a thick capsule with an increased number of follicles with distortion/expansion of the germinal centers by an atypical cell population showing plasmablastic morphology. (Figure 1) The atypical cells were large with smooth nuclear contours, vesicular and eccentrically placed nuclei, one or two prominent nucleoli and dense amphophilic cytoplasm – seen in clusters with focal confluence. Mitotic activity was high in the atypical cell population. In Case 2, the atypical cells showed greater pleomorphism with large and frequently multinucleated forms. In Case 1 particularly, there was prominent vascular proliferation as typically seen in multicentric Castleman disease (MCD), HHV8-positive. Both lymph nodes contained a heavy infiltrate of plasma cells in the inter-follicular areas. Additionally, in Case 1, focal clusters and sheets of the atypical cells were also identified in the inter-follicular areas, and in sinuses.
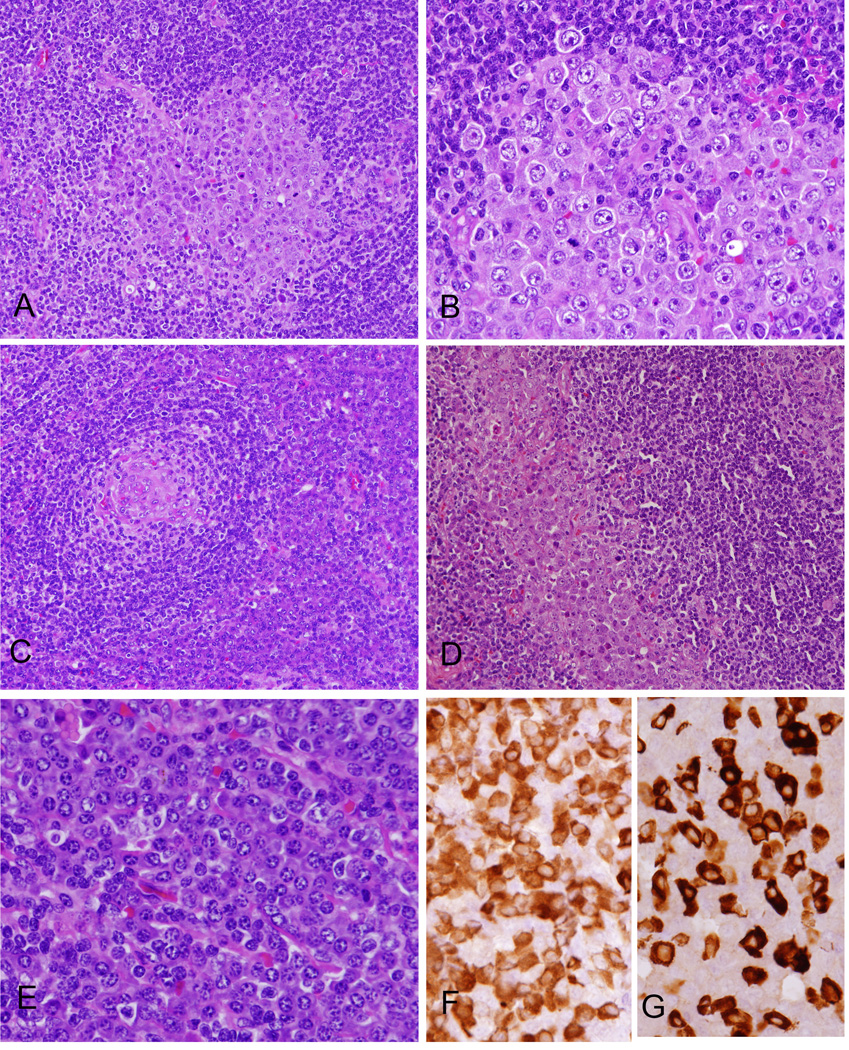
Figure 1. Morphologic features of KSHV- and EBV-associated GLPD.
(A) The atypical cells were largely confined to germinal centers. (B) The cells are large, showing plasmablastic morphology with smooth nuclear contours, vesicular and eccentrically placed nuclei, 1–2 prominent nucleoli and dense amphophilic. (C) Focal Castleman-like areas with regressed germinal centers surrounded by atypical cells are present. (D) Focally the atypical cells infiltrate sinuses, also highlighted with immunohistochemical stains (see Figure 2). (E) There is marked inter-follicular plasmacytosis. Plasma cells are polyclonal for Kappa (F) and lambda (G) light chains by immunohistochemistry, while the plasmablastic cells were negative (not shown).
The atypical plasmablastic cells showed dual positivity for HHV-8 and EBV, being positive for LANA and viral IL-6, as well as EBER in-situ hybridization (ISH). The cells were negative for the EBV latency proteins LMP1, EBNA2, BZLF-1 (indicating Latency I). They were also negative for kappa and lambda (by immunohistochemistry, further confirmed by ISH in Case 1). However, in keeping with plasmablastic morphology, they were positive for MUM-1/IRF-4. However, CD20, CD79a, PAX5, BCL6, CD10 and CD30 were negative. Interestingly, both cases showed aberrant expression of CD3 (partial), but were negative for other T-cell markers including CD2, CD5, CD7, CD4 and CD8. The heavy inter-follicular plasma cell population was highlighted by CD138, and was polyclonal for kappa and lambda light chains. CD23 highlighted the follicular dendritic meshwork and focal disruption of the follicles by the atypical cells was evident. CD30 was negative.
Flow cytometry performed in Case 1 showed a polytypic B-cell population (60.2% of all nucleated cells) and minor T-cell population (34.4% of all nucleated cells) with no aberrant T-cell antigen loss. PCR studies for immunoglobulin and T-cell receptor gene rearrangements were performed in both cases. Polyclonal rearrangement patterns were obtained in Case 1, while DNA amplification was unsuccessful in Case 2.
In case 2, one year after initial diagnosis, the patient underwent an inguinal lymph node biopsy. It showed an EBV-positive diffuse large B-cell lymphoma, which notably was negative for KSHV/HHV-8. PCR studies identified two significant clonal peaks for the IGκ locus, but a polyclonal pattern for IG frameworks II and III.
Discussion
Kaposi sarcoma–associated herpesvirus (KSHV), also known as human herpesvirus 8 (HHV8) is lymphotropic virus and plays a causative role in genesis of Kaposi sarcoma [6]. KSHV8/HHV8 has been associated with three distinct lymphoproliferative disorders: primary effusion lymphoma (PEL), multicentric Castleman disease (MCD), and most rarely, germinotropic lymphoproliferative disorder (GLPD) [1]. These lymphoproliferative disorders differ in terms of their clinical presentation, site(s) of involvement, EBV status, cytoplasmic immunoglobulin expression and rearrangement status of the immunoglobulin genes. PEL and MCD occur predominantly in immunodeficient patients. Patients have marked systemic symptoms and a poor prognosis [7, 8]. PEL involves predominantly the body cavities, along with non-cavitary sites of involvement, occurs in patients with human immunodeficiency virus (HIV) infection and does not show cytoplasmic immunoglobulin expression [7, 9]. In MCD, the HHV-8 infected cells express lambda light chain but are polyclonal by molecular testing. They can accumulate in small clusters, previously called microlymphomas [10]. In a small fraction of cases, progression to diffuse large B-cell lymphoma positive for HHV-8 occurs. However, co-infection with EBV is not seen [10–12].
In the group of KSHV-associated diseases, GLPD represents a novel entity, presenting with usually localized lymphadenopathy without immunodeficiency or immunosuppression and in the few reported cases, showing an overall favorable response to chemotherapy and radiation. In keeping with these features, both of our reported cases initially presented with localized lymphadenopathy without evidence of immunosuppression. Case 1 was later found to have diffuse lymphadenopathy involving the right axillary, right inguinal and left femoral areas by radiologic studies. However, there was a favorable response to chemotherapy and the patient remained largely asymptomatic a year and a half after the initial diagnosis. In case 2, a large localized mass involving the axillary region was surgically resected. This patient, however, progressed over the course of the year to develop generalized lymphadenopathy. A subsequent inguinal lymph node biopsy showed EBV-positive DLBCL, which was negative for KSHV, suggesting that it arose from an independent B-cell clone.
Molecular analysis of KSHV-positive plasmablasts in GLPD has demonstrated a polyclonal or oligoclonal pattern of immunoglobulin gene rearrangement, leading to the proposed terminology of “KSHV and EBV-associated germinotropic lymphoproliferative disorder (GLPD)”. Case 1 demonstrated the expected polyclonal pattern by PCR analysis; however, in case 2, DNA amplification was unsuccessful. In contrast to the three cases described by Du et al., that showed light chain restriction, both of our cases were negative for kappa and lambda when evaluated by immunohistochemistry, and also by ISH in one case.
Interestingly, both cases showed Castleman-like changes with marked plasmacytosis of the medullary cords resembling MCD – suggesting potential overlap with HHV-8 associated MCD. Both MCD and GLPD lack evidence of monoclonaity by PCR at the molecular level, and both show positivity for LANA and viral IL-6. However, only GLPD is co-infected by both HHV-8 and EBV. The latter finding overlaps with nodal involvement by PEL, making the differential diagnosis challenging, the major distinction being evidence of clonality.
There have been rare cases reported deviating from the major HHV-8 associated LPD delineated in the WHO classification. Recently, two cases of KSHV/HHV-8 associated lymphoma occurring in HIV-seronegative patients, without serous effusion or evidence of MCD have been reported. These cases showed a predilection for lymph nodes and displayed an anaplastic large cell morphology. They were completely devoid of lineage specific antigens; however, they demonstrated immunostaining with plasma cell reactive antibodies [13]. Also, cases of HHV8-positive large B-cell lymphoma with varying histological features including appearance suggestive of classical Hodgkin’s lymphoma in an immunocompetent patient and showing features of intravascular large B-cell lymphoma in an HIV-positive patient have been reported, expanding the spectrum of lymphoproliferative disorders that can be associated with HHV8 [14].
The origin of the plasmablasts in GLPD has been unclear. The presence of the atypical cells in the inter-follicular and mantle-zone areas raises the possibility of migration of neoplastic B-lymphocytes into germinal centers by ecotaxis/homing [15]. However, analysis by Du et al., of microdissected KSHV-positive cells showed evidence of somatic hypermutation and intraclonal variation, favoring a germinal center derivation [2]. Selective involvement of germinal centers has been reported in some aggressive B-cell lymphomas, including Burkitt’s lymphoma [16]. Previously, 3 cases of large B-cell lymphoma of the mediastinum showing marked tropism for germinal centers were described. These cases shared some clinical and morphological similarities with KSHV-associated GLPD. The tumor cells were lambda light chain restricted and expressed CD20. KSHV and EBV infection status were not explored, but other features including positivity for CD20 argue against GLPD [17].
KSHV-associated GLPD remains an intriguing disorder that responds to conventional chemotherapy in spite of dual KSHV and EBV co-infection. The role of viral IL-6, if any, remains elusive. As in PEL and KSHV-associated MCD, the activation of IL-6 receptor signaling through vIL-6 and human IL-6 may play a role in differentiation of KSHV-infected B-cells into plasmablasts and development of lymphoproliferative lesions [11, 18, 19]. The EBV latency proteins of the KSHV-infected plasmablasts in GLPD have not been previously studied. Both cases in our report were negative for the EBV latency proteins LMP1, EBNA2 and BZLF-1, indicating Latency I. Interestingly, plasmablastic lymphoma, which has a similar morphology and phenotype to GLPD also displays a Latency I phenotype for EBV[20]. In conclusion, our report expands the knowledge regarding the clinical and biological properties of GLPD, and show evidence of potential overlap with MCD and KSHV-associated lymphomas.
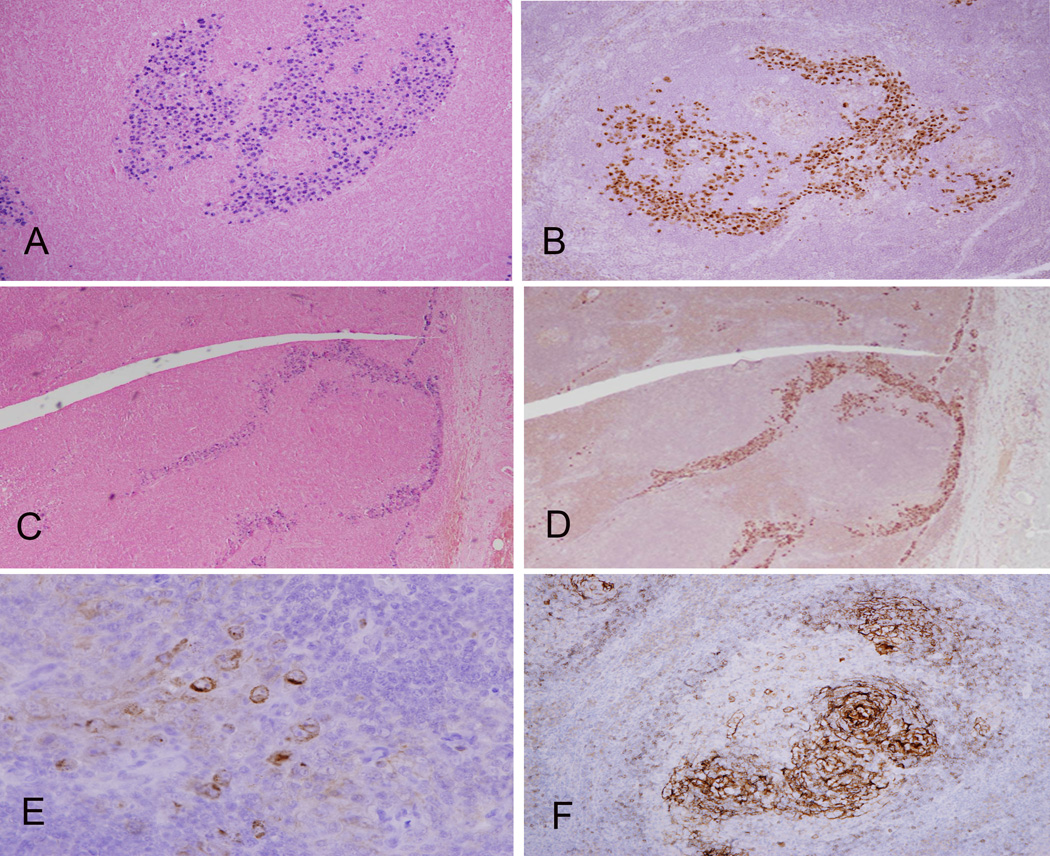
Figure 2. Localization of EBV and HHV-8 in GLPD.
The distribution of tumor cells within colonized follicles is highlighted by EBER in situ hybridization (A) and immunohistochemistry for the latent protein, LANA, of HHV-8 (B). However a sinusoidal distribution was seen focally with EBER (C) and staining for LANA (D). The atypical cells are focally positive for vIL-6 (E). CD23 staining shows an intact FDC meshwork within a partially involved follicle (F).
Acknowledgments
This work was supported by intramural research funding from the Center for Cancer Research, National Cancer Institute.
Footnotes
Disclosures: The authors have no other funding or conflicts of interest to disclose.
References
- 1.Cesarman E, Knowles DM. The role of Kaposi's sarcoma-associated herpesvirus (KSHV/HHV-8) in lymphoproliferative diseases. Semin Cancer Biol. 1999;9(3):165–174. doi: 10.1006/scbi.1998.0118. [DOI] [PubMed] [Google Scholar]
- 2.Du MQ, et al. KSHV- and EBV-associated germinotropic lymphoproliferative disorder. Blood. 2002;100(9):3415–3418. doi: 10.1182/blood-2002-02-0487. [DOI] [PubMed] [Google Scholar]
- 3.D'Antonio A, et al. KSHV- and EBV-associated germinotropic lymphoproliferative disorder: a rare lymphoproliferative disease of HIV patient with plasmablastic morphology, indolent course and favourable response to therapy. Leuk Lymphoma. 2007;48(7):1444–1447. doi: 10.1080/10428190701387039. [DOI] [PubMed] [Google Scholar]
- 4.Taris M, et al. KHSV/EBV associated germinotropic lymphoproliferative disorder: a rare entity, case report and review of the literature. Ann Pathol. 2014;34(5):373–377. doi: 10.1016/j.annpat.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 5.Oh J, et al. A case of successful management of HHV-8(+), EBV(+) germinotropic lymphoproliferative disorder (GLD) Int J Hematol. 2012;95(1):107–111. doi: 10.1007/s12185-011-0975-8. [DOI] [PubMed] [Google Scholar]
- 6.Sharp TV, Boshoff C. Kaposi's sarcoma-associated herpesvirus: from cell biology to pathogenesis. IUBMB Life. 2000;49(2):97–104. doi: 10.1080/15216540050022395. [DOI] [PubMed] [Google Scholar]
- 7.Nador RG, et al. Primary effusion lymphoma: a distinct clinicopathologic entity associated with the Kaposi's sarcoma-associated herpes virus. Blood. 1996;88(2):645–656. [PubMed] [Google Scholar]
- 8.Oksenhendler E, et al. High incidence of Kaposi sarcoma-associated herpesvirus-related non-Hodgkin lymphoma in patients with HIV infection and multicentric Castleman disease. Blood. 2002;99(7):2331–2336. doi: 10.1182/blood.v99.7.2331. [DOI] [PubMed] [Google Scholar]
- 9.Dupin N, et al. HHV-8 is associated with a plasmablastic variant of Castleman disease that is linked to HHV-8-positive plasmablastic lymphoma. Blood. 2000;95(4):1406–1412. [PubMed] [Google Scholar]
- 10.Du MQ, et al. Kaposi sarcoma-associated herpesvirus infects monotypic (IgM lambda) but polyclonal naive B cells in Castleman disease and associated lymphoproliferative disorders. Blood. 2001;97(7):2130–2136. doi: 10.1182/blood.v97.7.2130. [DOI] [PubMed] [Google Scholar]
- 11.Parravicini C, et al. Differential viral protein expression in Kaposi's sarcoma-associated herpesvirus-infected diseases: Kaposi's sarcoma, primary effusion lymphoma, and multicentric Castleman's disease. Am J Pathol. 2000;156(3):743–749. doi: 10.1016/S0002-9440(10)64940-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matolcsy A, et al. Immunoglobulin VH gene mutational analysis suggests that primary effusion lymphomas derive from different stages of B cell maturation. Am J Pathol. 1998;153(5):1609–1614. doi: 10.1016/S0002-9440(10)65749-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carbone A, et al. KSHV/HHV-8 associated lymph node based lymphomas in HIV seronegative subjects. Report of two cases with anaplastic large cell morphology and plasmablastic immunophenotype. J Clin Pathol. 2005;58(10):1039–1045. doi: 10.1136/jcp.2005.026542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferry JA, et al. HHV8-positive, EBV-positive Hodgkin lymphoma-like large B-cell lymphoma and HHV8-positive intravascular large B-cell lymphoma. Mod Pathol. 2009;22(5):618–626. doi: 10.1038/modpathol.2009.36. [DOI] [PubMed] [Google Scholar]
- 15.Warnke R, Levy R. Immunopathology of follicular lymphomas. A model of B-lymphocyte homing. N Engl J Med. 1978;298(9):481–486. doi: 10.1056/NEJM197803022980903. [DOI] [PubMed] [Google Scholar]
- 16.Mann RB, et al. Non-endemic Burkitts's lymphoma. A B-cell tumor related to germinal centers. N Engl J Med. 1976;295(13):685–691. doi: 10.1056/NEJM197609232951301. [DOI] [PubMed] [Google Scholar]
- 17.Suster S. Large cell lymphoma of the mediastinum with marked tropism for germinal centers. Cancer. 1992;69(12):2910–2916. doi: 10.1002/1097-0142(19920615)69:12<2910::aid-cncr2820691208>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 18.Oksenhendler E, et al. High levels of human herpesvirus 8 viral load, human interleukin-6, interleukin-10, and C reactive protein correlate with exacerbation of multicentric castleman disease in HIV-infected patients. Blood. 2000;96(6):2069–2073. [PubMed] [Google Scholar]
- 19.Jones KD, et al. Involvement of interleukin-10 (IL-10) and viral IL-6 in the spontaneous growth of Kaposi's sarcoma herpesvirus-associated infected primary effusion lymphoma cells. Blood. 1999;94(8):2871–2879. [PubMed] [Google Scholar]
- 20.Colomo L, et al. Diffuse large B-cell lymphomas with plasmablastic differentiation represent a heterogeneous group of disease entities. Am J Surg Pathol. 2004;28(6):736–747. doi: 10.1097/01.pas.0000126781.87158.e3. [DOI] [PubMed] [Google Scholar]