Abstract
Older adults living with HIV (OALWH) comprise a growing population with a range of complex and interconnecting medical and psychosocial needs. Based on the biopsychosocial model with its emphasis on a holistic approach to various aspects of people’s lives, the current study explored associations between physical health, psychological health, substance use, and overall quality of life. Drawing on data from 114 substance-using OALWH (aged 50 or older), we employed linear regression to show associations between the number of current comorbid health conditions on quality of life, over and above depression, substance use problems, and demographic characteristics (age, race/ethnicity, gender, sexual orientation, education, and relationship status). In both bivariate and multivariable contexts, the number of comorbid conditions was associated with reduced quality of life. Depression and substance use were also negatively associated with quality of life. These findings indicate that clinical and supportive care for OALWH, particularly when related to mental health and substance use, should also include an integrated focus on the comparatively high number of current comorbid conditions that often accompany, and potentially complicate, HIV treatment and quality of life.
Keywords: Aging, Comorbid, Depression, Substance use, Health
Introduction
Older adults living with HIV (OALWH) are a growing population [1], with recent estimates in the U.S. exceeding 275,000 [2]. This rise in the number of OALWH is expected to continue [1], consisting both of those who have recently contracted HIV, and those who have been living with HIV for many years. In some U.S. cities such as San Francisco, more than 50% of the total population of people living with an AIDS diagnosis are now aged 50 or older [3]. Accordingly, among the aims of the White House’s National HIV/AIDS Strategy [4] is the call to develop and deliver more age-appropriate care and services for older adults.
A growing body of research attests to the increased disease burden experienced by OALWH, with studies reporting that most OALWH are dealing with at least one other major health condition in addition to HIV, and an average of five non-HIV medications [5]. In the Research on Older Adults with HIV study, 77% of OALWH reported two or more comorbid conditions [6]. An average of 3.8 chronic comorbid conditions was reported by the 452 OALWH surveyed in Balderson and colleagues [7], while an average of more than four conditions was found in a study of the medical records of 374 OALWH, with older participants having significantly higher rates than younger participants [8]. Some of these comorbid conditions, such as HIV-associated neurocognitive disorders, arise as a direct result of prolonged HIV infection [9, 10], or from sustained courses of anti-retroviral therapy [11, 12]. Other conditions, such as diabetes, heart disease, and chronic non-viral hepatitis, arise as a normative part of the aging process [13]; however, these comorbid conditions may be exacerbated by concurrent HIV infection [14] and related treatment [15]. Furthermore, OALWH have been shown to experience more problems with mobility, self-care, and performing usual activities, compared to their HIV-negative counterparts [16].
Consistent with the biopsychosocial model, which emphasizes the connections between physical and mental health as well as the impact of social factors, recent research on OALWH increasingly points to the interconnectedness of physical health, psychosocial wellbeing, and issues of HIV-management. The burden of comorbid health conditions among OALWH has numerous and varying impacts on a range of health issues, such as mental health outcomes (especially depression) [17] and medication adherence [8]. Balderson and colleagues [7] found that higher numbers of chronic conditions in OALWH predicted greater stress and depression, and diminished physical and social functioning. Also, Vance and colleagues [8] noted the link between increased medication burden in OALWH and problematic HIV medication adherence, particularly in the presence of cognitive impairments. The relationship between comorbid health conditions and substance use among OALWH, however, has been relatively understudied.
The use of drugs and alcohol is a well-established predictor of HIV-medication adherence problems among HIV-positive people generally [18]. At the same time that the OALWH population is expanding, the number of older adults generally experiencing problems with substance use is also increasing [19], and rates of substance use are particularly high in OALWH compared to their HIV-negative counterparts [20–22]. Furthermore, the use of multiple substances is commonplace [23], persistent in many older adults [24], and associated with HIV medication non-adherence and self-reported viral load detectability [25]. Similar to their younger counterparts, associations in OALWH have been found between substance use and HIV medication adherence problems; however, for OALWH, it is increasingly likely that use occurs in the context of a network of adherence challenges due to their high rates of comorbid physical and mental health challenges.
In sum, many OALWH face a myriad of physical, mental, and social health challenges [6], and the existing literature highlights associations among these difficulties and quality of life [5, 7]. In this context, quality of life refers to the individual’s perceptions of their physical functioning, levels of felt energy, satisfaction with social connections, and psychological wellbeing. In previous studies, quality of life has been found to be the strongest correlate of depression in adults living with HIV/AIDS [26], and to be associated with chronic disease burden among OALWH [7].
The purpose of the current study was to enhance understanding of the interconnectedness of health-related outcomes among substance-using OALWH. We hypothesized that, at the bivariate level, a greater number of comorbid health conditions, greater depression, and greater substance use problems would each be associated with reduced quality of life. We also hypothesized that, controlling for demographic variables (age, race/ethnicity, gender and sexual orientation, education, and relationship status), the number of comorbid health conditions would be negatively associated with quality of life, over and above depression and substance use problems.
Methods
Procedure
Data were drawn from two in-person baseline interviews with 114 OALWH enrolled in a randomized controlled trial of the Wisdom In Spirituality Education (WISE) intervention, a psychosocial behavioral intervention aimed at improving medication adherence and reducing substance use in OALWH. Participants were recruited via a range of strategies: active recruitment at AIDS Service Organizations (ASOs) and Community Based Organizations (CBOs) where HIV-positive individuals receive services; passive recruitment at ASOs and CBOs; internet-based advertisements on listservs and websites targeting the HIV-positive community and/or service providers; participant referrals, and; individuals ineligible for other studies at our research center.
A total of 1462 individuals in the New York City area were screened for eligibility, and 303 (20.7%) were found to be eligible and willing to participate in the study, according to the following eligibility criteria: [1] being HIV-positive; [2] age 50 or older; [3] reporting lifetime alcohol/drug dependence in a drug category which participant is currently using in the prior 12 months); [4] being on a prescribed HAART medication regimen, and; [5] reporting sub-optimal adherence measured as self-report of missing at least 3 days in the prior 30 days. All eligibility criteria were confirmed at the first baseline assessment, with substance dependence criteria being confirmed using the Computerized Diagnostic Interview Schedule Version IV (CDIS-IV). Participants were excluded from the project if they demonstrated evidence of gross cognitive impairment using the Mini-Mental Status Examination (impaired if score <24), unstable, serious psychiatric symptoms, or current suicidal/homicidal ideation using the Structured Clinical Interview, DSM-IV Psychotic Screening Module. All study protocols were approved by the institutional review board of the research team.
Baseline interviews were conducted between December 2011 and October 2014. Of the 303 eligible and interested participants, 281 (92.7%) scheduled a baseline appointment, and 162 (57.7%) of these attended the first baseline appointment, gave their consent to participate, and were deemed eligible for the second baseline appointment. Of these, 142 (87.7%) attended the second baseline appointment, after which 125 (88%) were deemed eligible for, and randomized into, the study. Participants were compensated $30 for each baseline appointment. Five participants withdrew before the completion of the second baseline and, of the remaining 120, data were lost or incomplete for six cases, thus leaving a final analytic sample of 114 OAWLH.
Measures
Demographics
Participants reported their date of birth, gender, sexual orientation, race and ethnicity, level of education, annual income, employment status, and relationship status (whether partnered or single).
Comorbid Health Conditions
Participants were asked to indicate whether they had any of the following 25 chronic conditions in the past year (yes, no): arthritis, broken bones, brittle bones/osteoporosis, cancer, dermatological problems, diabetes, hearing loss, heart condition, hypertension, incontinence, menstrual problems, migraines, thyroid problems, nervous system disorders, pneumonia, non-cancer prostate problems, respiratory conditions, erectile problems, vaginal dryness, vaginal pain, shingles, staph infection, stroke, vision loss, and liver cirrhosis. Participant’s conditions were tallied to give a summed score of current conditions, consistent with previous research [27]. In the current analysis, anxiety and depression were excluded from the count of current conditions.
Quality of Life
The Medical Outcomes Study HIV Health Survey (MOS-HIV) [28] was used to measure the participant’s health-related quality of life across ten domains (e.g., pain, physical functioning, energy/fatigue, social functioning). Its 35 items ask participants to rate their health and daily functioning in relation to the past 4 weeks (e.g., “how much bodily pain have you generally had during the past 4 weeks?”) with responses including none, very mild, mild, moderate, severe, and very severe. Scores are converted to a total out of 100, with reverse-scoring of relevant domains such that higher scores indicate greater quality of life. The scale has been shown to be a good predictor of depression in HIV-positive adults [8]. In the current study, the scale showed strong internal consistency, α = 0.92.
Depression
Participants completed the 15-item Geriatric Depression Scale (GDS-15)[29] with a dichotomized response (yes, no). Scores can range from 0 to 15, with higher scores indicating greater depressive symptomatology. The GDS-15 has been validated to assess depression in OALWH [30], and has been used to screen late-life depression in HIV-positive adults [31]. Participants with scores greater than five received mental health referrals; those who scored greater than ten were provided with a clinical check-in and appropriate referrals. In the current study, the scale showed strong internal consistency, α = 0.83.
Substance Use Problems
The Shortened Inventory of Problems—Alcohol and Drugs scale (SIP-AD)[32] consists of 15 items assessing adverse intrapersonal and interpersonal consequences arising from use of alcohol and/or drugs in the past 3 months (e.g., “While drinking/using drugs, I have said harsh or cruel things to someone”). Participants rate the frequency of each on a scale of 0 (Never), 1 (Once or a few times), 2 (Once or twice a week), or 3 (Daily or almost daily). Possible scores range from a total of 0 to 45, with higher scores indicating greater problems. In the current study, the scale showed strong internal consistency, α = 0.95.
Participants
The final sample of 114 OALWH in the current study was mostly black (80.7%), and male (67.5%). Fifty (43.8%) participants identified as gay or bisexual males. More than half (53.5%) had some college education, and 80.8% earned less than $20,000 annually. Ages ranged from 50 to 66 years, with a mean age of 54.5 years (SD = 4.2). On average, participants had been HIV-positive for 17.4 years (SD = 6.7). These sample characteristics factored into our choice of covariates in subsequent analyses as described below.
Analytic Plan
Bivariate correlations among depression, substance use problems, and quality of life were calculated using Pearson’s product-moment coefficient, while correlations with age and number of comorbid conditions were assessed using Kendall’s Tau. Multivariable linear regression tested firstly demographic variables—age, race/ethnicity (referent = black), sexual and gender identity (referent = heterosexual males), education (referent = less than college), and relationship status (referent = single)—depression, and substance use problems as predictors of quality of life. In the second step of the regression analysis, number of comorbid conditions was entered, in order to enable inspection of R2-change.
Results
The demographic characteristics of the sample are displayed in Table 1. Participants reported having between 0 and 10 current comorbid health conditions (M = 2.8, SD = 2.3), excluding depression and anxiety. Table 2 displays bivariate correlations among quality of life, age, depression, substance use problems, and number of comorbid health conditions. Quality of life was negatively associated with all variables, while number of comorbid conditions was not correlated with age, depression, or substance use problems. Greater depression was associated with increased substance use problems.
Table 1.
Demographics, N = 114
| Total | ||
|---|---|---|
|
| ||
| N | % | |
| Race/ethnicity | ||
| Black | 92 | 80.7 |
| Latino | 8 | 7.0 |
| White | 11 | 9.6 |
| Multiracial/other | 3 | 2.6 |
| Gender and sexual orientation | ||
| Gay/bisexual male | 50 | 43.8 |
| Heterosexual male | 27 | 23.7 |
| Gay/bisexual female | 6 | 5.3 |
| Heterosexual female | 31 | 27.2 |
| Education | ||
| High school, GED, or less | 53 | 46.5 |
| Some college | 40 | 35.1 |
| Bachelor degree or more | 21 | 18.4 |
| Income | ||
| Less than $10 K | 46 | 40.4 |
| $10 K–$19 K | 46 | 40.4 |
| $20 K–$29 K | 15 | 13.2 |
| $30 K or more | 7 | 6.1 |
| Employment | ||
| Employed | 93 | 81.6 |
| Unemployed | 21 | 18.4 |
| Relationship status | ||
| Single | 60 | 52.6 |
| Partnered | 54 | 47.4 |
| Age (years) | M | SD |
| 54.5 | 4.2 | |
Table 2.
Bivariate correlations, means, and ranges (N = 114)
| 2 | 3 | 4 | 5 | M (SD) | Range | |
|---|---|---|---|---|---|---|
| 1. Quality of life | −0.13* | −0.52** | −0.30** | −0.22** | 73.1 (12.2) | 2.3–4.9 |
| 2. Age (years) | – | −0.02 | −0.02 | 0.04 | 54.5 (4.2) | 50–66 |
| 3. Depression | – | 0.35** | 0.01 | 5.1 (3.7) | 0–14 | |
| 4. Substance use problems | – | −0.02 | 21.5 (10.8) | 0–45 | ||
| 5. Comorbid health conditions | – | 2.8 (2.3) | 0–10 |
p <0.05,
p <0.01
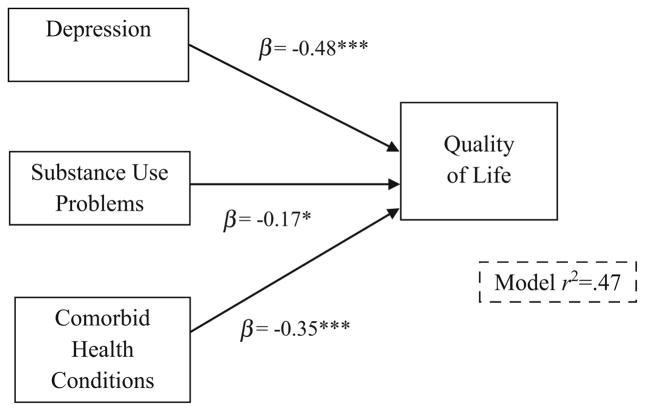
In the first step of our multivariable analyses, controlling for age, race/ethnicity, sexual and gender identity, education, and relationship status, depression was negatively associated with quality of life (β = −0.47, p <0.001), while substance use problems was not (β = −0.14, p = 0.11). This step of the model accounted for 36% of the variance in quality of life. In the second step, the number of comorbid conditions was entered and accounted for an additional 11% of the variance in quality of life. Age, depression, and substance use problems were negatively associated with quality of life. Over and above these covariates, the number of comorbid health conditions was negatively associated with quality of life (β = −0.35, p <0.001). Results of these analyses are displayed in Table 3, and depicted in Fig. 1.
Table 3.
Linear regressions on Quality of Life, N = 114
| Step 1
|
Step 2
|
|||||
|---|---|---|---|---|---|---|
| B | 95% CI | β | B | 95% CI | β | |
| Age | −0.03* | (−0.05, 0.00) | −0.19 | −0.02* | (−0.04, 0.00) | −0.15 |
| Race/ethnicity (referent = black) | 0.18 | (−0.06, 0.43) | 0.12 | 0.09 | (−0.13, 0.32) | 0.06 |
| Gender and sexual identity (referent = heterosexual males) | −0.17 | (−0.40, 0.06) | −0.12 | −0.10 | (−0.32, 0.11) | −0.07 |
| Education (referent =<college) | 0.01 | (−0.19, 0.21) | 0.01 | 0.02 | (−0.16, 0.20) | 0.02 |
| Relationship status (referent = single) | −0.14 | (−0.33, 0.06) | −0.11 | −0.17 | (−0.35, 0.01) | −0.14 |
| Depression | −0.08*** | (−0.11, −0.05) | −0.47 | −0.08*** | (−0.11, −0.05) | −0.48 |
| Substance use problems | −0.01 | (−0.02, 0.00) | −0.14 | −0.01* | (−0.02, 0.00) | −0.17 |
| Comorbid health conditions | −0.10*** | (−0.14, −0.06) | −0.35 | |||
| Model R2 | 0.36 | 0.47 | ||||
p ≤ 0.05;
p ≤ 0.01;
p ≤ 0.001
Fig. 1.
The impact of depression, substance use problems, and number of comorbid health conditions on quality of life *p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.001
Discussion
Findings from the current study illustrate the interconnected nature of physical, mental, and social health for OALWH in a manner that is highly consistent with the biopsychosocial model of health. Consistent with this framework, biological challenges (number of comorbid health conditions) and psychosocial health outcomes (depression and substance use problems) were each associated with reduced quality of life. These results indicate that quality of life in OALWH is substantially influenced by a range of health factors, whether physical or psychosocial, drawing attention to the critical role of co-occurring health challenges and the need for comprehensive care.
Consistent with previous research, our findings showed that the number of comorbid health conditions was associated with reduced quality of life [7], both at the bivariate level and alongside other known factors associated with reduced quality of life—depression [26] and substance use problems [33]. At the bivariate level however, comorbidity burden was not associated with depression in our sample, in contrast with previous studies [7, 17]. Given the multi-faceted nature of quality of life (encompassing domains such as social functioning, physical functioning, and general wellbeing), the impact of comorbid health conditions on its various domains requires further exploration in order to better inform interventions with OALWH. For example, comorbid health conditions may be impacting physical mobility, and research has shown associations between physical inactivity and depression [34], psychological distress [35], and the onset of other conditions like diabetes [36]. Accordingly, interventions targeting physical activity—which have been shown to improve physical and mental health among older adults generally [37, 38], and have been well-received by people living with HIV [39]—should be considered. Comorbid conditions may also be negatively impacting social functioning, the importance of which has been consistently highlighted for OALWH [40]. Accordingly, interventions aimed at maintaining or improving the quality and frequency of social interactions for OALWH should be prioritized.
At the bivariate level, we did not observe an association between comorbidity burden and substance use. That substance use problems were no more or less common among OALWH reporting high and low numbers of comorbid health conditions points to the complex and interrelated aspects of physical and mental health. Our finding of the negative association between substance use problems and quality of life also highlights the importance of addressing substance use among OALWH. This is in accordance with findings of previous research that substance use in OALWH is linked with a variety of mental health outcomes and health behaviors, including compromised medication adherence [25] and increased sexual risk-taking with casual partners [41]. Our findings suggest that interventions aimed at reducing substance use among OALWH need to take into account concurrent depression and comorbid health conditions. For example, interventions may ascertain and address how comorbid health conditions are impacting social support and mental health functioning aspects of quality of life, as these may be limiting the individual’s ability and willingness to reduce their substance use.
The current findings should be viewed in light of the study’s limitations—primarily, that our sample of OALWH were all substance-using individuals who were interested in, or motivated about the possibility of, reducing their substance use. The sample was mostly Black, of low SES, metropolitan-based, and with a high concentration of diagnoses of AIDS, which may limit the generalizability of our findings and may have contributed to restricted range on some of the variables of interest. The sample is also relatively small, and analyses may be unduly influenced by the number of demographic covariates included. Future research should attempt to test these associations on larger samples. Due to the eligibility criteria, all participants reported suboptimal medication adherence which prevented the examination of correlates or predictors of medication non-adherence. Also, comorbid health conditions were defined as those experienced in the past 12 months for which a doctor has issued a medical diagnosis. More exact data regarding the concurrent presence of symptoms with current HIV symptoms and/or treatment, and the severity associated with each condition would be helpful. Future research may focus on severity, as well as number, of comorbid conditions. Finally, longitudinal data to observe changes over time remains a focus of future research.
These findings highlight the relevance of depression, substance use problems, and comorbid health conditions on quality of life among OALWH, encouraging the development and refinement of interventions to improve general health and functioning in this population. These findings have implications for the development of comprehensive treatment programs for OALWH, including screening, monitoring, and prevention of physical conditions (such as low muscle mass and low bone mineral density) that could further diminish quality of life and which are found more commonly in people living with HIV [42]. They suggest that a whole-person approach informed by the biopsychosocial model is needed to address the interconnected nature of health challenges in OALWH. In sum, researchers and health care providers are encouraged by the current findings to approach OALWH with a comprehensive understanding of the interconnectedness of their medical, psychosocial, and behavioral challenges, and to view HIV within the wider context of each individual’s current health problems. An awareness of comorbid health conditions and of their potential to impact quality of life is required when addressing mental health and health behaviour outcomes in order to further improve care for this fast-growing and vulnerable population.
Acknowledgments
Funding: This study was funded by the National Institute of Drug Abuse (NIDA) (R01-DA029567); (PI: Jeffrey Parsons).
Footnotes
Compliance with Ethical Standards
Conflict of interest All authors declare that they have no conflict of interest.
Ethical Approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent Informed consent was obtained from all individual participants included in the study.
References
- 1.High KP, Brennan-Ing M, Clifford DB, et al. HIV and aging: state of knowledge and areas of critical need for research. A report to the NIH Office of AIDS Research by the HIV and Aging Working Group. J Acquir Immune Defic Syndr. 2012;60:S1–18. doi: 10.1097/QAI.0b013e31825a3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. [Accessed 2 Jan 2016];HIV Surveillance Report 2013. 2015 Feb;25 www.cdc.gov/hiv/library/reports/surveillance/index.html. [Google Scholar]
- 3.O’Keefe KJ, Scheer S, Chen M-J, Hughes AJ, Pipkin S. People fifty years or older now account for the majority of AIDS cases in San Francisco, California, 2010. AIDS care. 2013;25(9):1145–8. doi: 10.1080/09540121.2012.752565. [DOI] [PubMed] [Google Scholar]
- 4.The White House Office of National AIDS Policy. [Accessed 1 Dec 2015];National HIV/AIDS strategy: Updated to 2020. 2015 Jul; www.whitehouse.gov/sites/default/files/uploads/NHAS.pdf.
- 5.Rodriguez-Penney AT, Iudicello JE, Riggs PK, et al. Co-Morbidities in persons infected with HIV: increased burden with older age and negative effects on health-related quality of life. AIDS Patient Care STDS. 2013;27(1):5–16. doi: 10.1089/apc.2012.0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brennan M, Karpiak SE, Cantor MH, Shippy RA. Older adults with HIV: an in-depth examination of an emerging population. New York: Nova Science Publishers; 2009. [Google Scholar]
- 7.Balderson BH, Grothaus L, Harrison RG, McCoy K, Mahoney C, Catz S. Chronic illness burden and quality of life in an aging HIV population. AIDS Care. 2013;25(4):451–8. doi: 10.1080/09540121.2012.712669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vance DE, Mugavero M, Willig J, Raper JL, Saag MS. Aging with HIV: a cross-sectional study of comorbidity prevalence and clinical characteristics across decades of life. J Assoc Nurses AIDS Care. 2011;22(1):17–25. doi: 10.1016/j.jana.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 9.Cherner M, Ellis RJ, Lazzaretto D, et al. Effects of HIV-1 infection and aging on neurobehavioral functioning: preliminary findings. AIDS. 2004;18:27–34. [PubMed] [Google Scholar]
- 10.Ernst T, Chang L. Effect of aging on brain metabolism in antiretroviral-naive HIV patients. AIDS. 2004;18:61–7. [PubMed] [Google Scholar]
- 11.Marra CM, Zhao Y, Clifford DB, et al. Impact of combination antiretroviral therapy on cerebrospinal fluid HIV RNA and neurocognitive performance. AIDS. 2009;23(11):1359. doi: 10.1097/QAD.0b013e32832c4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gannon P, Khan MZ, Kolson DL. Current understanding of HIV-associated neurocognitive disorders pathogenesis. Curr Opin Neurol. 2011;24(3):275–83. doi: 10.1097/WCO.0b013e32834695fb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Önen NF, Overton ET, Seyfried W, et al. Aging and HIV infection: a comparison between older HIV-infected persons and the general population. HIV Clin Trials. 2010;11(2):100–9. doi: 10.1310/hct1102-100. [DOI] [PubMed] [Google Scholar]
- 14.Guaraldi G, Orlando G, Zona S, et al. Premature age-related comorbidities among HIV-infected persons compared with the general population. Clin Infect Dis. 2011;53(11):1120–6. doi: 10.1093/cid/cir627. [DOI] [PubMed] [Google Scholar]
- 15.Orlando G, Meraviglia P, Cordier L, et al. Antiretroviral treatment and age-related comorbidities in a cohort of older HIV-infected patients. HIV Med. 2006;7(8):549–57. doi: 10.1111/j.1468-1293.2006.00420.x. [DOI] [PubMed] [Google Scholar]
- 16.Miners A, Phillips A, Kreif N, et al. Health-related quality-of-life of people with HIV in the era of combination antiretroviral treatment: a cross-sectional comparison with the general population. Lancet HIV. 2014;1(1):e32–40. doi: 10.1016/S2352-3018(14)70018-9. [DOI] [PubMed] [Google Scholar]
- 17.Havlik RJ, Brennan M, Karpiak SE. Comorbidities and depression in older adults with HIV. Sex Health. 2011;8(4):551–9. doi: 10.1071/SH11017. [DOI] [PubMed] [Google Scholar]
- 18.Hinkin CH, Barclay TR, Castellon SA, Levine AJ, Durvasula RS, Marion SD, Longshore D. Drug use and medication adherence among HIV-1 infected individuals. AIDS Behav. 2007;11(2):185–94. doi: 10.1007/s10461-006-9152-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Substance Abuse and Mental Health Service Administration, Office of Applied Studies, USA. [Accessed 2 Jan 2016];An examination of trends in illicit drug use among adults aged 50–59 in the United States. 2009 www.ncjrs.gov/App/Publications/abstract.aspx?ID=250130.
- 20.Rabkin J, McElhiney M, Ferrando S. Mood and substance use disorders in older adults with HIV/AIDS: methodological issues and preliminary evidence. AIDS. 2004;18(S1):S43–8. [PubMed] [Google Scholar]
- 21.Justice AC. HIV and aging: time for a new paradigm. Curr HIV/AIDS Rep. 2010;7(2):69–76. doi: 10.1007/s11904-010-0041-9. [DOI] [PubMed] [Google Scholar]
- 22.Skalski LM, Sikkema KJ, Heckman TG, Meade CS. Coping styles and illicit drug use in older adults with HIV/AIDS. Psychol Addict Behav. 2013;27(4):1050–8. doi: 10.1037/a0031044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kedia S, Sell MA, Relyea G. Mono–versus polydrug abuse patterns among publicly funded clients. Subst Abuse Treat Prev Policy. 2007;2:33–42. doi: 10.1186/1747-597X-2-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Midanik LT, Tam TW, Weisern C. Concurrent and simultaneous drug and alcohol use: results of the 2000 National Alcohol Survey. Drug Alcohol Depen. 2007;90:72–80. doi: 10.1016/j.drugalcdep.2007.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parsons JT, Starks TJ, Millar BM, Boonrai K, Marcotte D. Patterns of substance use among HIV-positive adults over 50: implications for treatment and medication adherence. Drug Alcohol Depen. 2014;139:33–40. doi: 10.1016/j.drugalcdep.2014.02.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gore-Felton C, Koopman C, Spiegel D, Vosvick M, Brondino M, Winningham A. Effects of quality of life and coping on depression among adults living with HIV/AIDS. J Health Psych. 2006;11(5):711–29. doi: 10.1177/1359105306066626. [DOI] [PubMed] [Google Scholar]
- 27.Karpiak SE, Shippy RA, Cantor MH. Research on older adults with HIV. New York: AIDS Community Research Initiative of America; 2006. [Google Scholar]
- 28.Wu AW, Revicki DA, Jacobson D, Malitz FE. Evidence for reliability, validity and usefulness of the Medical Outcomes Study HIV Health Survey (MOS-HIV) Qual Life Res. 1997;6(6):481–93. doi: 10.1023/a:1018451930750. [DOI] [PubMed] [Google Scholar]
- 29.Yesavage JA, Brink T, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiat Res. 1983;17(1):37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 30.Kalichman SC, Sikkema KJ, Somlai A. Assessing persons with human immunodeficiency virus (HIV) infection using the Beck Depression Inventory: disease processes and other potential confounds. J Personal Assess. 1995;64(1):86–100. doi: 10.1207/s15327752jpa6401_5. [DOI] [PubMed] [Google Scholar]
- 31.Heckman TG, Barcikowski R, Ogles B, et al. A telephone-delivered coping improvement group intervention for middle-aged and older adults living with HIV/AIDS. Ann Behav Med. 2006;32(1):27–38. doi: 10.1207/s15324796abm3201_4. [DOI] [PubMed] [Google Scholar]
- 32.Blanchard KA, Morgenstern J, Morgan TJ, Labouvie EW, Bux DA. Assessing consequences of substance use: psychometric propoerties of the inventory of drug use consequences. Psychol Addict Behav. 2003;17(4):328–31. doi: 10.1037/0893-164X.17.4.328. [DOI] [PubMed] [Google Scholar]
- 33.Sassoon SA, Rosenbloom MJ, Fama R, Sullivan EV, Pfefferbaum A. Selective neurocognitive deficits and poor life functioning are associated with significant depressive symptoms in alcoholism–HIV infection comorbidity. Psychiatry Res. 2012;199(2):102–10. doi: 10.1016/j.psychres.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Strawbridge WJ, Deleger S, Roberts RE, Kaplan GA. Physical activity reduces the risk of subsequent depression for older adults. Am J Epidemiol. 2002;156(4):328–34. doi: 10.1093/aje/kwf047. [DOI] [PubMed] [Google Scholar]
- 35.Cairney J, Faulkner G, Veldhuizen S, Wade TJ. Changes over time in physical activity and psychological distress among older adults. Can J Psychiatry. 2009;54(3):160–9. doi: 10.1177/070674370905400304. [DOI] [PubMed] [Google Scholar]
- 36.Jefferis BJ, Whincup PH, Lennon L, Wannamethee SG. Longitudinal associations between changes in physical activity and onset of Type 2 Diabetes in older British men: the influence of adiposity. Diabetes Care. 2012;35(9):1876–83. doi: 10.2337/dc11-2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bann D, Hire D, Manini T, et al. Light intensity physical activity and sedentary behavior in relation to body mass index and grip strength in older adults: cross-sectional findings from the lifestyle interventions and independence for elders (LIFE) study. PLoS ONE. 2015;10(2):e0116058. doi: 10.1371/journal.pone.0116058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Loprinzi PD. Objectively measured light and moderate-to-vigorous physical activity is associated with lower depression levels among older US adults. Aging Ment Health. 2013;17(7):801–5. doi: 10.1080/13607863.2013.801066. [DOI] [PubMed] [Google Scholar]
- 39.Hand GA, Phillips KD, Dudgeon WD, Lyerly GW, Durstine JL, Burgess SE. Moderate intensity exercise training reverses functional aerobic impairment in HIV-infected individuals. AIDS Care. 2008;20(9):1066–74. doi: 10.1080/09540120701796900. [DOI] [PubMed] [Google Scholar]
- 40.Grov C, Golub SA, Parsons JT, Brennan M, Karpiak SE. Loneliness and HIV-related stigma explain depression among older HIV-positive adults. AIDS Care. 2010;22(5):630–9. doi: 10.1080/09540120903280901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Starks TJ, Millar BM, Parsons JT. Predictors of condom use with main and casual partners among HIV-positive men over 50. Health Psychol. 2015;34(11):1116–22. doi: 10.1037/hea0000236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Erlandson KM, Allshouse AA, Jankowski CM, MaWhinney S, Kohrt WM, Campbell TB. Functional impairment is associated with low bone and muscle mass among persons aging with HIV-infection. J Acquir Immune Defic Syndr. 2013;63(2):209. doi: 10.1097/QAI.0b013e318289bb7e. [DOI] [PMC free article] [PubMed] [Google Scholar]